1. Introduction
Multiple sclerosis (MS) is one of the most common immune-mediated demyelinating and neurodegenerative diseases of the CNS, however, the exact etiology is unknown. Functional gastrointestinal disorders in MS are common and the prevalence is higher with longer disease duration [
1]. There are also reports on increased incidence of inflammatory bowel diseases (IBD), including both Crohn’s disease (CD) and ulcerative colitis (UC), among MS patients which indicates a role of the gastrointestinal (GI) tract in the development of the disease [
2,
3]. We have previously demonstrated increased permeability and inflammation of the intestine in mice with experimental autoimmune encephalomyelitis (EAE), an animal model of MS [
4]. This finding was later confirmed by clinical study on MS patients [
5]. We also observed an activation of Th17 cells, but also increased levels of other IL-17- producing cells in the lamina propria (LP) and the gut lymphoid organs [
4].
Increasing amounts of evidence suggest a co-localization and a crosstalk between T cells and neutrophils during inflammation [
6,
7]. Along with their beneficial role in innate immunity, recruitment and accumulation of neutrophils in the intestine is often associated with mucosal damage and disease development [
8], suggesting that granulocytes may also play a role for intestinal pathology in MS. There is evidence suggesting a pathogenic role of neutrophils in development of extra-intestinal autoimmune diseases, e.g., in an MS model [
9]. Cytokines released by Th17 cells in association with goblet cell alterations, as well as elevated neutrophil infiltration in the gut followed by increased activity of myeloperoxidase (MPO), have been reported in IBD [
10,
11]. Elevated levels of IL-17 in CSF and blood of MS patients correlate with clinical exacerbations and neutrophil infiltration into the CNS [
12]. Neutrophils are essential in phagocytizing bacteria and release of antimicrobial proteins, such as calprotectin in the innate immune response. Calprotectin is a calcium and zinc binding heterocomplex of the S100A8 and S100A9 proteins, constituting approximately 60% of cytoplasmic content in neutrophils. Released calprotectin binds specifically to endothelial cells leading to loss of barrier function [
13,
14]. Increased serum levels of calprotectin have previously been reported in several inflammatory conditions, including IBD, cystic fibrosis, rheumatoid arthritis, and multiple sclerosis [
14,
15,
16,
17,
18]. As a consequence of intestinal inflammation, an increased concentration of calprotectin in feces is considered as a marker of inflammation, which is used for diagnosis and monitoring of IBD [
19]. Mild increase of fecal calprotectin also shown in different type of IBS [
20,
21]. Increased levels of calprotectin have also been detected in CSF of MS patients during the acute phase of the disease [
22].
Based on these premises, we investigated the infiltration and activity of neutrophils in the intestine, examined the level of fecal calprotectin and determined goblet cell number in EAE mice. We explored if fecal calprotectin might serve as marker of intestinal inflammation in EAE as it does in gastrointestinal inflammatory disorders.
2. Results
2.1. Increased Calprotectin in Plasma, Cecal Content and Feces
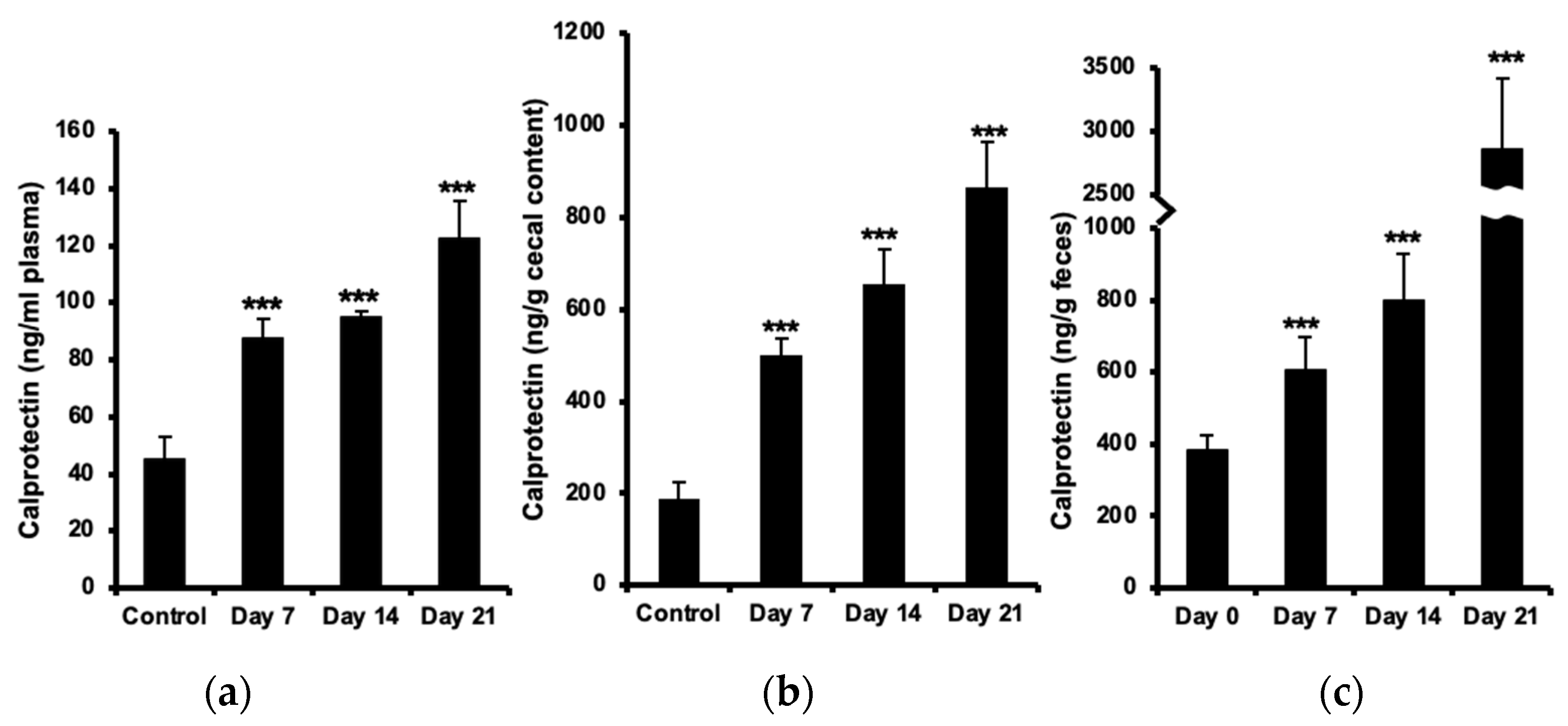
The concentration of plasma calprotectin increased (almost 2-fold) already before the onset of neurological symptoms at day 7 post immunization and further (up to 3-fold) at day 21, as compared with healthy controls (
Figure 1a). Calprotectin levels in cecal content was two-fold elevated at day 7 and more than four-fold at day 21 (
Figure 1b). The increase in fecal calprotectin was even more marked, reaching an 8-fold increase (almost 3 µg/g feces) at day 21 (
Figure 1c).
2.2. Increased Number of Neutrophils in the Intestine
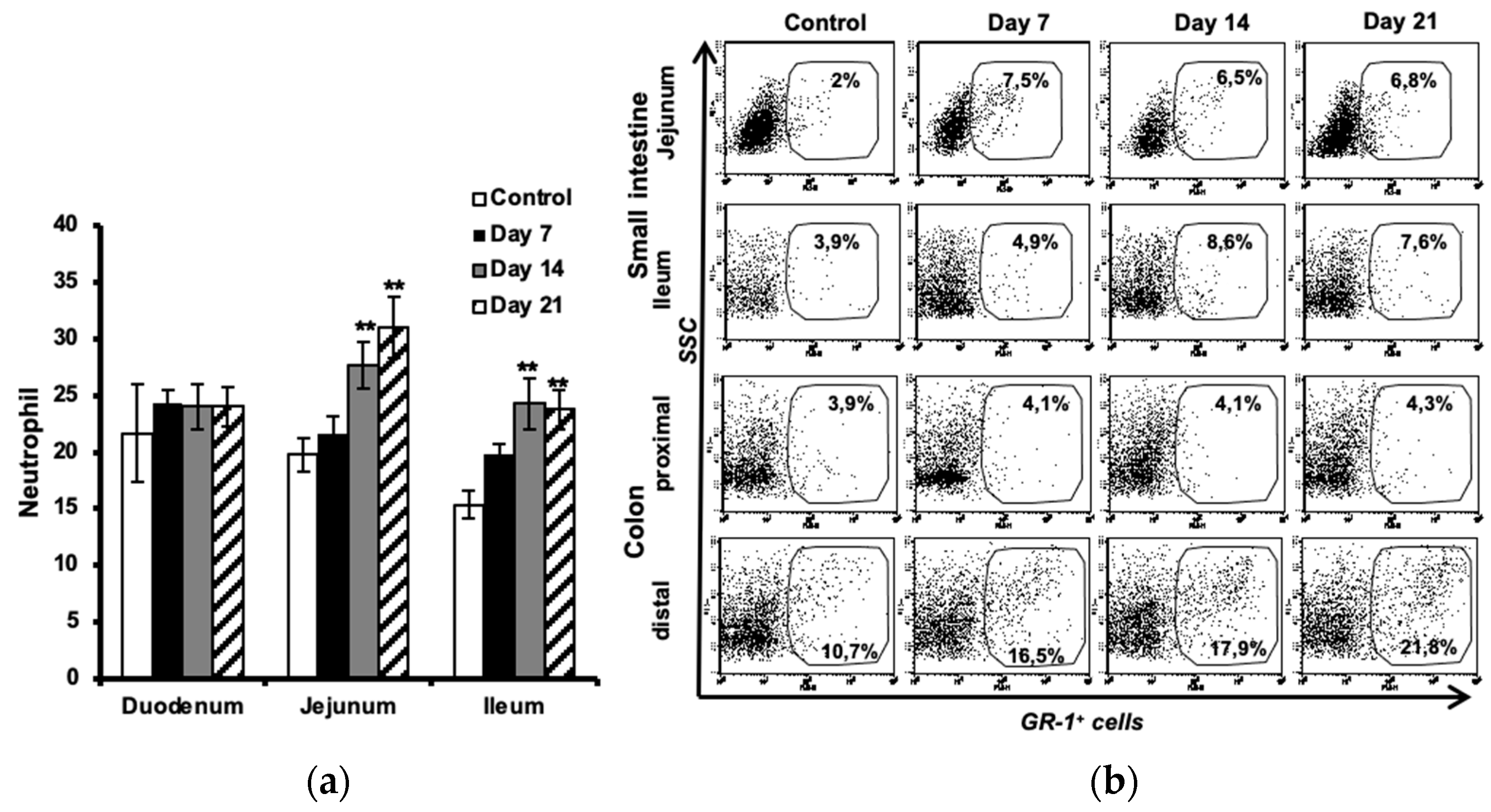
Histological evaluation of neutrophils in the small intestine showed increased number of these cells in lamina propria of the jejunum and ileum at the onset of the disease (day 7), which further augmented during the development of neurological symptoms (days 14 and 21 post immunization) as compared to the control (
Figure 2a).
Flow cytometric analysis of GR-1-expressing granulocytes confirmed a significant increase of neutrophils in the jejunum (more than 3 times) already at day 7 and with almost the same ratio during progression of disease. Increased numbers of neutrophils were observed in the ileum at day 7 which then increased more than 2 times at day 21. Further analysis in the colon revealed no significant changes in number of neutrophils in the proximal part but showed increased amount of these cells in the distal part at day 7 post-immunization, which then increased two folds at day 21 during the established and chronic phase of disease (
Figure 2b). All values are compared to results from unimmunized controls.
2.3. Increased IL-17 Expression in Intestinal Neutrophils
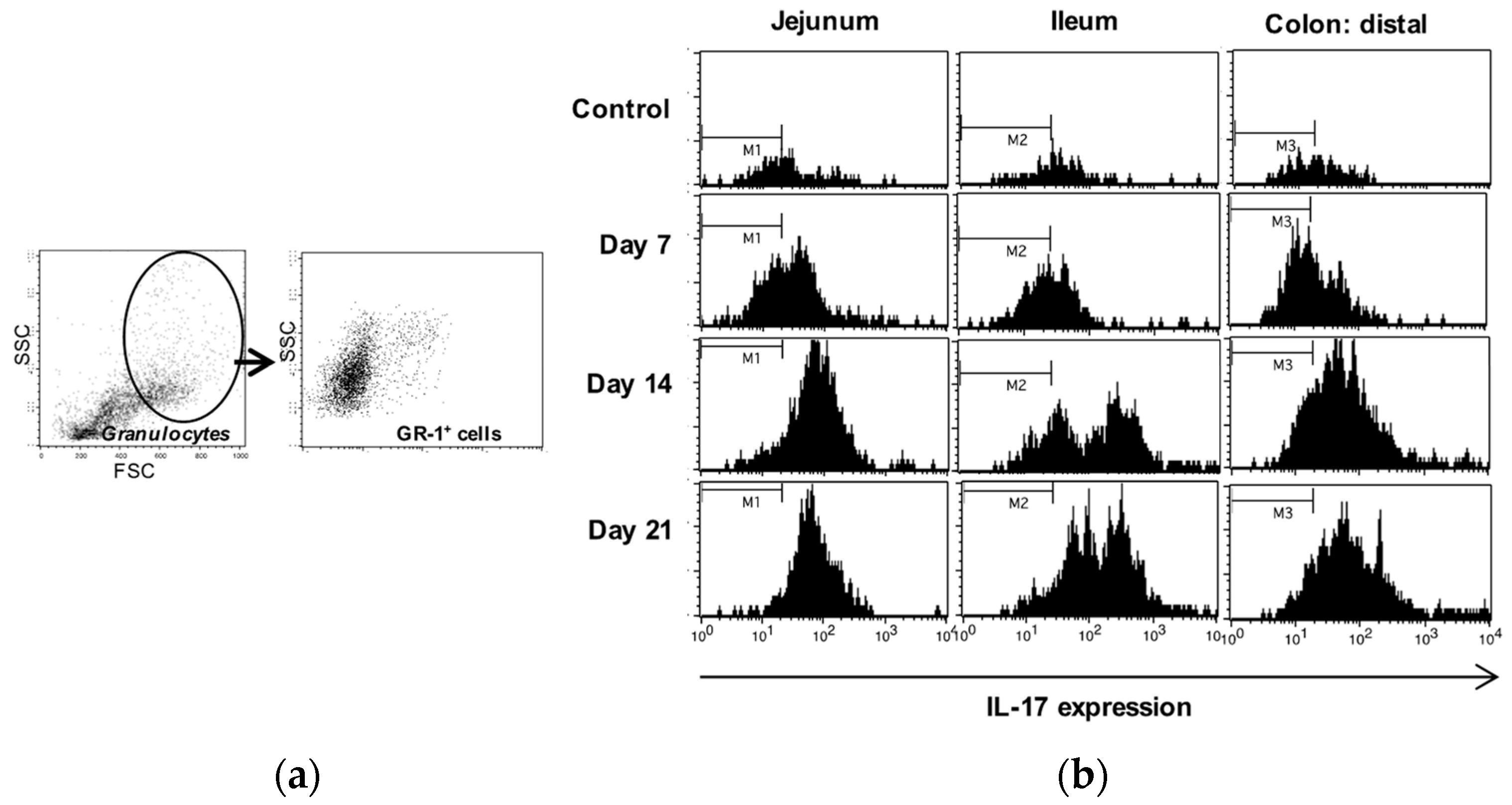
The IL-17 expression in neutrophils isolated from small intestinal and colon samples was analyzed using flow cytometry. The cell populations were sequentially gated for granulocytes (by light scattering properties), the neutrophil surface marker GR-1 (
Figure 3a) and then for expression of IL-17 (
Figure 3b). The results showed a minor upregulation of IL-17 in neutrophils already before the onset of disease (day 7) in jejunum and ileum and also in distal colon, to become marked at day 14 and 21. No differences between EAE and control animals were observed in the proximal colon (data not shown).
2.4. Enhanced Myeloperoxidase Activity in the Intestine
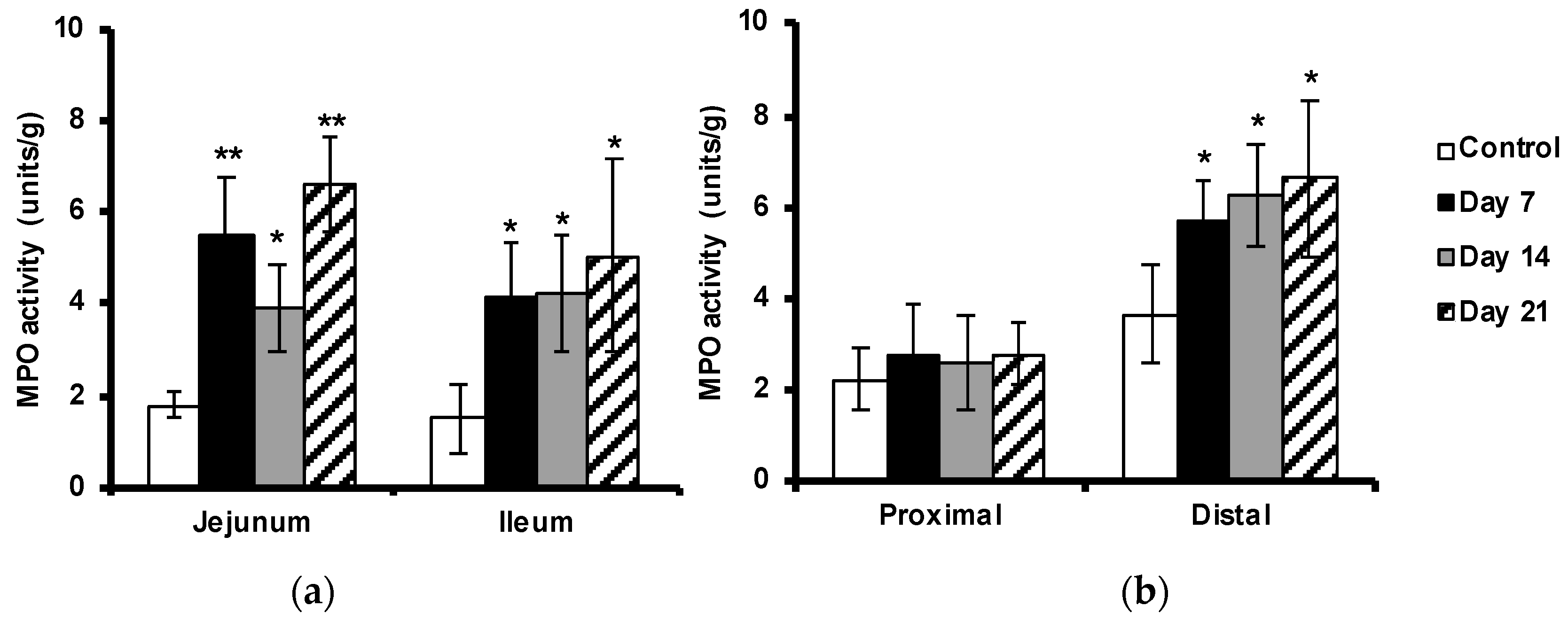
The MPO activity in the tissues was used to indicate neutrophil activation. MPO activity was increased in the small intestine and in the distal colon, but not significantly in the proximal colon, as compared with the non-immunized controls (
Figure 4a,b). In concert with the previously observed neutrophil infiltration, MPO activity was already raised before the onset of disease (at day 7) with more than 2-fold in small intestine, and about 50% in distal part of colon, compared to samples isolated from control animals.
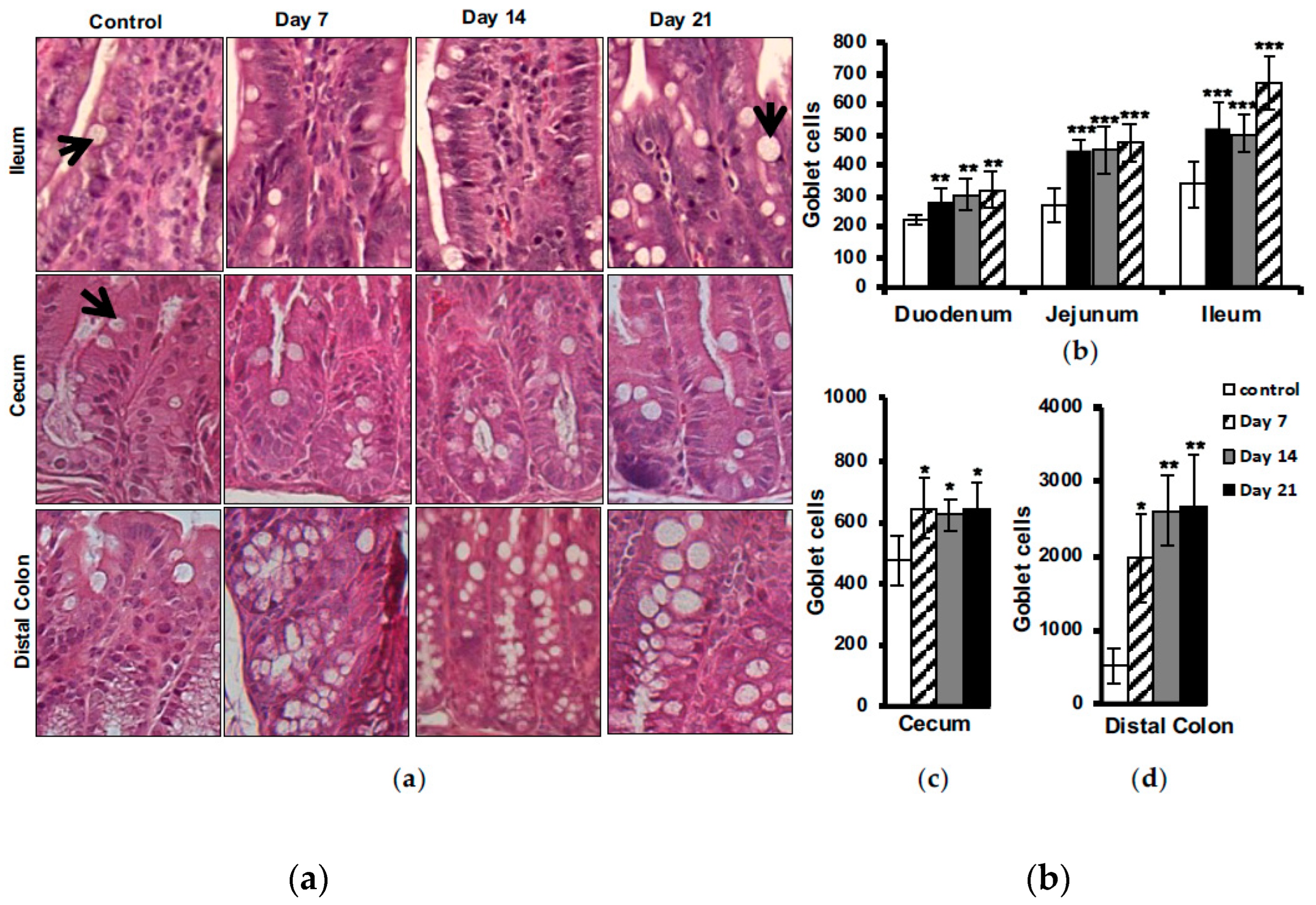
2.5. Increased Number of Intestinal Goblet Cells
Histological evaluation of goblet cells in different parts of the intestine showed an increased number of these cells in EAE animals at day 7 post immunization, with some further elevation to be seen at day 21 in the small intestine (
Figure 5a,b), cecum (
Figure 5a,c) and distal colon (
Figure 5a,d). No differences between EAE and control animals were observed in the proximal colon (data not shown).
3. Discussion
Fecal calprotectin is elevated in infectious and inflammatory conditions and has been suggested as a reliable, non-invasive marker which is specifically able to distinguish inflammatory from non-inflammatory conditions in different intestinal complications [
23,
24,
25]. In this work we report an increased level of fecal calprotectin in EAE mice, correlating temporally with disease development and innate immune alterations in the intestine. Our results correlate well with previous observations in CD and UC patients indicating a strong association between fecal calprotectin levels and the disease activities [
26]. Furthermore, we also revealed increased levels of calprotectin in the cecum contents and blood plasma of these animals. The data showed changes in calprotectin concentrations early during the disease development which then increased during the progress of the disease. The results are comparable with previous observations in MS patients showing elevated levels of calprotectin in plasma and CSF which correlated well with the disease activity and the presence of phagocyting cells in the active CNS lesions [
22,
27,
28].
Since increased intestinal permeability has been documented in patients with IBD, and luminal calprotectin excretion reflects the migration of neutrophils through the intestinal epithelium via intercellular junctions, a relationship between intestinal permeability and fecal calprotectin has been suggested [
29]. We have previously reported increased intestinal permeability and an altered small intestinal mucosal structure including inflammation in the EAE mice [
4]. Increased intestinal permeability in patients with multiple sclerosis has been also shown previously but no significant increase of calprotectin which could be duo to the anti-inflammatory medication [
5,
30]. In fact, it has been shown that Natalizumab, a common medication to decrease relapse frequencies in relapsing remitting MS, can reduce the intestinal inflammation with no effect on intestinal permeability [
31].
In order to explore a correlation between increased intestinal permeability and luminal calprotectin excretion, we further examined the GI tract and found increased numbers of infiltrating neutrophils in jejunal and ileal part of the small intestine and the distal part of the colon. Increased MPO activity in these intestinal segments confirmed the accumulation and activation of neutrophils in EAE mice. MPO activity has shown to be directly relative to the neutrophil number in animal models of intestinal inflammation [
32]. MPO is the most abundant peroxidase enzyme in the neutrophil azurophilic granules which is synthesized during myeloid differentiation and upon activation is involved in the induction of neutrophil apoptosis [
33,
34]. Previous results indicate the proinflammatory cytokines, e.g., IFN-γ and TNF-α, or other stimuli may regulate mucosal barrier function and provide signals for recruitment and activation of neutrophils and other inflammatory cells [
4,
29]. Epithelial damage may also reduce transepithelial resistance and allow leakage of luminal material into the lamina propria. A phenomenon called ‘leaky gut’ has been suggested as a preexisting primary mucosal defect in CD and has previously been demonstrated by us in EAE mice [
4,
35]. Our results show increased neutrophil recruitment to the intestinal tissue starting early during progress of CNS inflammation, concomitant with increased IL-17 expression and MPO activity. There is evidence of cross-talk between Th17 cells and activated neutrophils where they can trigger activation and reciprocal recruitment via release of chemokines [
7]. Th17 cells were found in gut tissue from CD and synovial fluid from rheumatoid arthritis patients [
7,
36]. Recent studies have also demonstrated a key role of these cells in the development of EAE during the early stages of the disease [
9]. Activated neutrophils are accumulating in the CNS, both prior and during the acute phase of EAE. IL-17 has been suggested to trigger pathogenic pathways mediating the disruption of blood-brain barrier and release of neutrophil-recruiting cytokines and growth factors leading to accumulation of neutrophils in the CNS. We have previously shown increased amounts of IL-17 producing cells in the gut and associated lymphoid tissues in the EAE mice. This may explain our observation on increased infiltration of neutrophil into the GI tract of these animals. Our further analysis on these neutrophils also revealed a markedly upregulated expression of IL-17 which may be triggered as an outcome of the available cytokine milieu. There is also evidence indicating that IL-17 may stimulate release of MPO from neutrophils [
37], which further explain increased MPO activity we observed in the intestinal tissues from EAE mice.
Goblet cells are present in the whole intestine and constitute the main source of mucins providing a dynamic protecting barrier influenced by inflammatory conditions [
38]. Goblet cell hyperplasia has been described in a number of infections caused by parasites, bacteria and viruses. They play an important role in gut barrier defense mainly by producing mucin and participate in immune responses by presenting luminal antigens to lymphoid cells. A regulatory role of T cells in infection-induced goblet cell hyperplasia has been suggested. IL-22, a cytokine produced by Th17 cells, has recently been identified to trigger goblet cell mucus production [
39,
40]. Nevertheless, depletion of goblet cells and their mucus production is a hallmark of IBD pathology [
38]. It may be speculated that the goblet cell depletion seen in IBD is a late manifestation of a long-standing and chronic inflammatory condition.
We report the reverse situation in EAE, a significant increase in goblet cell number, correlating with accumulation of intestinal neutrophils. The goblet cell hyperplasia we observed in the gut of EAE mice, may be mediated by increased activation of Th17 cells and release of their cytokines, in turn leading to recruitment of neutrophils and goblet cell hyperplasia. Furthermore, our finding may explain the increase of Akkermansia muciniphila in EAE and also MS patients. Akkeramensia consume mucin, and by increased amount of goblet cells there is more food for this bacterium which favoring increased amount of Akkeramensia. Increased goblet cells mostly indicate presence of a danger in gut lumen due to, e.g., bacterial invasion and exposure to LPS, which triggers goblet cell hyperplasia and subsequent mucus production flushing out the bacteria [
41]. In our previous study we showed an affected intestinal mucosal barrier with increased permeability early during development of EAE. We have further demonstrated an altered tight junction regulation and hypothesized an increased paracellular permeability exposing the body more to the environmental factors and harmful microbial invasion promoting the intestinal inflammation [
4]. This scenario may stimulate proliferation and differentiation of intestinal goblet cells and also result to recruitment of, phagocytes, e.g., neutrophils, to actively implicated and begin the immunological defense against the invasion. Observations from IBD patients have shown increased neutrophil migration into the gut leading to apoptosis and release of calprotectin, as a consequence of intestinal inflammation [
29].
In conclusion, we have shown that intestinal components of innate immunity are affected in EAE, including neutrophils and goblet cells, in conjunction with elevated calprotectin concentrations. Our data prompt a study on fecal calprotectin as a clinically useful marker of intestinal inflammation, and possibly also as a marker of neurological disease activity in MS patients. Furthermore, similarities between IBS and our findings in the EAE could suggest the EAE as a good model for IBS, however further investigation is needed.
4. Materials and Methods
4.1. Animals
Female C57BL/6 mice (8–10 weeks old) were obtained from (Taconic M & B A/S, Denmark). The mice were bred under specific pathogen-free condition in a controlled environment (20 ± 1ºC, 50% ± 10% relative humidity, 12:12- hour light dark cycle). The trials followed the European Community regulations for animal experiments and were approved by the local Ethical Review Committee for Animal Experiments (Permit Number: M211-11).
4.2. Induction and Assessment of EAE
A synthetic myelin peptide oligodendrocyte glycoprotein (MOG), amino acids 35–55 (MEVGWYRSPFSRVVHLYRNGK-COOH, Schafer-N, Denmark) was used to induce EAE. Mice were immunized by an intradermal injection with an emulsion containing 100 µg of the peptide in complete Freund’s adjuvant (H37RA, Difco laboratories, USA) together with i.p. injections of 200 ng pertussis toxin (Sigma-Aldrich, Sweden) at days 0 and 2. To follow the progression of disease the animals were weighed and examined daily for clinical signs of EAE in a blinded fashion[
42]. Since a disease incidence of approximately 80% is expected and the animals usually lose around 10% of their body weight, preceding by a few days the disease onset, which appears 8-10 days after immunization, only mice with weigh loss were examined at day 7. By day 14 and 21, animals with signs of disease were included in the experiment. Unimmunized healthy mice were used as control. At the end of the experiments the animals were anesthetized with a mixture of Ketamin (0.5 mg/g body weight; Ketalar, Parke-Davis, Sweden) and Azaperon (0.4 mg/g body weight; Stresnil, Janssen-Cilag Pharma, Austria), blood was taken from the vena cava into tubes containing 1.5 mg EDTA and 20 000 IU aprotinin (Trasylol, Bayer, Germany) that were ice-chilled until centrifugation at 3000 x g for 15 min to separate the plasma. The small intestine, cecum, cecal content and the colon from each animal were dissected for analysis.
4.3. Calprotectin Measurement
Feces was collected by temporally moving the animal to a separate clean cage at day 0 (before inducing the disease) and day 7, 14 and 21 after inducing EAE. The samples were kept at −20 °C until analysis. The concentration of calprotectin was determined by using a commercially available S100A8/S100A9 enzyme-linked immunosorbent assay (ELISA) kit (Immundiagnostik, Germany). A 4-parameter-algorithm used to form the standard curve and to the calculate data. Data were presented in ng/ml for plasma and in ng/g for feces and cecum content.
4.4. Microscopic Analysis of Neutrophils and Goblet Cells
Dissected small intestine, cecum and colon from 4 animals in each group (Control, EAE Day 7, Day 14, Day 21), was washed with phosphate buffered saline (PBS) and fixed in 4% phosphate buffered formaldehyde for 24 h and then stored in 70% ethanol until further processing. After embedding into paraffin, the tissues were cut laterally into 5 µm thick sections, deparaffinized and stained with hematoxylin and eosin (H&E) according to standard procedures. The sections were photographed by using an Olympus PROVIS microscope (objective 20x for goblet cells and 40x for neutrophils) equipped with an Olympus DP50 camera (Olympus, Japan). Cells were counted by using the ImageJ software (NIH, USA). The number of goblet cells was determined (counting vacuoles) in three entire sections from duodenum (the most proximal), jejunum (middle part) and ileum (the most distal) part of the small intestine, cecum as well as proximal and distal part of large intestine from each animal. Neutrophils were counted in the lamina propria of 5 randomly chosen villi from 3 different sections in each part of small intestine from the animals at either 7, 14 or 21 days after EAE induction and from the healthy control animals.
4.5. Flow Cytometry Analysis of Neutrophils
Flow cytometry analysis of neutrophils in the small intestine and colon was performed using our previous protocol [
42]. In brief, at the end of each experiment the mouse intestine was isolated, cleaned from fat and connective tissues, washed thoroughly with PBS to remove all content, opened longitudinally, cut into 0.5 cm pieces (the parts with Peyer’s patches were excluded) and shaken at 220 rpm in 25 ml EDTA solution for 30 min at 37 °C. Samples were centrifuged at 1500 x g and the supernatant was discarded, this process was repeated twice. The tissues were then washed with harvest medium consisting of RPMI 1640, heat-inactivated fetal bovine serum, HGPG (HEPES, L-glutamine, penicillin/streptomycin and gentamycin) for 5 min before incubation with collagenase (100 U/ml) for 45 min at 37 °C on the shaker followed by centrifugation. All cells were then incubated with anti-CD16/CD32 followed by FITC-conjugated anti-mouse Ly-6G (Gr-1) for neutrophils. For analysis of intracellular cytokines, cells were fixed with Cytofix/Cytoperm solution and stained with PE-conjugated anti-IL-17A, (eBioscience, USA). A FACSort flow cytometer; was used for acquisition of data and analysis was made with CELLQuest software (BD Biosciences, USA)[
42].
4.6. MYELOPEROXIDASE Activity
Tissue sections from the proximal and distal parts of the small and large intestines were collected after flushed with PBS to clean from their contents and snap-frozen in isopentane on dry-ice and then stored at -80 °C until measurements. Samples were weighed, homogenized in 0.02 M phosphate buffer (PB), pH 7.4 and centrifuged at 13500 x g for 5 minutes. The pellet was resuspended in 0.05 M PB, pH 6.0, containing 0.5% hexadecyltrimethylammonium bromide and frozen at −20°C for 2 h, then thawed at 25 °C, followed by sonication. Thereafter, the samples were placed in a water bath at 60 °C for 2 h and then centrifuged again. The supernatants were used and tetramethylbenzidine liquid substrate system (Sigma-Aldrich) was added as a peroxidase substrate and the mixture was incubated in the dark for 10 min at room temperature. To stop the reaction 0.5 M H2SO4 was used. The enzyme activity was determined spectrophotometrically, as the MPO catalyzed change in absorbance at 450 nm in the redox reaction of H2O2 at 25 °C. Values are expressed as MPO units per gram tissue.
4.7. Statistics
Statistical evaluation was performed using StatView software (SAS, USA). Calprotectin and MPO activity data were analyzed by using ANOVA with Bonferroni/Dunn testing. The results of the histological analysis were compared using a nonparametric Mann-Whitney test. In all statistical analyses, p ≤ 0.05 was taken as the level of significance.
Author Contributions
Conceptualization, M.N., S.L. and B.W.; methodology, MN, SL and BW; data curation, validation and visualization M.N. and S.L.; formal analysis and investigation, M.N., S.L.; resources S.L. and B.W.; writing—original draft preparation, M.N.; writing—review and editing, M.N., S.L., B.W.; supervision, S.L., B.W.; funding acquisition, M.N., S.L and B.W. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was partly funded by grant from the Royal Physiographic Society in Lund, Sweden.
Institutional Review Board Statement
The animal study protocol was approved by the local Ethical Review Committee for Animal Experiments (Permit Number: M211-11).
Acknowledgments
We would like to thank Agnes Paulus for her contribution in analysis of goblet cells.
Conflicts of Interest
MN is currently employed by ImmuneBiotech AB. SL is founder of ImmuneBiotech AB. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Marrie, R.A.; Leung, S.; Tyry, T.; Cutter, G.R.; Fox, R.; Salter, A. Functional gastrointestinal disorders negatively affect health-related quality of life in MS. Neurol Clin Pract. 2019, 9, 381–390;. https://doi.org/10.1212/CPJ.0000000000000668. [CrossRef]
- Alkhawajah, M.M.; Caminero, A.B.; Freeman, H.J.; Oger, J.J.F. Multiple sclerosis and inflammatory bowel diseases: What we know and what we would need to know! Mult Scler 2013, 19 (3): 259-265;. https://doi.org/10.1177/1352458512461393. [CrossRef]
- Norton, C.; Chelvanayagam, S. Bowel problems and coping strategies in people with multiple sclerosis. British Journal of Nursing. 2010, 19(4):220, 221-6. https://doi.org/10.12968/bjon.2010.19.4.46783. [CrossRef]
- Nouri, M.; Bredberg, A.; Weström, B.; Lavasani, S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One. 2014, 3;9(9):e106335. https://doi.org/10.1371/journal.pone.0106335. [CrossRef]
- Sjöström, B.; Bredberg, A.; Mandl, T.; Alonso-Magdalena, L.; Ohlsson, B., Lavasani, S.; Nouri, M.; Henriksson, G. Increased intestinal permeability in primary Sjögren’s syndrome and multiple sclerosis. J Transl Autoimmun. 2021, 4: 100082. https://doi.org/10.1016/j.jtauto.2021.100082. [CrossRef]
- Bert, S.; Nadkarni, S.; Perretti, M. Neutrophil-T cell crosstalk and the control of the host inflammatory response. Immunol Rev. 2023, 314(1):36-49. https://doi.org/10.1111/imr.13162. [CrossRef]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; Cassatella, M.A. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010, 115(2):335-43. https://doi.org/10.1182/blood-2009-04-216085. [CrossRef]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5(4):354-66. https://doi.org/10.1038/mi.2012.24. [CrossRef]
- Wojkowska, D.W., Szpakowski, P., Ksiazek-Winiarek, D., Leszczynski, M., Glabinski, A. Interactions between neutrophils, Th17 Cells, and chemokines during the initiation of experimental model of multiple sclerosis. Mediators Inflamm. 2014, 2014:590409. https://doi.org/10.1155/2014/590409. [CrossRef]
- Zhang, H.; Xue, Y.; Wang, H.; Huang, Y.; Du, M.; Yang, Q.; Zhu, M.J. Mast cell deficiency exacerbates inflammatory bowel symptoms in interleukin-10-deficient mice. World J Gastroenterol.2014, 9106–9115. https://doi.org/10.3748/wjg.v20.i27.9106. [CrossRef]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. Journal of Clinical Investigation. 2008, 118, 534–544. https://doi.org/10.1172/JCI33194. [CrossRef]
- V. Volin, M.; Shahrara, S. Role of TH-17 Cells in Rheumatic and Other Autoimmune Diseases. Rheumatology (Sunnyvale). 2011, 20;1(104): 2169. https://doi.org/10.4172/2161-1149.1000104. [CrossRef]
- Steinbakk, M.; Naess-Andresen, C.F.; Fagerhol, M.K.; Lingaas, E.; Dale, I.; Brandtzaeg, P. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet 1990, 336(8718):763-5. https://doi.org/10.1016/0140-6736(90)93237-j. [CrossRef]
- Konikoff, M.R.; Denson, L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006, 12(6):524-34. https://doi.org/10.1097/00054725-200606000-00013. [CrossRef]
- Golden, B.E.; Clohessy, P.A.; Russell, G.; Fagerhol, M.K.; Blood, G.R.; Golden, B.E. Calprotectin as a marker of inflammation in cystic fibrosis. Arch Dis Child. 1996, 74(2):136-9. https://doi.org/10.1136/adc.74.2.136. [CrossRef]
- Madland, T.M.; Hordvik, M.; Haga, H.J.; Jonsson, R.; Brun, J.G. Leukocyte protein calprotectin and outcome in rheumatoid arthritis: A longitudinal study. Scand J Rheumatol. 2002, 31(6):351-4. https://doi.org/10.1080/030097402320817077. [CrossRef]
- Frosch, M.; Metze, D.; Foell, D.; Vogl, T.; Sorg, C.; Sunderko, C.; Roth, J. Exp Dermatol. 2005, 14(4):259-65. https://doi.org/10.1111/j.0906-6705.2005.00271.x. [CrossRef]
- Olsson, A.; Gustavsen, S.; Hasselbalch, I.C.; Langkilde, A.R.; Sellebjerg, F.; Oturai, A.B.; Søndergaard, H.B. Biomarkers of inflammation and epithelial barrier function in multiple sclerosis. Mult Scler Relat Disord. 2020, 46:102520. https://doi.org/10.1016/j.msard.2020.102520. [CrossRef]
- Fagerhol, M.K. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet. 2000, 356(9244):1783-4. https://doi.org/10.1016/S0140-6736(00)03224-4. [CrossRef]
- Melchior, C.; Aziz, M.; Aubry, T.; Gourcerol, G.; Quillard, M.; Zalar, A.; Coëffier, M.; Dechelotte, P.; Leroi, A.M.; Ducrotté, P. Does calprotectin level identify a subgroup among patients suffering from irritable bowel syndrome? Results of a prospective study. United European Gastroenterol J. 2017, 5(2):261-269. https://doi.org/10.1177/2050640616650062. [CrossRef]
- Shulman, R.J.; Eakin, M.N.; Czyzewski, D.I.; Jarrett, M.; Ou, C.N. Increased Gastrointestinal Permeability and Gut Inflammation in Children with Functional Abdominal Pain and Irritable Bowel Syndrome. Journal of Pediatrics. J Pediatr. 2008, 153(5):646-50. https://doi.org/10.1016/j.jpeds.2008.04.062. [CrossRef]
- Berg-Hansen, P.; Vandvik, B.; Fagerhol, M.; Holmøy, T. Calprotectin levels in the cerebrospinal fluid reflect disease activity in multiple sclerosis. J Neuroimmunol. 2009, 216(1-2):98-102. https://doi.org/10.1016/j.jneuroim.2009.09.006. [CrossRef]
- Andréasson, K.; Scheja, A.; Saxne, T.; Ohlsson, B.; Hesselstrand, R. Faecal calprotectin: A biomarker of gastrointestinal disease in systemic sclerosis. J Intern Med. 2011, 270(1):50-7. https://doi.org/10.1111/j.1365-2796.2010.02340.x. [CrossRef]
- Røseth, A.G.; Kristinsson, J.; Fagerhol, M.K.; Schjønsby, H.; Aadland, E.; Nygaard, K.; Roald, B. Faecal calprotectin: A novel test for the diagnosis of colorectal cancer? Scand J Gastroentero. 1993, 28(12):1073-6. https://doi.org/10.3109/00365529309098312. [CrossRef]
- Klingberg, E.; Carlsten, H.; Hilme, E.; Hedberg, M.; Forsblad-D’Elia, H. Calprotectin in ankylosing spondylitis - Frequently elevated in feces, but normal in serum. Scand J Gastroenterol. 2012, 47(4):435-44. https://doi.org/10.3109/00365521.2011.648953. [CrossRef]
- Smith, L.A.; Gaya, D.R. Utility of faecal calprotectin analysis in adult inflammatory bowel disease. World J Gastroenterol. 2012, 14;18;(46):6782-9. https://doi.org/10.3748/wjg.v18.i46.6782. [CrossRef]
- Bogumil, T.; Rieckmann, P.; Kubuschok, B.; Felgenhauer, K.; Brück, W. Serum levels of macrophage-derived protein MRP-8/14 are elevated in active multiple sclerosis. Neurosci Lett. 1998, 247(2-3):195-7. https://doi.org/10.1016/s0304-3940(98)00263-8. [CrossRef]
- Floris, S.; Van Der Goes, A.; Killestein, J.; Knol, D.L.; Barkhof, F.; Polman, C.H.; Dijkstra, C.D.; De Vries, H.E.; Meilof, J.F. Monocyte activation and disease activity in multiple sclerosis. A longitudinal analysis of serum MRP8/14 levels. J Neuroimmunol. 2004, 148(1-2):172-7. https://doi.org/10.1016/j.jneuroim.2003.11.005. [CrossRef]
- Berstad, A., Arslan, G., Folvik, G.: Relationship between Intestinal Permeability and Calprotectin Concentration in Gut Lavage Fluid. Scand J Gastroenterol. 2000, 35(1):64-9. https://doi.org/10.1080/003655200750024551. [CrossRef]
- Becker, A.; Abuazab, M.; Schwiertz, A.; Walter, S.; Faßbender, K.C.; Fousse, M.; Unger, M.M. Short-chain fatty acids and intestinal inflammation in multiple sclerosis: modulation of female susceptibility by microbial products? Auto Immun Highlights. 2021;7;12(1):7. https://doi.org/10.1186/s13317-021-00149-1. [CrossRef]
- Nelson, S.M.L.; Nguyen, T.M.; Mcdonald, J.W.D.; Macdonald, J.K. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2018, 8(8):CD006097. https://doi.org/10.1002/14651858.CD006097.pub3. [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative Assay for Acute Intestinal Inflammation Based on Myeloperoxidase Activity Assessment of Inflammation in Rat and Hamster Models. Gastroenterology. 1984, 87(6):1344-50.
- Droeser, R.A.; Hirt, C.; Eppenberger-Castori, S.; Zlobec, I.; Viehl, C.T.; Frey, D.M.; Nebiker, C.A.; Rosso, R.; Zuber, M.; Amicarella, F.; Iezzi, G.; Sconocchia, G.; Heberer, M.; Lugli, A.; Tornillo, L.; Oertli, D.; Terracciano, L.; Spagnoli, G.C. High Myeloperoxidase Positive Cell Infiltration in Colorectal Cancer Is an Independent Favorable Prognostic Factor. PLoS One. 2013, 8(5):e64814. https://doi.org/10.1371/journal.pone.0064814. [CrossRef]
- Arnhold, J.; Flemmig, J. Human myeloperoxidase in innate and acquired immunity, Arch Biochem Biophys. 2010, 500(1):92-106. https://doi.org/10.1016/j.abb.2010.04.008. [CrossRef]
- Ma, T.Y. Intestinal epithelial barrier dysfunction in Crohn’s disease. Proc Soc Exp Biol Med. 1997, 214(4):318-27. https://doi.org/10.3181/00379727-214-44099. [CrossRef]
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014, 2014:928461. https://doi.org/10.1155/2014/928461. [CrossRef]
- Silverpil, E.; Glader, P.; Hansson, M.; Lindén, A. Impact of interleukin-17 on macrophage phagocytosis of apoptotic neutrophils and particles. Inflammation. 2011, 34(1):1-9. https://doi.org/10.1007/s10753-010-9201-8. [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr Gastroenterol Rep. 2010, 12(5):319-30. https://doi.org/10.1007/s11894-010-0131-2. [CrossRef]
- Kim, J.J.; Khan, W.I. Goblet cells and mucins: Role in innate defense in enteric infections, Pathogens. 2013, 2(1):55-70. https://doi.org/10.3390/pathogens2010055. [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Stevens, S.; Flavell, R.A. Innate and Adaptive Interleukin-22 Protects Mice from Inflammatory Bowel Disease. Immunity. 2008, 29(6):947-57. https://doi.org/10.1016/j.immuni.2008.11.003. [CrossRef]
- Toward, T.J.; Broadley, K.J. Goblet cell hyperplasia, airway function, and leukocyte infiltration after chronic lipopolysaccharide exposure in conscious guinea pigs: Effects of rolipram and dexamethasone. J Pharmacol Exp Ther. 2002, 302(2):814-21. https://doi.org/10.1124/jpet.102.033951. [CrossRef]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010, 2;5(2):e9009. https://doi.org/10.1371/journal.pone.0009009. [CrossRef]
Figure 1.
Increased calprotectin in blood plasma, cecal content and feces during progression of EAE. Calprotectin was measured using an ELISA kit for levels in plasma (a), cecal content (b) and feces (c) in unimmunized animals (control), and in EAE mice before (day 0) or at 7 (day 7), 14 (day 14) and 21 days (day 21) after immunization. The results are expressed as mean ±SD, (n= 7-10). *** indicate a statistical sign at p-value ≤0.001.
Figure 1.
Increased calprotectin in blood plasma, cecal content and feces during progression of EAE. Calprotectin was measured using an ELISA kit for levels in plasma (a), cecal content (b) and feces (c) in unimmunized animals (control), and in EAE mice before (day 0) or at 7 (day 7), 14 (day 14) and 21 days (day 21) after immunization. The results are expressed as mean ±SD, (n= 7-10). *** indicate a statistical sign at p-value ≤0.001.
Figure 2.
Increased number of neutrophils in the intestine during progression of EAE. Sections from duodenum, jejunum and ileum part of the small intestine, taken from unimmunized control animals and EAE mice at days 7, 14 and 21 post immunization, were stained with H&E. Microscopical quantitative analysis of neutrophils in the lamina propria were performed (a). Each bar represents mean ± SD of neutrophil count in five randomly chosen villi from three different sections per animal, (n=4). **represents a p-value ≤0.01 in comparison with the controls. Single cell suspension from jejunum, ileum, proximal and distal colon at 7, 14 and 21 days after EAE induction were used for neutrophil analysis by flow cytometry. Gating was done sequentially for granulocytes (by light scattering properties) and the neutrophil surface marker GR-1 (b). Dot plots from one representative experiment from three experiments.
Figure 2.
Increased number of neutrophils in the intestine during progression of EAE. Sections from duodenum, jejunum and ileum part of the small intestine, taken from unimmunized control animals and EAE mice at days 7, 14 and 21 post immunization, were stained with H&E. Microscopical quantitative analysis of neutrophils in the lamina propria were performed (a). Each bar represents mean ± SD of neutrophil count in five randomly chosen villi from three different sections per animal, (n=4). **represents a p-value ≤0.01 in comparison with the controls. Single cell suspension from jejunum, ileum, proximal and distal colon at 7, 14 and 21 days after EAE induction were used for neutrophil analysis by flow cytometry. Gating was done sequentially for granulocytes (by light scattering properties) and the neutrophil surface marker GR-1 (b). Dot plots from one representative experiment from three experiments.
Figure 3.
Increased IL-17 expression in intestinal neutrophils during progression of EAE. Single cell suspensions from small intestine (jejunum, ileum), and colon (distal) were used for flow cytometry analysis. The cells were gated as indicated for granulocyte (by light scattering properties) and further for expression of neutrophil surface marker, GR-1 (a). The expression of IL-17 was then investigated in GR-1+ cells in jejunum (b), ileum of the small intestine, and in colon distal, taken from unimmunized controls, and EAE mice at days 7, 14 and 21 after immunization. M1, 2 and 3 bars indicate the highest levels of IL-17 expression in cells isolated from control animals, representing a way to facilitate comparison between the groups. Data are representative of one of three independent experiments.
Figure 3.
Increased IL-17 expression in intestinal neutrophils during progression of EAE. Single cell suspensions from small intestine (jejunum, ileum), and colon (distal) were used for flow cytometry analysis. The cells were gated as indicated for granulocyte (by light scattering properties) and further for expression of neutrophil surface marker, GR-1 (a). The expression of IL-17 was then investigated in GR-1+ cells in jejunum (b), ileum of the small intestine, and in colon distal, taken from unimmunized controls, and EAE mice at days 7, 14 and 21 after immunization. M1, 2 and 3 bars indicate the highest levels of IL-17 expression in cells isolated from control animals, representing a way to facilitate comparison between the groups. Data are representative of one of three independent experiments.
Figure 4.
Increased MPO activity in the intestine during progress of EAE. MPO activity was measured in the jejunum and ileum parts of the small intestine (a) and the proximal and distal parts of colon (b) in control unimmunized mice and EAE mice at days 7, 14 and 21 after immunization. A colorimetric method was used and the results are expressed as mean ±SD (n= 4) * represents a p-value ≤0.05 and ** a p-value ≤0.01.
Figure 4.
Increased MPO activity in the intestine during progress of EAE. MPO activity was measured in the jejunum and ileum parts of the small intestine (a) and the proximal and distal parts of colon (b) in control unimmunized mice and EAE mice at days 7, 14 and 21 after immunization. A colorimetric method was used and the results are expressed as mean ±SD (n= 4) * represents a p-value ≤0.05 and ** a p-value ≤0.01.
Figure 5.
Increased goblet cell number in the intestine of EAE animals. H&E-stained sections from ileum part of small intestine cecum and distal colon (a) of unimmunized control and of EAE animals at days 7, 14 and 21 post immunization (objective 20X), arrows show goblet cells. The amount of goblet cells was counted performing microscopic quantitative analysis on sections from duodenum, jejunum and ileum of the small intestine (b), cecum (c) and distal colon (d). Each bar represents mean ± SD of three analyzed sections per animal, (n=4). * Represents a p-value ≤0.05, ** a p-value ≤0.01, and *** a p-value ≤0.001.
Figure 5.
Increased goblet cell number in the intestine of EAE animals. H&E-stained sections from ileum part of small intestine cecum and distal colon (a) of unimmunized control and of EAE animals at days 7, 14 and 21 post immunization (objective 20X), arrows show goblet cells. The amount of goblet cells was counted performing microscopic quantitative analysis on sections from duodenum, jejunum and ileum of the small intestine (b), cecum (c) and distal colon (d). Each bar represents mean ± SD of three analyzed sections per animal, (n=4). * Represents a p-value ≤0.05, ** a p-value ≤0.01, and *** a p-value ≤0.001.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).