1. Introduction
Cancer-associated fibroblasts (CAFs) play an important role in the tumour microenvironment, exhibiting functions in tumour migration, growth, metastasis, progression, and resistance to chemotherapy [
1]. Fibroblast activation protein (FAP) is a membrane-bound glycoprotein that is specifically overexpressed in activated fibroblast, including CAFs [
2]. FAPI-46, in particular, has emerged as a very promising theranostic tool in cancer. When labelled with the positron-emitting gallium-68, FAPI-46 has showed improved tumour-to-organ ratios, allowing for high contrast PET imaging of multiple types of tumours [
3]. In this context, there have been several attempts to optimize the GMP production and quality control of [
68Ga]GaFAPI-46, and its therapy counterpart [
177Lu]LuFAPI-46, to meet the increasing clinical demand for these radiopharmaceuticals [
4,
5,
6,
7].
Gallium-68 is available worldwide through the
68Ge/
68Ga generator [
8]. Despite being convenience, generators have limitations in terms of the maximum number of elutions and activity per elution, high cost, and the possibility of contamination with long-lived parent radionuclide germanium-68 (half-life 271 days) [
9]. On the other hand, cyclotron production of gallium-68 takes advantage of the extensive network of medical cyclotrons available that can produce considerable amounts and perform consecutive production cycles [
10]. The production of [
68Ga]GaFAPI-46 using gallium-68 from a cyclotron has not been reported, to our knowledge. The validation of its GMP production can help us to meet the growing demand for this radiopharmaceutical worldwide. In this work, we describe a method for the GMP automated synthesis and full validation of this process. We believe that this could serve as a roadmap for other laboratories that are trying to implement the routine synthesis of this radiopharmaceutical.
2. Results
2.1. [68Ga]GaFAPI-46 synthesis
Gallium-68 was produced using a standard medical cyclotron (IBA Cyclone Kiube, Louvain-la-Neuve, Belgium) by the irradiation of a zinc-68 solution for 70 to 80 minutes. Purification and synthesis were performed on an IBA Synthera extension fully automated platform (Louvain-la-Neuve, Belgium). The starting activity at the EOP was 4.31 ± 0.36 GBq (n=3), and the peptide quantity was 50 µg/batch (11.67 ± 0.97 µg/GBq). All syntheses were successfully completed within 25 minutes after the EOP, and all quality control parameters were in line Phar. Eur. specifications. Notably, the highest activities in the final product vial, with approximately 2.5 GBq of [
68Ga]GaFAPi-46, produced comparable results in terms of radiochemical purity to the lower activity vials (
Table 1).
Table 2 summarizes the results obtained from Spreckelmeyer (2020) and Alfeimi (2022) studies, which used different synthesis modules and generator produced gallium-68 to synthetize [
68Ga]GaFAPI-46 [
6,
7]. In comparison to these studies, we achieved similar results in terms of radiochemical yield (RCY) and radiochemical purity (RCP), with values of 90.53% and 98.94% (by radio-High-Performance Liquid Chromatography, radio-HPLC), respectively, but with up to three times more activity at the EOS.
2.2. Validation of analytical methods
Validation was performed for the 3 key QC techniques used to assess the quality of [
68Ga]GaFAPI-46: HPLC, Thin Layer chromatography (TLC) and Gas chromatography (GC). Method validation and analysis for sterility was conducted by a certified outsourcing company (Microbios, Barcelona, Spain). pH and endotoxin analysis were performed as described in the relevant monographs of Ph. Eur. [
11]. HEPES test was based on previously established procedures for other
68Ga-based radiopharmaceuticals (
e.g., monograph of GALLIUM (
68Ga) PSMA-11 INJECTION).
2.2.1. HPLC, TLC and GC methods validation
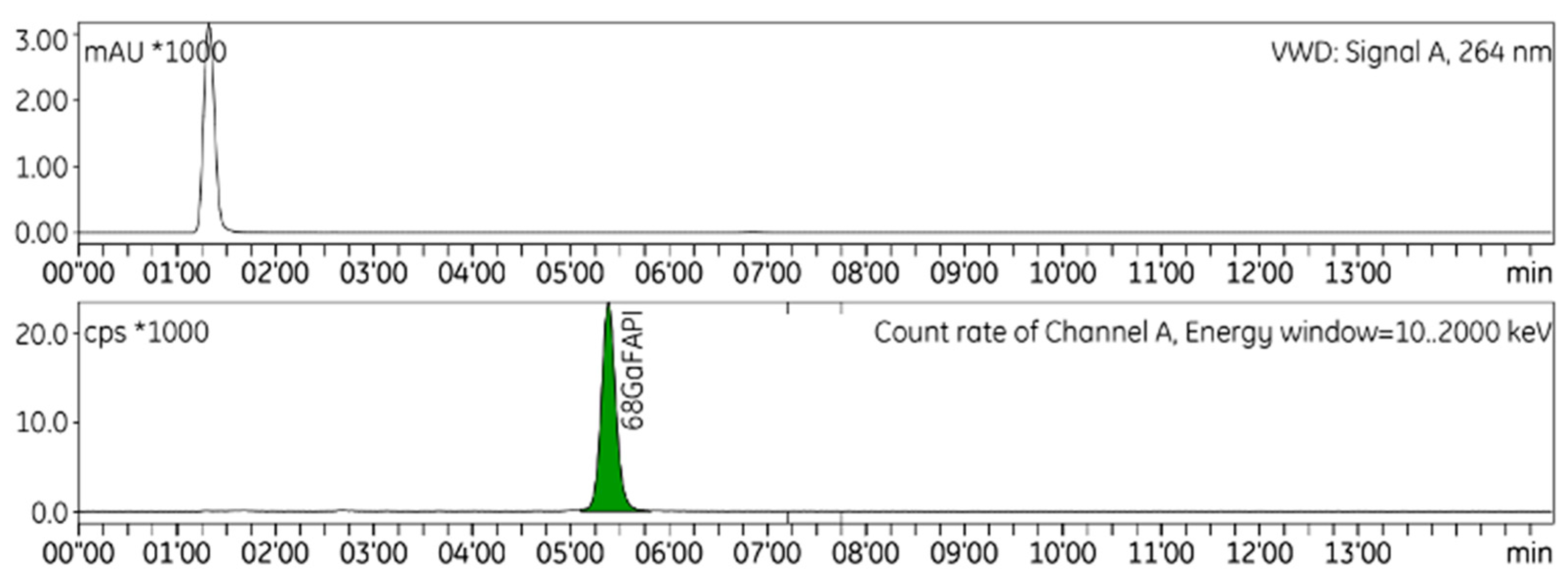
HPLC is used to identify and quantify radiochemical impurities in the drug product (
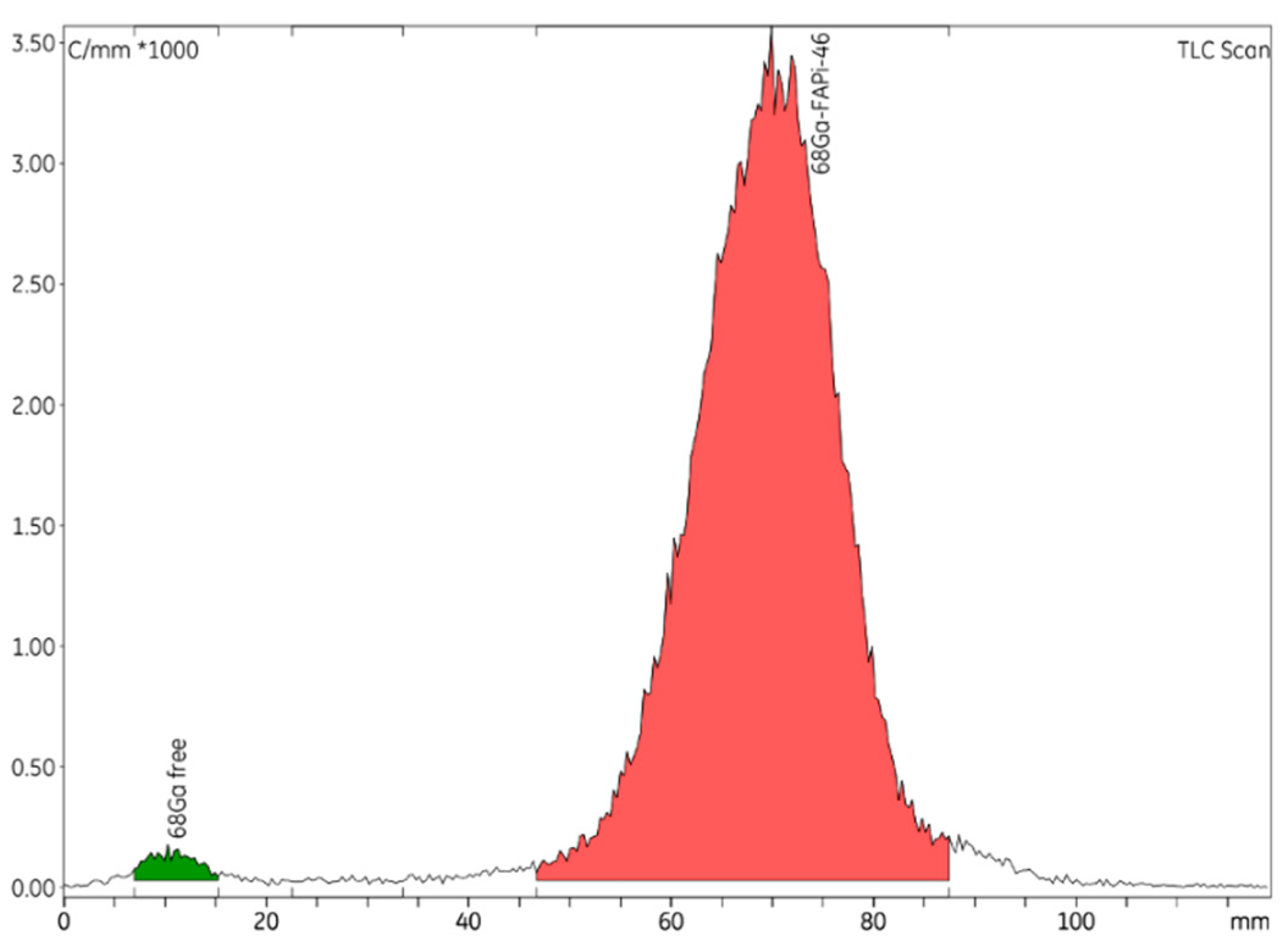
Figure 1). The radio-TLC technique is used to establish the radiochemical purity of [
68Ga]GaFAPI-46 (
Figure 2), and GC to quantify the presence of ethanol in the final formulation. To validate the analytical methods, the following parameters were checked: accuracy, repeatability, selectivity/specificity, quantification limit (LOQ), linearity, and range [
12]. A summary of the validation of HPLC, TLC and GC methods results can be seen in
Table 3.
2.3. Quality control
Quality control (QC) tests for [
68Ga]Ga-FAPI-46 were established based on the current requirements for other
68Ga-based radiopharmaceuticals monographs (e.g., monograph of GALLIUM (
68Ga) PSMA-11 INJECTION).
Table 4 displays the results of three exemplificative QC batches of [
68Ga]GaFAPI-46, which were also used to validate the synthesis process of the final drug product.
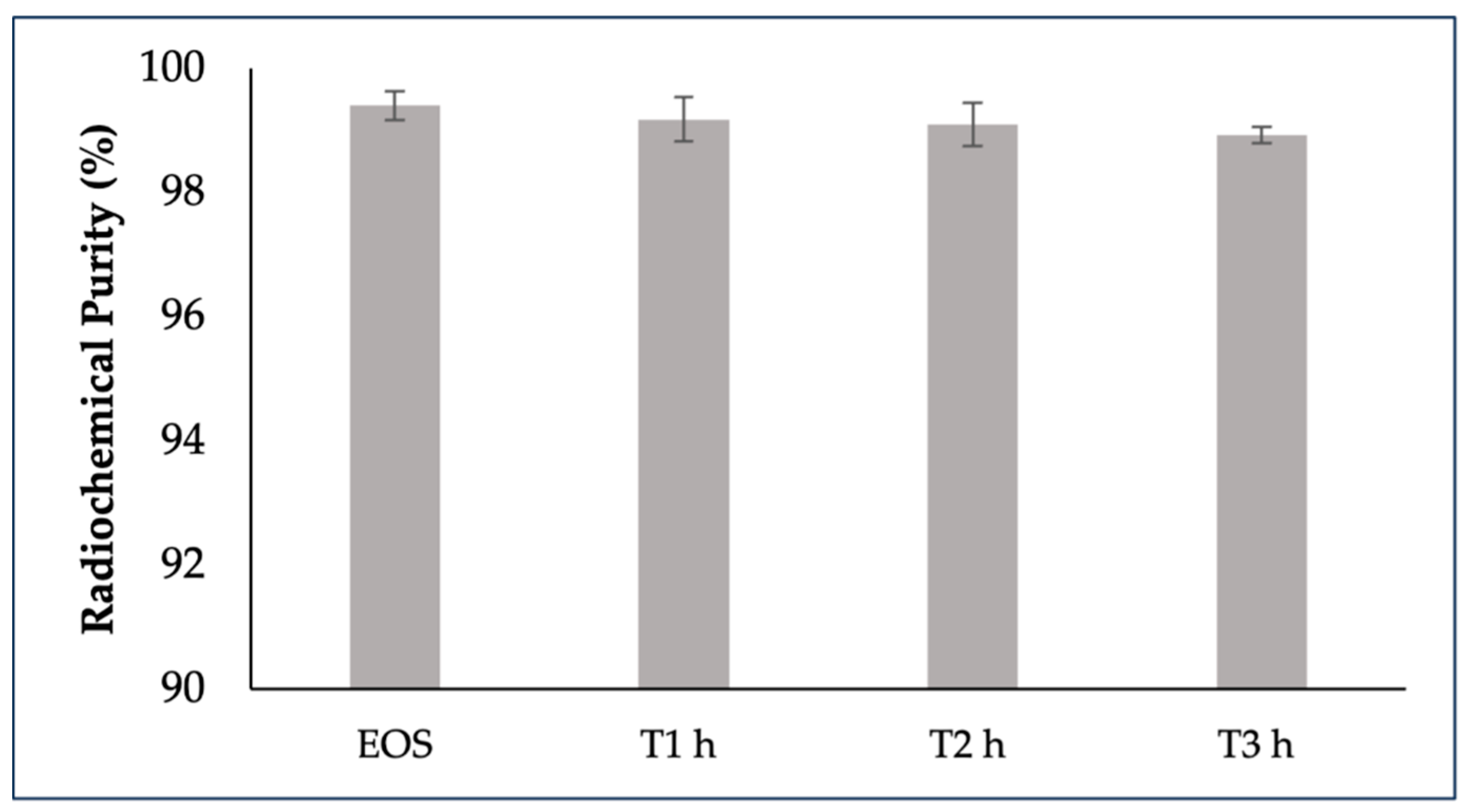
2.4. [68Ga]GaFAPI-46 stability
The stability of [
68Ga]Ga-FAPI-46 in 10% (v/v) ethanol formulation at room temperature was tested up to 3 h by radio-HPLC, [
68Ga]Ga-FAPI-46 was not stable under those conditions at higher activities. During this time period, two radioactive side-products were detectable, with increased percentage at higher activities. The stability of [
68Ga]Ga-FAPI-46 was achieved by adding 500 mg of sodium ascorbate to the final formulation. Moreover, the radiochemical purity of all batches remained above 95%, stable over a 3h period of incubation at room temperature (RT), regardless of vial activity (
Figure 3).
3. Discussion
Typically, the synthesis of
68Ga-based radiopharmaceuticals is performed using 1.85 GBq (from the generator elution). Protocols for fully automated production of [
68Ga]GaFAPI-46 using generators have been published with up to 1.7 GBq of [
68Ga]GaFAPi-46 [
7] and, generally, over 90% radiochemical yield (
Table 2). In this article, we present the results and validation of a fully automated synthesis method of [
68Ga]GaFAPI-46 produced from cyclotron. Furthermore, we have validated the specific QC analytical methods for this tracer.
When using gallium-68 from a cyclotron to produce [
68Ga]GaFAPi-46, the starting activities of gallium-68 can be as high as 5 GBq after purification. With the increasing demand for
68Ga-based radiopharmaceuticals in the last decade, cyclotron production of gallium-68 enables a better response to the clinical doses of gallium-68 which are routinely necessary. The automated synthesis of [
68Ga]GaFAPI-46 using Synthera® Extension (
Table 1) was found to be highly reproducible. Our results demonstrate that the use of 50 µg of FAPI-46 yields a similar radiochemical yield as in previously described studies [
6,
7], even with triple the activity.
The addition of sodium ascorbate in the final formulation of the product prevented radiolysis of the radiopharmaceutical. The formulation of [
68Ga]GaFAPI-46 in saline with 10% vol ethanol was found to be unstable. Although this effect has already been formerly described, it had not been observed to this extent. The use of ascorbic acid in the reaction has been reported to improve stability for
68Ga- and
177Lu-based radiopharmaceuticals [
4,
5,
13]. Additionally, the use of sodium ascorbate in the final formulation has been shown to enhance stability in [
177Lu]LuFAPI-46. Consistent with these findings, the use of sodium ascorbate prevented degradation of [
68Ga]GaFAPI-46 over a 3h period, even at high activities of up to 3.0 GBq. Results of synthesis validation, summarized in
Table 4, demonstrate that all the tested quality parameters were in accordance with the Phar. Eur.
4. Materials and Methods
The FAPI-46 precursor and the standard [natGa]Ga-FAPI-46 were manufactured by ABX (Radeberg, Germany) and were made available, free of charge, by SOFIE Biosciences (Dulles, VA, USA). An aqueous stock solution of 1 mg/mL was prepared, and aliquots of 50 µg were stored at -15° C. All chemicals were of analytical grade, and the solvents for high-pressure liquid chromatography (HPLC) were purchased as HPLC grade. Enriched zinc-68 (66 mg/mL and 98.0% isotopic enrichment) for gallium-68 production, as well as all the chemicals and tubing kits for gallium-68 purification, were purchased from Fluidomica (Cantanhede, Portugal).
4.1. Irradiation and purification of [68Ga]GaCl3
A regular production of gallium-68 via cyclotron liquid target was achieved using the method described previously [
10]. In summary, a solution of 66 mg/mL of zinc-68 was irradiated for a duration of up to 70 minutes using a Cyclone Kiube variable energy cyclotron (IBA; Louvain-la-Neuve, Belgium). Following the irradiation, the resulting target solution was transferred to a shielded hot-cell and automatically purified using the method formerly detailed [
14,
15].
4.2. Synthesis of [68Ga]GaFAPI-46 using a Synthera® Extension synthesizer
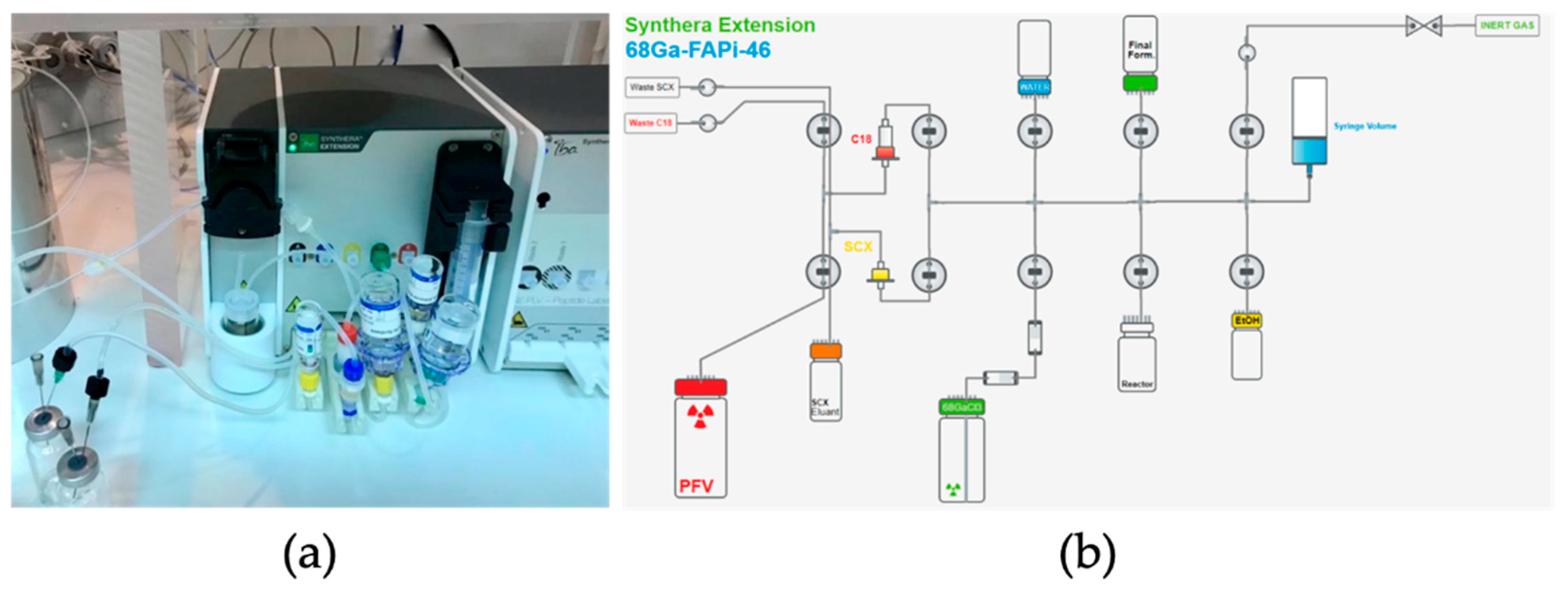
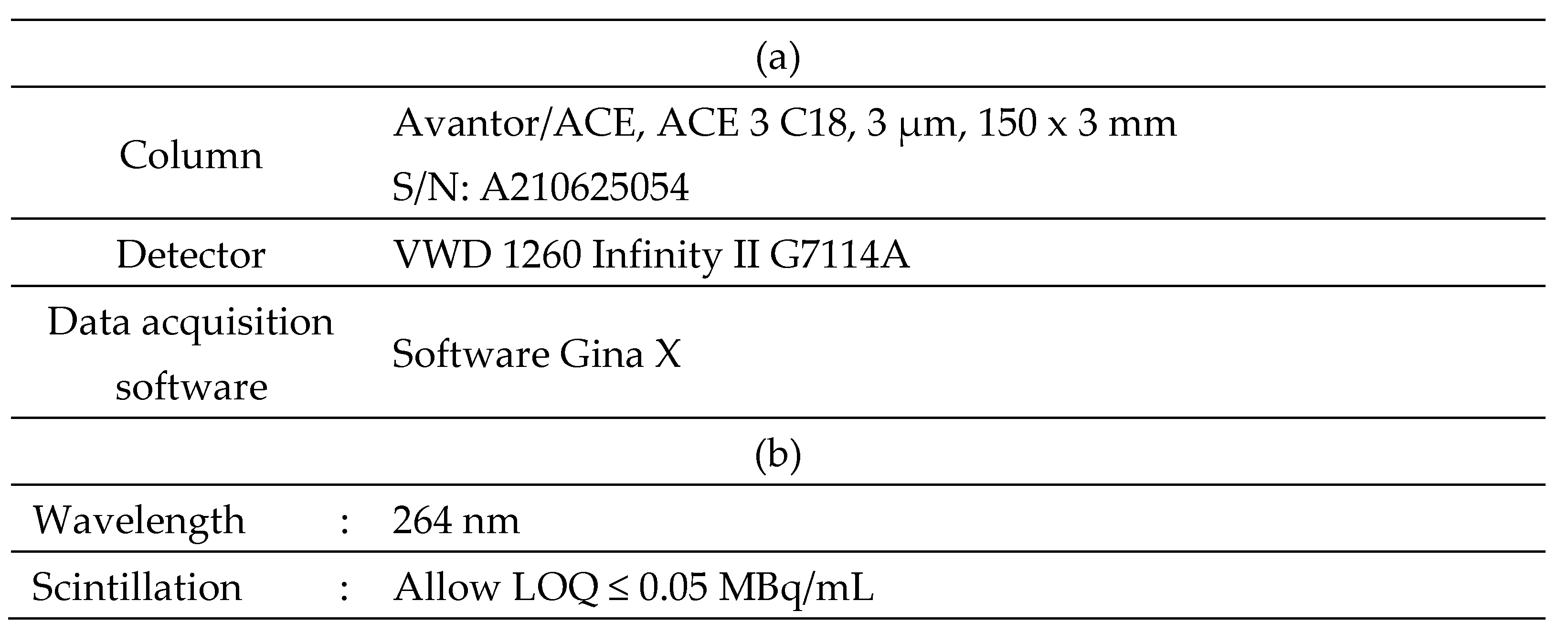
For the fully automated synthesis of [
68Ga]GaFAPI-46 using IBA (Louvain-la-Neuve, Belgium) Synthera® Extension module, shown in
Figure 1a, we used single-use labelling cassettes and reagent kits supplied by Fluidomica. The reagent kit included a SXC bound elute cartridge, a C18 plus short cartridge, HEPES buffer (0.5 M), saline, ethanol, and a sodium ascorbate vial (500 mg). The C18 cartridge required preconditioning with 10 mL of ethanol and 10 mL of water, and drying with air before use. Before galium-68 purification, the reaction mixture, consisting of 1 mL of 0.5 M HEPES buffer, 10 mg of ascorbic acid, and 50 µg of FAPI-46, was introduced into the reactor vial.
The initial step in the synthesis process involved concentrating the [68Ga]GaCl3 solution using an SCX cartridge and a peristaltic pump to prevent cross-contamination of the tubing system with free gallium-68. The loaded SCX cartridge was later eluted with a 5 M NaCl (in 0.05 M HCl) solution. To label [68Ga]GaFAPI-46, the gallium-68 solution was mixed with 50 µg of FAPI-46 precursor and heated to 90o C for 5 minutes. Following the labelling reaction, the solution was passed through the C18 cartridge, eluted with a mixture of 2 mL water/ethanol (1:1) and filtered into the final product vial, which was infused with sodium ascorbate.
Figure 4.
Set up to produce [68Ga]GaFAPI-46 on Synthera Extension Module. (a) Synthera Extension module with kit and reagents installed; (b) Layout scheme of the automatic module for the synthesis of [68Ga]GaFAPI-46.
Figure 4.
Set up to produce [68Ga]GaFAPI-46 on Synthera Extension Module. (a) Synthera Extension module with kit and reagents installed; (b) Layout scheme of the automatic module for the synthesis of [68Ga]GaFAPI-46.
4.3. Radionuclidic identity and purity
4.3.1. HPGe Analysis
The radionuclidic purity (RNP) of gallium-68 at the end-of-beam (EOB) was determined through γ-spectroscopy of the final solution using a High Purity Germanium detector (HPGe), several hours after the EOB. The HPGe was calibrated with 154Eu and 133Ba radioactive sources and placed in a low-background shielding. γ-spectra were acquired using point-source-like samples with a dead-time below 4%. GammaVision (ORTEC Inc.) software was used to determine photopeak areas.
4.3.2. Half-life measurements
The half-life was determined by measuring, gallium-68 in the Ionization Chamber with a time interval of 5 min.
4.4. Radiochemical Purity and Identity
4.4.1. HPLC Analysis
The HPLC method used in this study was previously described by Eryilmaz et al. [
5].
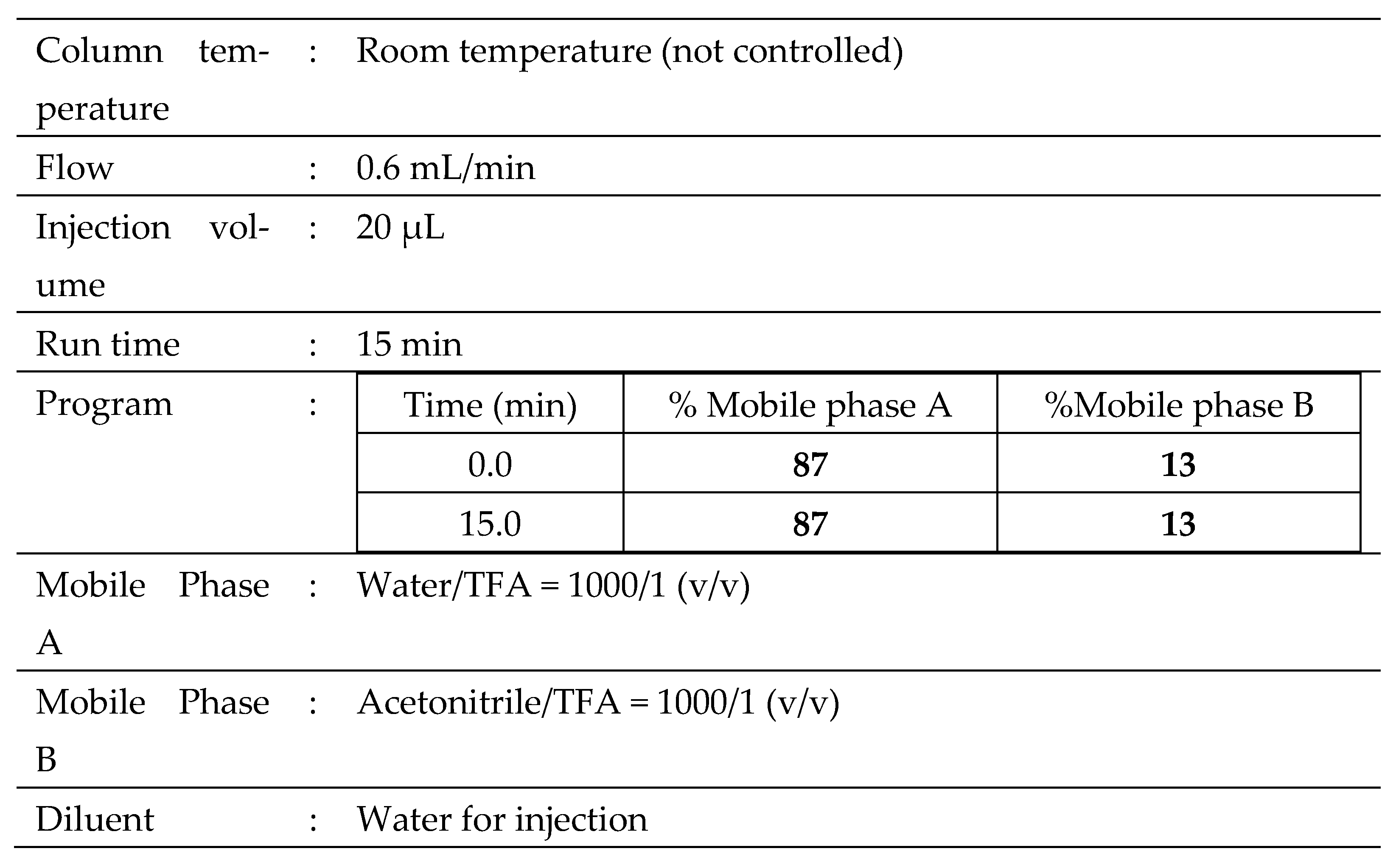
Table 5 A and B specify the equipment and operating conditions used during all HPLC analyses.
4.4.2. TLC Analysis
As described in the European Pharmacopeia (Eur. Phar.), an ammonium acetate (77 g/L):methanol (50:50 v/v) solution was used as the mobile phases for iTLC, and iTLC-SG strips were used as the stationary phases. The colloidal species of gallium-68 were detected at Rf < 0.1 and the product [68Ga]GaFAPI-46 was detected at Rf > 0.5.
Table 6.
TLC method used to determine radiochemical purity of [68Ga]GaFAPI-46 drug product. (a)
Specifications of the TLC equipment used, model miniGita; (b) Operation conditions of the method.
Table 6.
TLC method used to determine radiochemical purity of [68Ga]GaFAPI-46 drug product. (a)
Specifications of the TLC equipment used, model miniGita; (b) Operation conditions of the method.
| (a) |
| Detector |
Scintillation |
| Data acquisition software |
TLC Control software, version 2.30 |
| (b) |
| Detector |
Scintillation |
: |
Allow LOQ ≤ 0.5 MBq/mL |
| Others |
Chromatographic paper |
: |
Agilent iTLC-SG |
| |
Application volume |
: |
5 µL |
| |
Elution length |
: |
80 mm (origin: 1.0 cm from the bottom end; elution front: 2.0 cm from the top end) |
| |
Mobile Phase A |
: |
Ammonium acetate 1.0 M / methanol = 1/1 (v/v) |
| |
Diluent |
: |
Water for injection |
4.5. Residual Solvents
4.5.1. Ethanol
The presence of ethanol was evaluated by gas chromatography (GC) using an Agilent 6850 RaytestGmbh (Straubenhardt, Germany) GC system.
4.5.2. HEPES
This system uses TLC aluminum foil as the stationary phase and a mixture of water and acetonitrile (25:75 v/v) as the mobile phase. A reference solution containing 200 µg of HEPES in 10 mL of water was eluted in the strip along with the sample and the strip is then exposed to iodine vapor for 4 min for HEPES detection.
Table 7.
GC method used to quantify the presence of EtOH in the final drug product. Specifications of the GC equipment used, model 6850A, and operation conditions of the method.
Table 7.
GC method used to quantify the presence of EtOH in the final drug product. Specifications of the GC equipment used, model 6850A, and operation conditions of the method.
| Injector |
| Mode |
Split |
| Temperature |
250 °C |
| Split ratio |
15:1 |
| Gas |
Helium |
| Liner |
Cone liner with glass wool, 4.0 mm ID, PN#5183-4647. |
| Oven |
| Equilibrium time |
0.00 min |
| Run time |
15.0 min |
| Detector |
| Temperature |
260 °C |
| Mode |
Constant Makeup |
| Makeup flow |
30 mL/min (He) |
| Hydrogen flow |
30 mL/min |
| Air flow |
300 mL/min |
Column
(HP-Fast Residual Solvent; PN#1095V-420E or equivalent) |
| Mode |
Constant flow |
| Flow |
3.0 mL/min |
| Length |
30 m |
| Internal diameter |
530 µm |
| Film Thickness |
1.0 µm |
4.6. Stability of [68Ga]GaFAPI-46
The stability of [
68Ga]GaFAPI-46 in its final formulation, consisting of 10% EtOH (v/v) with ascorbic acid, was evaluated by HPLC and the stability measurements were quantified using the previously validated method. The protocol published by Fonseca et al. was followed with minor changes [
16]. Aliquots were taken at different time points and measured using the HPLC method, up to three hours after the end-of-synthesis.
5. Conclusion
In this study, we demonstrated that [68Ga]GaFAPI-46 can be produced according to GMP using liquid targets in a medical cyclotron, resulting in significantly higher production levels compared to 68Ge/68Ga generators. The high radionuclidic and radiochemical purity, as well as the stability of the final drug product, indicate that this method could serve as an alternative to the conventional gallium-68 generators. These findings provide a roadmap for future [68Ga]GaFAPI-46 implementations aimed at meeting the routinely necessary clinical dose requirements.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1: Repeatability results of HPLC method for (a) [
68Ga]GaFAPI-46 and (b) [
natGa]GaFAPI-46; Table S2: Repeatability results of radioTLC method for [
68Ga]GaFAPI-46; Table S3: Repeatability results of GC method for ethanol content in [
68Ga]GaFAPI-46; Figure S1: LOQ representative chromatograms of HPLC; TLC and GC methods; Figure S2: Linearity plots of (a) HPLC, (b) TLC and (c) GC methods.
Author Contributions
Conceptualization, A.I.F., V.H.A. and F.A.; methodology, A.I.F. and V.H.A.; resources, I.H.; investigation, A.I.F.; writing—original draft preparation, A.I.F.; writing—review and editing, A.J.A., F.A. and V.H.A.; supervision—A.J.A., F.A. and A.F.; project administration, A.J.A. and F.A. All authors have read and agree to the published version of the manuscript.
Funding
This work was founded by the Portuguese Foundation for Science and Technology (FCT) through PhD grants (grant number PD/BDE/150681/2020 and PD/BDE/150331/2019) and by ICNAS-P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Acknowledgments
The authors gratefully acknowledge SOFIE Biosciences (Dulles, VA, USA) for providing the peptide for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; et al. Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 19. [Google Scholar]
- Loktev, A.; Lindner, T.; Burger, E. M.; Altmann, A.; Giesel, F.; Kratochwil, C.; et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, A.; Balzer, M.; Amthauer, H.; Brenner, W.; Spreckelmeyer, S. Optimization of 177Lu-labelling of DOTA-TOC, PSMA-I&T and FAPI-46 for clinical application. EJNMMI Radiopharm. Chem. 2023, 8. [Google Scholar]
- Eryilmaz, K.; Kilbas, B. Fully-automated synthesis of 177Lu labelled FAPI derivatives on the module modular lab-Eazy. EJNMMI Radiopharm. Chem. 2021, 6(1). [Google Scholar]
- Alfteimi, A.; Lützen, U.; Helm, A.; Jüptner, M.; Marx, M.; Zhao, Y.; et al. Automated synthesis of [68Ga]Ga-FAPI-46 without pre-purification of the generator eluate on three common synthesis modules and two generator types. EJNMMI Radiopharm. Chem. 2022, 7(1). [Google Scholar] [CrossRef] [PubMed]
- Spreckelmeyer, S.; Balzer, M. : Poetzsch, S.; Brenner, W. Fully-automated production of [68Ga]Ga-FAPI-46 for clinical application. EJNMMI Radiopharm. Chem 2020, 5(1). [Google Scholar] [CrossRef] [PubMed]
- Notni, J.; Wester, H. J. Re-thinking the role of radiometal isotopes: Towards a future concept for theranostic radiopharmaceuticals. J. Label. Compd. Radiopharm. 2018, 61(3), 141–153. [Google Scholar] [CrossRef] [PubMed]
- INTERNATIONAL ATOMIC ENERGY AGENCY, Gallium-68 Cyclotron Production, IAEA-TECDOS-1863, IAEA, Vienna (2019).
- Alves, F.; Alves, V. H.; Carmo, S. J. C.; Neves, A. C. B.; Silva, M.; Abrunhosa, A. J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32(17). [Google Scholar] [CrossRef]
- European Pharmacopoeia 10.0. 2.2.4. Approximate PH of Solutions.
- Gillings, N.; Todde, S.; Behe, M.; Decristoforo, C.; Elsinga, P.; Ferrari, V.; et al. EANM guideline on the validation of analytical methods for radiopharmaceuticals. EJNMMI Radiopharm. Chem 2020, 5(1). [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Beaudoin, J.-F.; Benesty, O. B.; Ait-Mohand, S.; Dumulon-Perreault, V.; Rousseau, É.; et al. Ga-DOTATATE Prepared from Cyclotron Produced Gallium-68:An Integrated Solution from Cyclotron Vault to Safety Assessment and Diagnostic Efficacy in Neuroendocrine Cancer Patients. J. Nucl. Med. 2022, 64(2), 232–238. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, A.; Alves, V.; Neves, Â.; Alves, F.; Gameiro, C.; Geets, J.-M. Fully automated liquid target production of [68Ga]GaCl3 in line with GMP requirements. In International Symposium on Trends in Radiopharmaceuticals Vienna, Austria, 2020.
- Alves, V. H.; do Carmo, S. J. C.; Alves, F.; Abrunhosa, A. J. Automated Purification of Radiometals Produced by Liquid Targets. Instrum. 2018, 2(17), 1–9. [Google Scholar] [CrossRef]
- Fonseca, A. I.; Alves, V. H.; do Carmo, S. J. C.; Silva, M.; Hrynchak, I.; Alves, F.; Falcão, A.; Abrunhosa, A. J. Production of GMP-compliant clinical amounts of copper-61 radiopharmaceuticals from liquid targets. Pharmaceuticals 2022, 15(6), 723. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).