1. Introduction
Arsenic contamination of water is a global problem. Arsenic is an element with transition metal properties and as such can occupy both cationic and anionic positions in the compounds it forms [
1]. Arsenic reacts quickly to changes in environmental conditions and easily changes its oxidation state. In aqueous solutions, depending on the Eh-pH conditions, arsenic forms different arsenite and arsenate oxyanions [
2].
Most natural waters have pH values between 5.5 and 9. Under these conditions, the compounds H
3AsO
30 for As(III) and H
2AsO
4- and HAsO
42- for As(V) are stable [
3]. Arsenic acid H3AsO40 is stable in strongly acidic environments (pH < 2) and is not characteristic for natural underground and surface waters. Under oxidizing conditions at pH=2÷6.9, the arsenate oxyanion H
2AsO
4- is stable. As the pH increases to 11.5, the dominant oxyanion becomes HAsO
42-. Complete dissociation to AsO
43- in natural conditions is rarely achieved because extremely high pH values (>11.5) are required [
3].
In reducing conditions for most natural waters, the arsenite form is dominant, and for pH up to 9, it is represented by arsenic acid ions H3AsO30. When the pH values increase, it dissociates to H2AsO3- and HAsO32-, and complete dissociation is reached in situations with pH values above 13.5.
Most of the ecological problems depend on the mobilization of arsenic species under the influence of natural processes - reactions in natural waters, biological activity, volcanic emissions, etc. Some anthropogenic factors such as the mining industry, the burning of fossil fuels, and the use of arsenic-containing pesticides, cause a local strong increase in the concentration of arsenic in water. In Bulgaria, significant water contamination with arsenic has been noted in large reservoirs (Ogosta dam), water sources (Poibrene village), soils (Pirdop aria), and mine and industrial wastewater.
Based on continued research on the effects of inorganic arsenic on human health, in 1993 the World Health Organization reduced the permissible concentration of arsenic for safe water from 50 µg/L to 10 µg/L [
4]. This necessitates the introduction of water treatment methods that are effective for both high and very-low arsenic-containing waters. For this purpose, several methods have been developed to remove arsenic from contaminated water, as each of them has its advantages and disadvantages [
2,
5]. Two of the most widely used, developed, and studied methods for arsenic removal are coagulation and flocculation, and adsorption and chemisorption. The coagulation and flocculation were widely investigated, considering various factors such as the type of coagulant and the existing arsenic species [
6,
7,
8], solubility [
9], amount of coagulant [
10,
11,
12], pH [
11,
13,
14] etc. The adsorption and chemisorption of various arsenic species are processes of even higher research interests. The adsorption ability of various adsorbents such as alumina, activated carbon, carbon molecular sieves, various modified zeolites, polymer adsorbents, silica gel have been widely studied [
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36].
The present work aims to develop a suitable solution for the purification of waters with a low and high concentration of arsenic. The extraction efficiency of natural zeolite (clinoptilolite) rocks coated with pure Fe and Al hydroxides, oxo-hydroxides and Mg-Fe layered double hydroxide (LDH) for aresinte and arsenate adsorption in dynamic conditions (sorption column) and static conditions (constant volume system) was studied.
2. Materials and Methods
2.1. Used materials
2.1.1. Aqueous solutions preparation
The stock solution of As(III) was prepared under laboratory conditions by dissolving NaAsO2 in 1 L of tap water in order to obtain a solution of As(III) with a concentration of 4 mg/L. The amount of 200 ml of this solution was separated was diluted in 20 L of water to obtain a solution of As(III) with a concentration of 44 µg/L. Half of this solution (10 L) was oxidized with H2O2 to give an As(V) solution. The concentration of dissolved As(V) is 44 µg/L. Equal volumes of these two solutions were mixed and a new solution was obtained in which ratio As(III): As(V) is 1:1.
2.1.2. Sorbent preparation and characterization
All chemical reagents used were with grade “pure for analysis”. The phase composition of the sorbents was investigated by powder X-ray diffraction (XRD) analysis. The powder XRD patterns were recorded on diffractometer D8 Advance, Bruker. Filtered Co-Kα radiation was used in the range 2Θ 4–80°, step 0.02° 2Θ, and exposure time per step 1.5 s. The specialized software Diffrac.Eva was used for qualitative and semi-quantitative phase composition determination. The morphology and chemical composition of covering layers were investigated by Scanning Electron Microscope (SEM) JEOL – model JSM-6010PLUS/LA fitted with energy dispersive spectrometer (EDS).
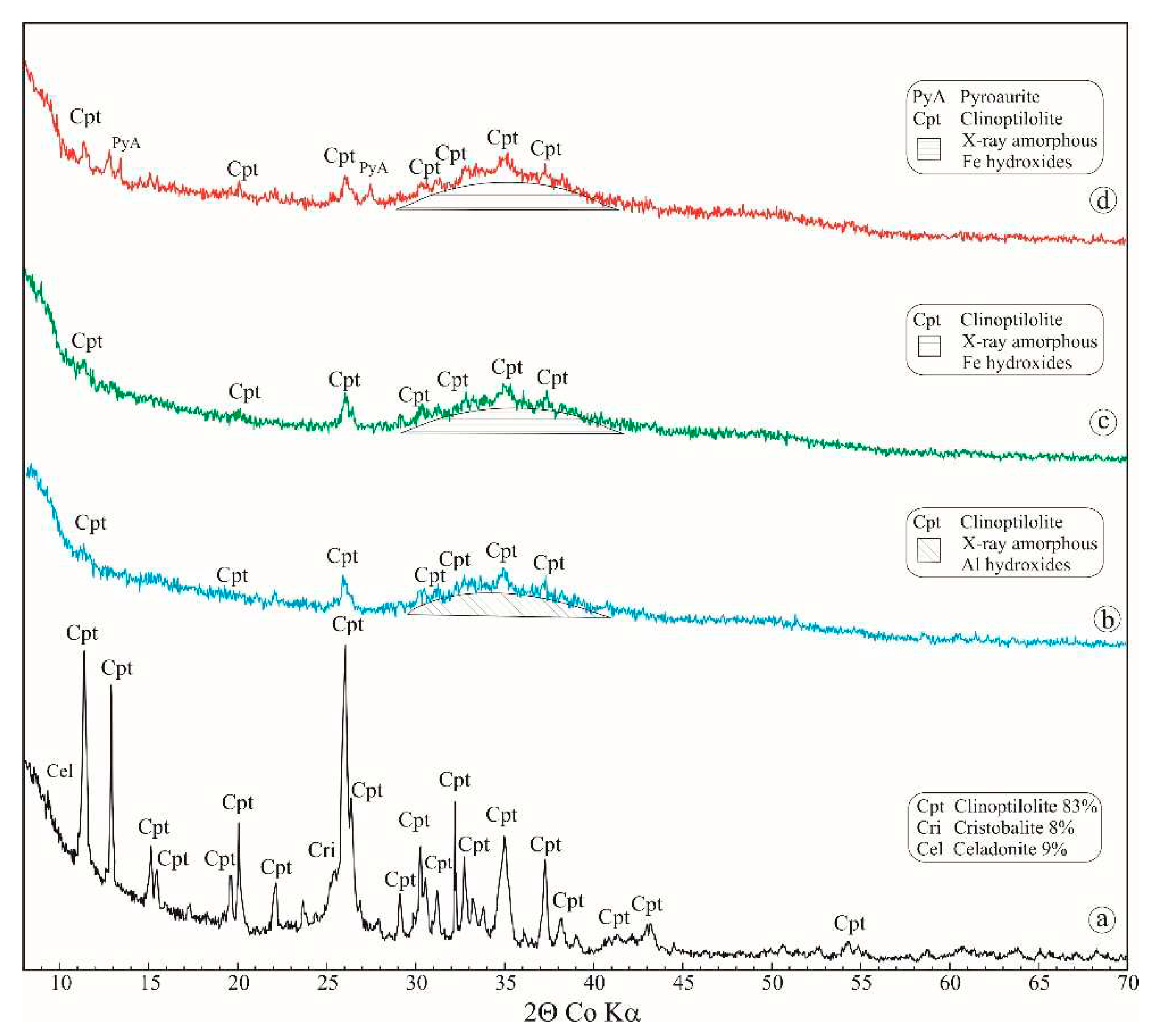
The rock material used for preparation of the sorbents is clinoptilolite tuff from the Beli Plast deposit, Eastern Rhodopes, Bulgaria. The mineral composition of the rock includes 80-88% clinoptilolite, 3-10% montmorillonite and celadonite, and 2-8% α-cristobalite (
Figure 1a).
Four clinoptilolite tuff (Cpt) samples of 200 g fraction up to 2.5 mm in size were used. One sample without further processing was tested as a direct sorbent (
Figure 2a). The other three samples were coated with oxy-hydroxides and hydroxides of iron (Fe-Cpt), aluminum (Al-Cpt) and iron-magnesium LDH (Mg,Fe-Cpt) (
Figure 2b).
The sorbent Fe-Cpt was obtained by soaking 200 g of clinoptilolite tuff with 80 ml of 1 M Fe(NO
3)
3 solution for 30 min under vigorous stirring. After supernatant decantation, the solid sample was treated with 150 ml of 1 M NaOH solution. After alkalization, the entire coating procedure was repeated with 40 ml of Fe(NO
3)
3 and alkalization with 60ml of 1M NaOH to obtain sufficiently dense layer of iron oxy-hydroxides and hydroxides (
Figure 3a). The phase identification of the newly formed hydroxides and oxo-hydroxides cannot be determined due to their very small sizes (several unit cells) giving diffraction as an X-ray amorphous halo (
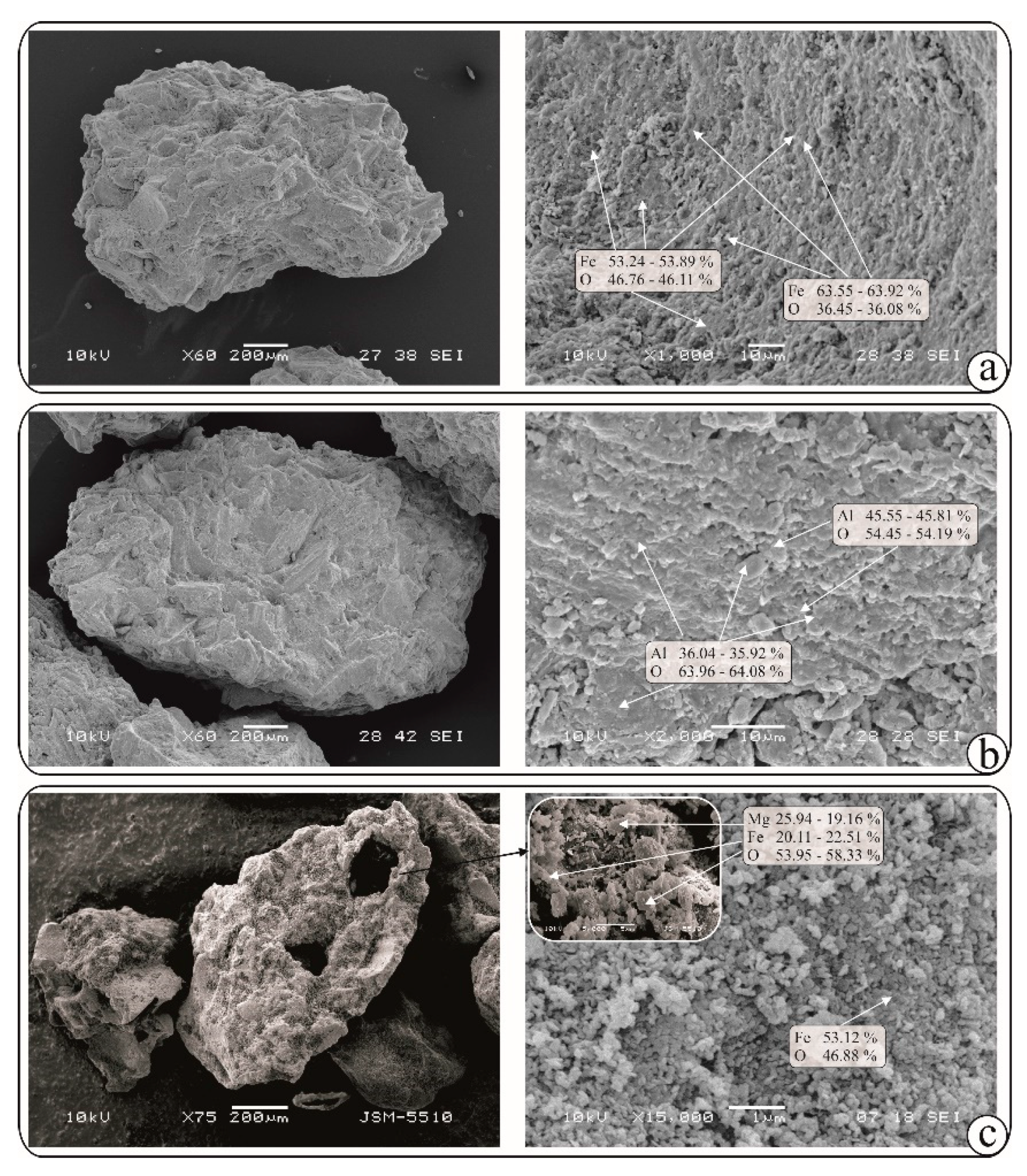
Figure 1b). The chemical composition, determined by EDS, shows presence only of Fe and O. The obtained ratio of the two elements (Fe:O) in different parts of the covering layer is 2:1 or 3:1, which implies forming of Fe-oxyhydroxides and Fe-hydroxides, respectively (
Figure 3a).
The preparation of sorbent Al-Cpt follows the above-described methodology. A sample of 200 g of clinoptilolite tuff was soaked with 80 ml of 1 M AlCl3 solution. The particles remain in the solution with continuous stirring for 30 min. After separation, the sample was treated with 150 ml of 1M NaOH solution. The procedure was repeated with 40 ml of AlCl3 and 60 ml of 1M NaOH to obtain a thicker layer (
Figure 3b) of aluminum oxy-hydroxides and hydroxides. The crystal sizes of the covering Al phases are again very small size (
Figure 1c). Like the Fe-Cpt, the composition of Al-Cpt covering layer contents only Al and O in ratio 2:1 or 3:1, which implies forming of Al-oxyhydroxides and Al-hydroxides, respectively. (
Figure 3b)
Following the same procedure the sorbent Mg,Fe-Cpt was prepared: a sample of 200 g of clinoptilolite tuff was wetted with a 120 ml mixed solution of 1M Fe(NO
3)
3 and 1M MgCl
2 with ratio Fe: Mg=2.5:1. The particles remained in the solution for 30 min under continuous vigorous stirring. After the separation of solid phase, an alkalization with 200 ml of 1M NaOH for 5 h follows. The obtained covering phase has Mg:Fe ratio like the layered double hydroxide (LDH) – pyroaurite (Mg
6Fe
2(OH)
16[CO
3].4H
2O). Indeed the obtained Mg,Fe-Cpt sorbent owns covering layer with quite different morphology - plate crystals typical for LDH are visible at some places (
Figure 3c). In addition, the presence of pyroaurite is registered in the powder diffraction pattern of this product (
Figure 1d). The calculated value of pyroaurite 003 and 110 d-spacing suggests for formation of pyroaurite with Mg:Fe=3:1, which is greater than the initial one. The obtained ratio Mg:Fe in plate crystals varies from 3.1:1 to 3.4:1. The residual part of Fe forms Fe-oxyhydroxides and Fe-hydroxides, which are detectable in some parts of covering layers (
Figure 3c).
After the last alkalization, all three obtained samples were washed until pH 8.5 of the wash water and dried for 12 hours at room temperature.
Two fractions – granular fraction 0.5-2.5 mm (denoted as Fe-Cpt, Al-Cpt, and Mg,Fe-Cpt) and powder fraction <0.5 mm (denoted as Fe-Cpt-f, Al-Cpt-f, and Mg,Fe-Cpt-f) were prepared by sieving of all thee prepared sorbents.
2.2. Sorption experiments
2.2.1. Arsenic uptake from aqueous solution under dynamic conditions in a sorption column
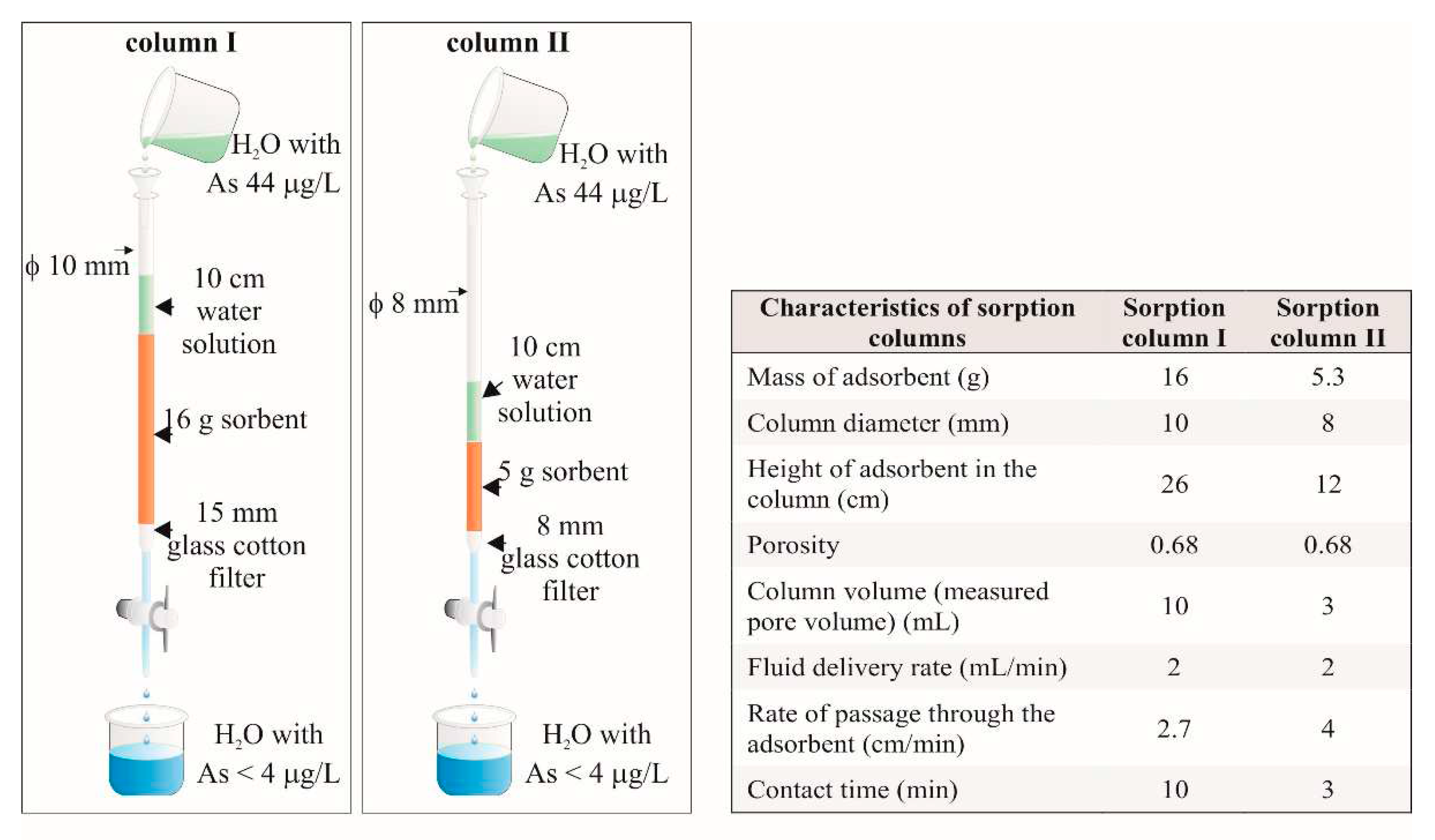
Two sorption columns were constructed using burettes with a volume of 25 ml and a diameter of 10 mm (for sorption column I) and 0.8 mm (for sorption column II). A glass cotton filter with a thickness of 15 mm for sorption column I and 8 mm for sorption column II is placed in the lower part of the burettes (
Figure 4).
During the experiments, a constant water column with a height of about 10 cm is maintained over the sorbent to prevent the column drying.
An amount of 1.92 L of solution with arsenic content of 44 µg/L was passed through sorption column I and 4 L of the same solution through the sorption column II. The concentration of arsenic in the effluents passed through the columns was monitored over a certain number of 1 ml volumes doses (one dose = 1 ml).
2.2.2. Arsenic uptake from aqueous solution under static conditions in a constant volume system
The experiment with fine fractions Fe-Cpt-f, Mg,Fe-Cpt-f, and Al-Cpt-f follows the procedure: 1 g of each sorbent was placed in 300 ml of As(III) solution with a concentration of 44 µg/L, the obtained suspension is stirred using an electromagnetic stirrer. The adsorption activity efficiency was studied depending on the time – 30 min, 60 min, 90 min, and 450 min.
2.2.3. Co-precipitation
The experiment was carried out with solutions with an As(III) or As(V) concentration of 4 mg/L following the procedure. To 50 ml of these solutions, 5 ml of 1M Fe(NO3)3 or 1 ml of 1M AlCl3 and 1M NaOH were added until complete precipitation. After the completion of each precipitation, the pH was measured, and the resulting slurries were filtered, and supernatant analyzed for arsenic content.
To accelerate the flocculation, the experiment was repeated by adding 0.1 g of micronized clinoptilolite tuff (size fraction <20 µm) to the solutions. The pH after complete precipitation was determined, followed by filtration of the precipitate and supernatant analyses for arsenic content.
2.2.4. Elution of arsenic from the spent sorbents
The extraction elution of the adsorbed arsenic from Fe-Cpt was carried out by wetting 4 g of the spent sorbent with 1 M NaOH. After 1 h, 12 ml of distilled water was passed through the wetted sorbent in the column. The As concentration of outflow was analyzed.
2.3. Data analysis methods
Adsorption data were analyzed using Langmuir and Freundlich adsorption isotherms. The isotherms relate the amount adsorbed per unit mass of adsorbent (S) to the equilibrium concentration of the adsorbate in the liquid phase ().
2.3.1. Langmuir isotherm
The Langmuir model assumes that the maximum adsorption occurs when a saturated monolayer of solute molecules is present on the surface of the adsorbent is saturated with monolayer of solute molecules. In this case, the adsorption energy is constant and there is no migration of the adsorbed molecules in the surface plane.
The mathematical expression that describes the Langmuir isotherm is:
Where and (Langmuir constants) indicate the maximum adsorption capacity () and the energy referred to as the heat of adsorption (), respectively.
2.3.2. Freundlich isotherm
The Freundlich model is an empirical expression that considers the adsorbent surface heterogeneity and describes the exponential distribution of adsorption sites and their energies.
The mathematical expression that gives the Freundlich isotherm is:
where
and
N are Freundlich constants that indicate the adsorption capacity and adsorption intensity, respectively.
Freundlich isotherms are more widely used, but unlike the Langmuir model, do not provide information on monolayer adsorption capacity.
2.4. Monitoring of As-content in water
Electrothermal atomic absorption spectrometry (ETAAS) was used for the determination of arsenite or arsenate content. Electrothermal atomic absorption measurements were carried out on a Perkin-Elmer (Norwalk, CT, USA) Zeeman 3030 spectrometer with an HGA-600 graphite furnace. The light source was an electrodeless discharge lamp for As. The spectral bandpass for all analytes was 0.7 nm. Pyrolytic graphite-coated tubes with pyro-coated platforms were used as atomizers. Sample aliquots of 20 μL were injected into the graphite furnace using autosampler AS-70. All measurements were carried out with at least three replicates and the values measured were based on integrated absorbance. ETAAS measurements of As were performed in the presence of 10 μL 250 μg/mL Pd solution with the use of 700ºC as loss-free pretreatment temperature.
3. Results and discussion
3.1. Adsorption in sorption columns
3.1.1. Adsorption of As(III) in a sorption column
The sorption properties of the unmodified clinoptilolite tuff were tested in order to determine its effect on the arsenic adsorption The unmodified clinoptilolite tuff (sorbent Cpt) showed poor adsorption efficiency of 5% for dissolved As(III) in the first 100 ml of the solution. The increase of the volume of the passed solution leads to the process of desorption of As(III). After passing the volume of 500 ml, the arsenic concentration in the effluate after sorption column is higher than that of the supplied initial solution. Since the amount of retained arsenite is negligibly small, the sorption efficiency of unmodified clinoptilolite tuff is unsignificant and its influence on the overall process can be neglected. The initially recorded adsorption of a certain amount of arsenite from the solution can be explained by the presence of clay minerals in the composition of the clinoptilolite tuff.
The data of the As(III) adsorption on Al-Cpt show that in the first 500 ml of the solution passed through the sorption column I, the concentration of As(III) is below the limit of detection of analytical method used (
Table 1).
The calculated amounts of the adsorbed As(III) show that within the first 50 doses (500 ml) the efficiency of the adsorbent is about 100%. In the next 140 doses (1.42 L), a slight decrease of the adsorbed amount (57.23 µg) compared to the supplied initial amount (62.48 µg) is observed (91.6 % degree of sorption efficiency).
Results obtained for As(III) adsorption on the Fe-Mg-Cpt, showed that the concentration of As(III) in the solution passed through the column remained below the detection limit for the first 100 doses (1L) of water passed. At the same amount of sorbent and under the same experimental conditions, Fe-Mg-Cpt has about 100% efficiency for a twice higher volume of solution than Al-Cpt. It should be noted that the sorbent active component (Fe) is about 2.5 times less than that in Fe-Cpt sorbent. LDHs have layered structures made up of positively charged hydroxide layers for the compensation of which exchangeable anions are placed in the interlayer space [
37,
38]. No special precautions were taken in the present experiment to remove the most selective carbonate anion but considering the purity of the chemicals and the high alkalinity of the synthesis, it can be suggested that the compensating anion is a hydroxyl group. The high activity of the sorbent can be assumed to be due to the inclusion of part of the arsenite anions at the expense of OH in the interlayer space of Mg,Fe-LDH from the covering layer. Unfortunately, in the powder X-ray diffractogram, due to the small size of the particles, the LHD lines are broad and with very weak intensity and, which makes their use difficult. Therefore, the occurrence of anion exchange cannot be categorically confirmed.
The highest degree of As(III) adsorption was obtained with Fe-Cpt sorbent. After passing the entire amount of water (1.92 L), the concentration of As(III) in the effluate spent solution remained below the limit of detection. It can be assumed that the entire amount of As(III) passed through the column was extracted from the solution and adsorbed on the sorbent. The extraction efficiency of the sorbent is assumed to be about 100% for the entire course of the experiment.
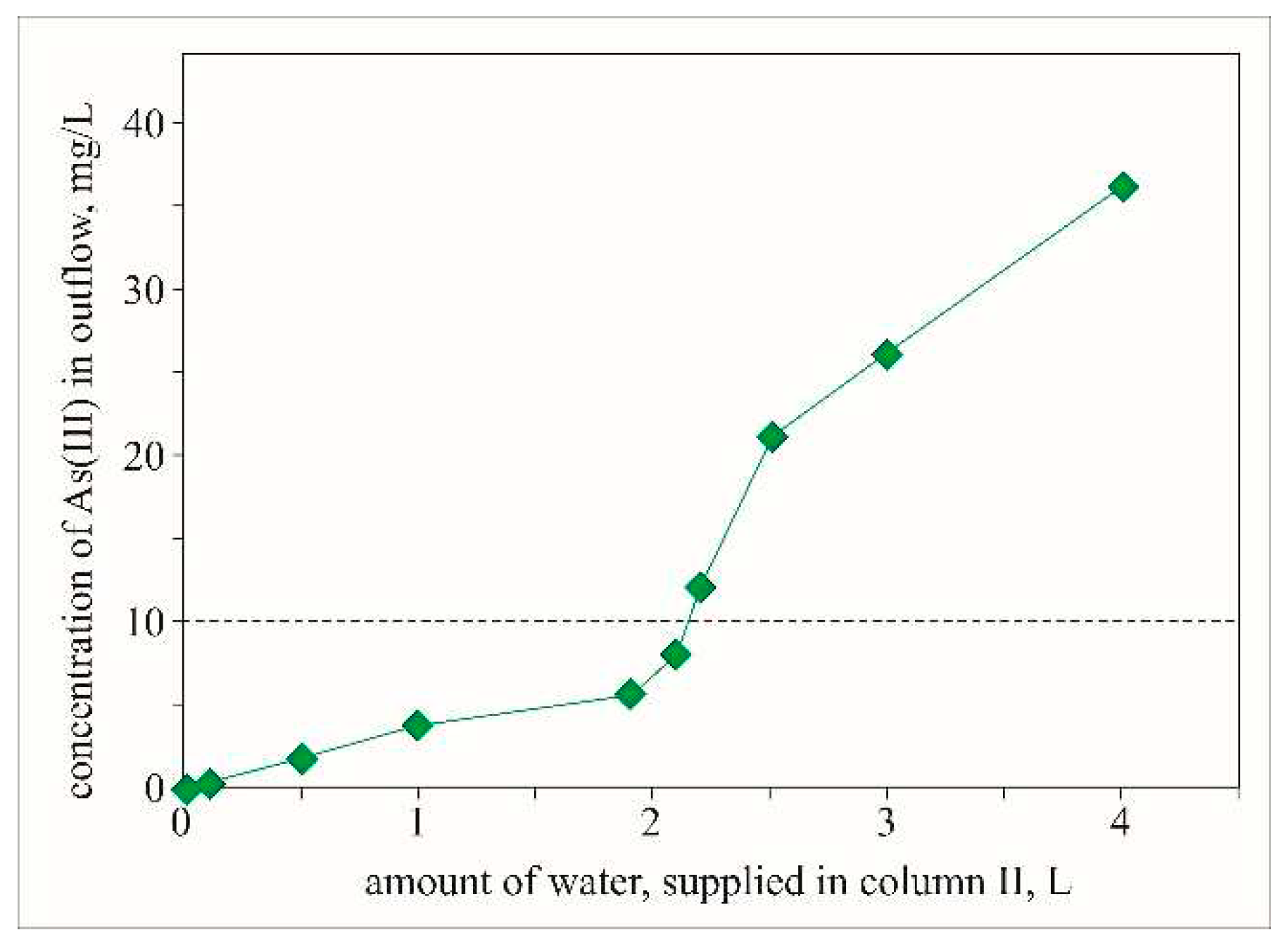
To follow the course of adsorption and evaluate the effectiveness of the sorbent, it is necessary to continue the adsorption process until all adsorption centers are occupied. For this reason, a new sorption column (sorption column II) was constructed. The amount of sorbent in this column is 3 times lower compared to sorption column I. The reduced amount of sorbent determines a 3-fold smaller dose, which provides a 3-fold higher amount of solution passing per gram of sorbent for the same experimental time. On the other hand, the reduced dose leads to a reduction in the contact time between the solution and the sorbent.
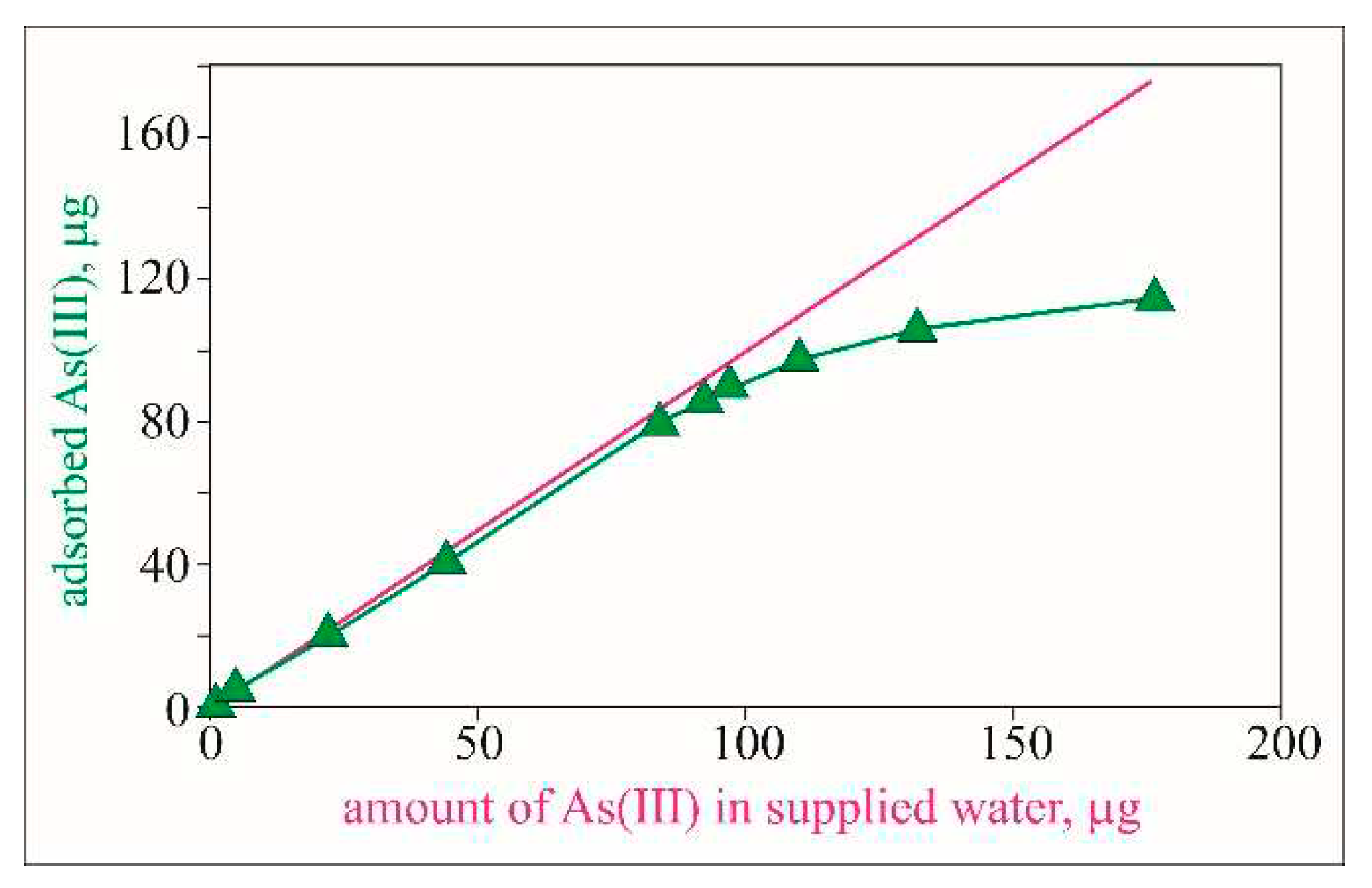
The obtained results suggest that during the experiment, the amount of adsorbed As(III) decreases and almost complete exhaustion of the sorbent is reached. According to the obtained data the adsorbed amount of arsenic approaches 100% of the supplied amount at the middle of the experiment and by the end of the experiment, it gradually decreases (
Figure 5).
The behavior of the adsorption concerning the amount of water solution passed (
Figure 6), shows that up to the passage of about 700 doses, the efficiency of the adsorbent is close to 95%, as 87.04 µg of arsenic is adsorbed from 92.4 µg of supplied As (
Table 2). Within these 700-715 doses, the concentration of arsenic in the effluate spent solution is below the permissible value of 10 µg/L for drinking water. The estimated amount of adsorbed arsenic is about 17 µg/g (
Table 2), which is in very good agreement with the results obtained in a study by Andrews (2009). However, this author reaches adsorption of 22 µg/g in the same range of doses but with three times longer contact time. In the next 633 doses, the efficiency of the sorbent decreases until it is almost completely exhausted. Within this volume, only 24% of the total amount of arsenic (114.14 µg) is adsorbed.
3.1.2. Adsorption of As(V) in a sorption column
The experiment was carried out on the adsorption of As(V) solution by Al-Cpt, Mg,Fe-Cpt, and Fe-Cpt sorbents. The aqueous solution was passed through adsorption column I (
Table 3).
Based on the observed adsorption data it can be concluded that the sorbents Fe-Cpt and Mg,Fe-Cpt retained their high extraction efficiency for the adsorption of As(V) – 100% and 92.9%, respectively. Unlike the two Fe-containing sorbents, the Al-Cpt sorbent has very low extraction efficiency (64.75%). In the first dose of water passed through the sorption column, the concentration of dissolved arsenic is below the detection limit. In contrast to the adsorption of As(III), already after the 10th dose (100 ml of aqueous solution passed through the column) the concentration of arsenate ions in the effluate waste solution exceeded the maximum permissible concentration of 10 µg/L.
3.1.3. Adsorption of As(III) + As(V) in a sorption column
The mixed 44μg/L As(III) + As(V) solution was passed through sorption column I. The selected sorbent was Fe-Cpt because it shows the highest efficiency toward the adsorption of As (
Table 4). The experimental data showed a high efficiency of the sorbent (adsorbed 84.41 µg out of 84.48 µg sold, which is close to 90%, similar to that of As(III) adsorption.
3.1.5. Data analysis
Langmuir and Freundlich's isotherms are usually used for analysis of adsorption data from aqueous solutions. Langmuir (
) and Freundlich (
) constants are indicators of sorption capacity. In both models, the amount adsorbed per unit mass of adsorbent (
S) is related to the equilibrium concentration of the adsorbate in the liquid phase (
). The amount of adsorbed arsenic was calculated using the formula:
where
is the initial and
- equilibrium concentration of As(III) (µg/L); V is the volume passed (L) and m is the mass of adsorbent (g).
The linear form of the Langmuir equation is usually used to calculate the adsorption parameters:
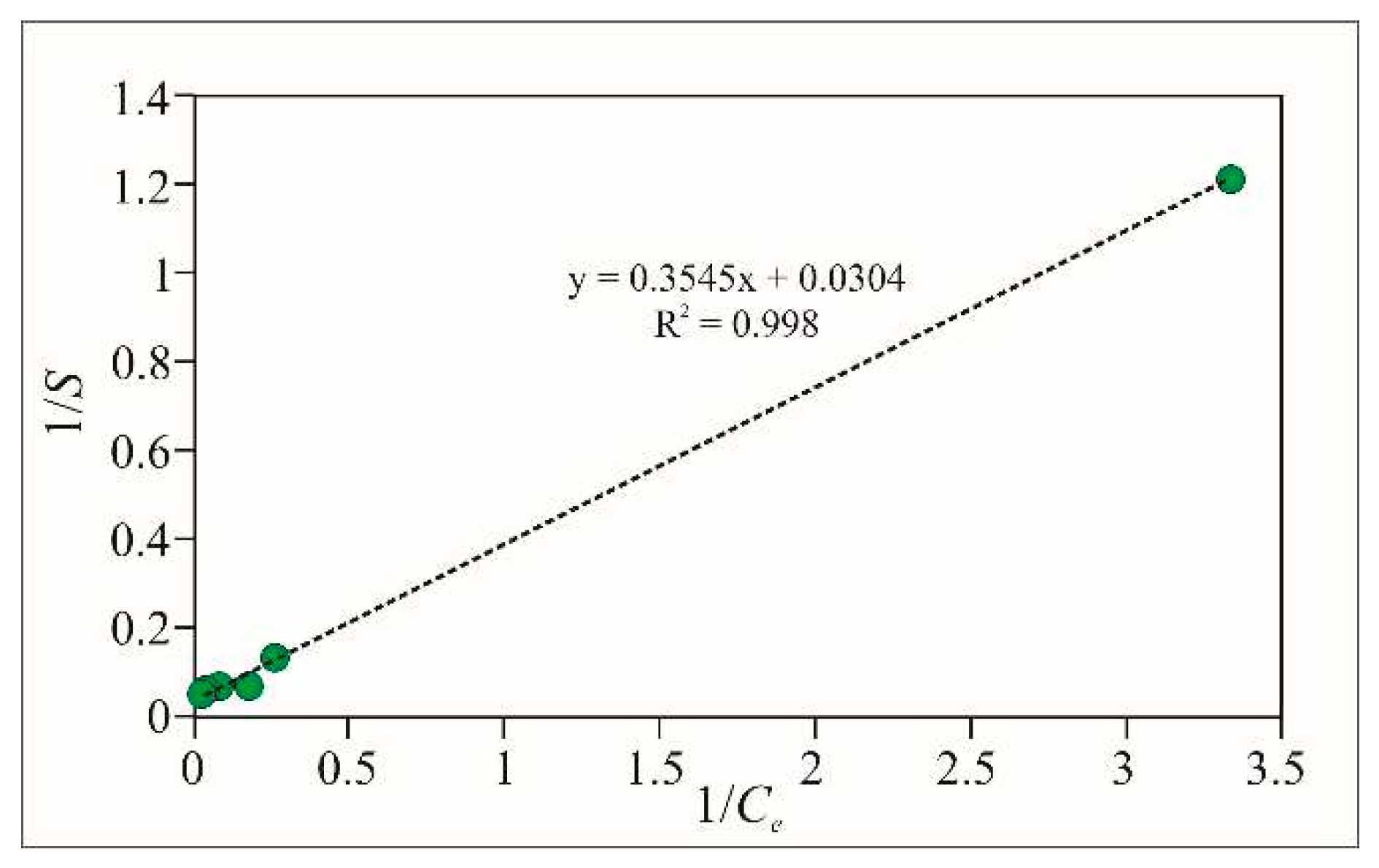
The Langmuir constants can be determined graphically by plotting the reciprocal values of the equilibrium concentration of the adsorbate in the solution (
) on the abscissa, and the reciprocal values of the adsorbed amount per gram of adsorbent (
) on the ordinate (
Figure 7).
The calculated values of the coefficients for As(III) are: for Fe-Cpt = 0.0858 L/µg and = 32.90 µg/g; for Al-Cpt = 0.0558 L/µg and = 21.98 µg/g and for Mg,Fe-Cpt = 0.0496 L/µg and = 24.75 µg/g.
The correlation factor R
2 for all three sorbents is close to one (0.998, 0.996, and 0.97 for Fe-Cpt, Al-Cpt, and MgFe-Cpt, respectively), which indicates that the adsorption of arsenite ions very well fits to the Langmuir model. The calculated value for
= 0.0858 L/µg for Fe-Cpt is in good agreement with the literature data [
25,
32,
33,
34,
35] for similar adsorbents (Fe-OH-coated various zeolites and other natural materials). The calculated maximum adsorption capacity (32.9mg/g) was less than the capacity (79mg/g) of Fe-treated activated carbon [
25] but greater than that for the goethite (25mg/g) and is comparable with data published by other authors [
30,
35,
36]. The results for adsorption of arsenate are like the data obtained for Fe-coated sorbents.
The Freundlich model assumes non-uniformity of adsorption sites. This non-uniformity is due to the existence of adsorption sites with different energies (non-equivalent/different adsorption sites) or is caused by the action of repulsive forces between adsorbed atoms and molecules.
The linear form of the mathematical expression of the Freundlich isotherm is represented by the logarithmic equation:
where
and
N are Freundlich constants.
is an index of adsorption capacity and
N being an index of adsorption intensity. The coefficients can be calculated from the log (
) to log (
S) plot, where
is (the perpendicular/horizontal projection of the line onto the abscissa) and
N reflects the slope of the line.
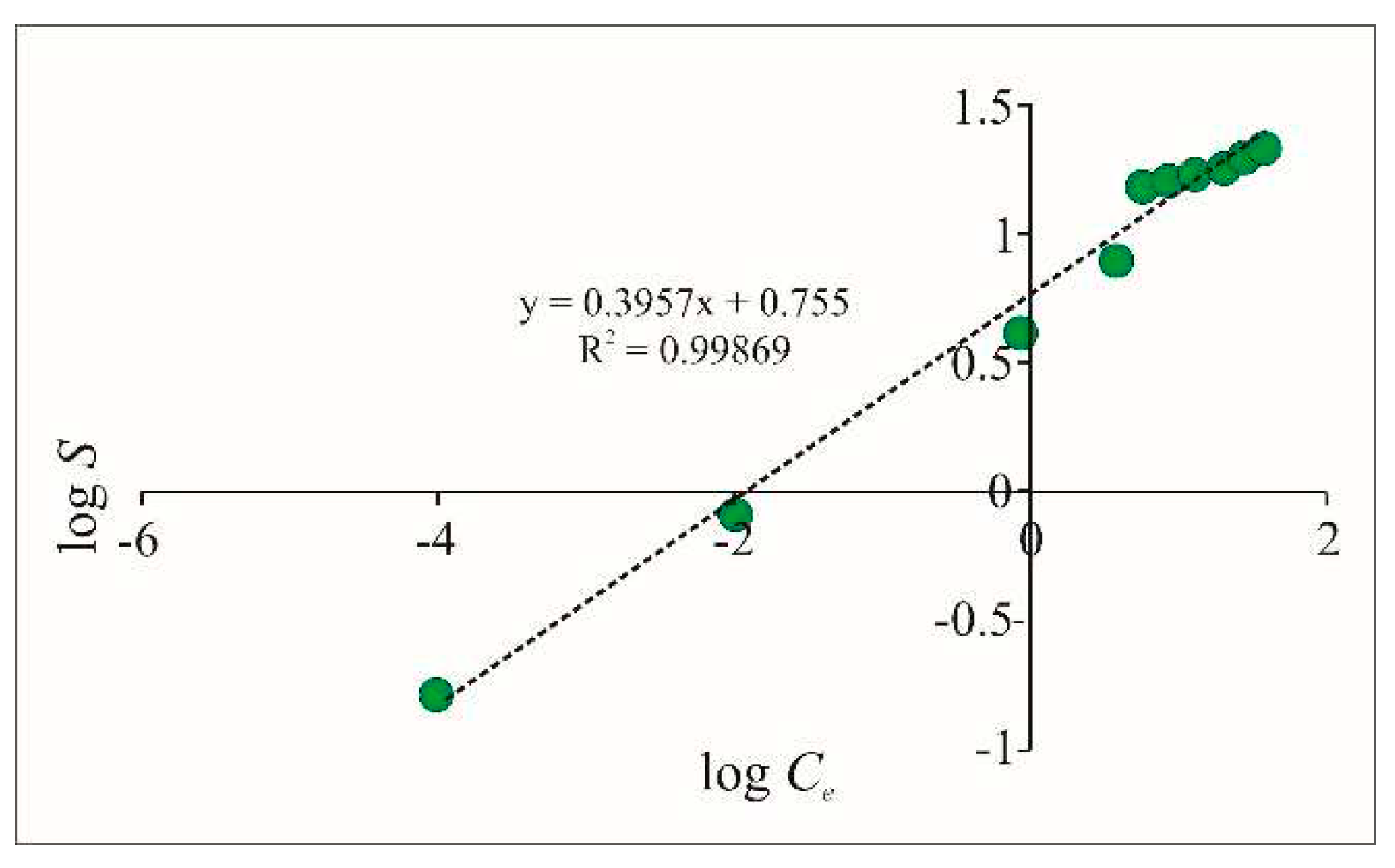
From the constructed graph for the studied Fe-Cpt adsorbent (
Figure 8), the values of the two constants were calculated:
= 5.689 and
N = 0.396.
As higher is the value of the Freundlich constant , the higher activity of the adsorption sites is assumed. The constant N is an empirical constant that depends on the nature of the adsorption sites. For values of N greater than 1, the interactions are associated with non-equivalent adsorption sites. For values of N less than 1, the Freundlich model approaches the Langmuir model for homogeneity of adsorption sites.
The obtained correlation factor is R2 = 0.9869, which gives reason to assume that the Freundlich isotherm is also applicable to characterize the studied adsorption process. The obtained values for and especially for N (N < 1), however, indicate that the adsorption in the present experiment follows the Langmuir model. This is in complete agreement with the studies of many authors who indicate that isotherms associated with anion adsorption generally reflect pseudo-Langmuir sorbate/sorbent ratios. This indicates that there is one dominant mode of adsorption binding, most often ligand exchange with complex formation.
3.1.6. Binding mechanisms
Since the active surface of the sorbents is composed of iron or aluminum hydroxides and oxyhydroxides, the adsorption of arsenic proceeds with the formation of complexes with the cations from the active surface [
24].
The starting As(III) solution has a measured pH of 6.8, which defines a neutral to slightly acidic nature of the solution. In the range of pH values 6-9 As(III) is mainly represented by the neutral form of arsenic acid H3AsO3-, but a small amount of dissociated arsenite ions (H2AsO3-) is also probably present.
For the present study, the adsorption of As(III) most likely takes place by the mechanism of inner-sphere binding, as for the H
3AsO
30 ions a reaction takes place between a basic and an acidic species with the formation of complexes, and for the monovalent H2AsO3 ions - at the expense of protonated hydroxyl groups:
Under the conditions of the experiment with a solution of As(V) (pH 6.9), the dominant ions of arsenic are H
2AsO
4- and HAsO
42- [
2]. The mechanisms of inner- and outer-sphere connection can be described by reactions:
The analysis of the obtained Langmuir and Freundlich isotherms shows that the adsorption follows the Langmuir model i.e., it proceeds on equivalent adsorption sites. Probably the main active mechanism is adsorption with inner sphere complex formation. This statement is supported by the results of the arsenic elution from the spent sorbent and by the X-ray powder diffraction data. The elution results show a very easy complete recovery of the adsorbed arsenic with a small amount of NaOH (20g spent sorbent was washed with 100ml 0.1M NaOH). In addition, in the powder diffractograms of the spent Fe-Cpt-f and Al-Cpt-f sorbents, the presence of arsenites was not observed, i.e. the base-acid reaction proceeded with the formation of iron hydrogen-arsenite complexes.
3.2. Arsenic uptake from a constant volume system (batch system) static conditions
Adsorption of arsenic in a constant volume system has a very high efficiency (
Table 5). The amount of adsorbed arsenic in the batch system from the sorbents Al-Cpt-f, Fe-Mg-Cpt-f, and Fe-Cpt-f is much greater than the amount of retained arsenic on the corresponding granular sorbents for the same amount of As(III) or As(V) solution (300 ml). Some authors [
29] have established similar differences for arsenic adsorption on the two different systems. The high efficiency of the constant volume systems is due to the long contact time between the sorbents and the solutions (in the present study – 7h 30min) on the one hand, and on the other hand, due to the small sizes of the sorbents, which provide a large adsorption surface. The static experiment was performed with the fine fraction obtained as a waste product after the sieving of the coated sorbents.
Despite the good adsorption characteristics, the method in a batch system is not suitable for the treating of large amounts of water. Its disadvantage is related to the fact that the installations would take up a lot of space, and for the full process to run, continuous stirring is required, which is associated with additional energy consumption. The flocculation and filtration of the suspension is an additional disadvantage of adsorption in a constant volume system. However, the high efficiency of the method could be useful for solutions with a high arsenic concentration such as technological waste products, solutions obtained during the regeneration of sorbents from sorption columns, etc.
3.3. Arsenic uptake by precipitation reactions
Another method that can be used for the removal of high concentration of arsenic is the co-precipitation method. In the present study, the experiments of precipitation of aluminum or iron oxy-hydroxides in aqueous solutions with 4200 μg/L As(III) and As(V) solutions without or with addition of a small amount of clinoptilolite tuff (fraction <20 μm) were carried out.
The results show that the reactions in the presence of iron cations work better than in the presence of aluminum cations. After precipitation experiment with iron salts, a concentration of 3 μg/L As(III) or As(V) was determined in supernatant solutions. The results of precipitation reactions with Al salts show quite worse performance for arsenite and arsenate uptake. The concentration of As(III) and As(V) in aqueous solution is 400 μg/L and 450 μg/L, respectively. In the experiment with the addition of a micron fraction of clinoptilolite tuff, the flocculation of the suspension was accelerated 5 times, and the concentration of As(III) in the supernatant solution was 600 μg/L for reactions with Al and 5 μg/L for reactions with Fe, respectively. The results for As(V) uptake by precipitation reactions are in the same range – the concentration of As(III) in the solution was 550 μg/L for reactions with Al and 3 μg/L for reactions with Fe, respectively.
4. Conclusions
The results of the experiment with the granular sorbents show good possibilities for obtaining water with an arsenic concentration below the permissible value of 10 µg/L for drinking waters. The determined efficiency of the Fe-containing sorbents for arsenite and arsenate uptake from wastewater with 44mg/L concentration is 400 L of water per kg sorbent at a rate of 2-2.4 L/h/kg.
It was found that the powdered sorbent showed a much higher adsorption capacity than the granular one in the batch system. As this requires further processing of the water by flocculation and filtration, it is more suitable for small quantities of water with a very high concentration of arsenic.
For waters with high concentrations of arsenic, the extraction method by precipitation reactions is most effective and suitable.
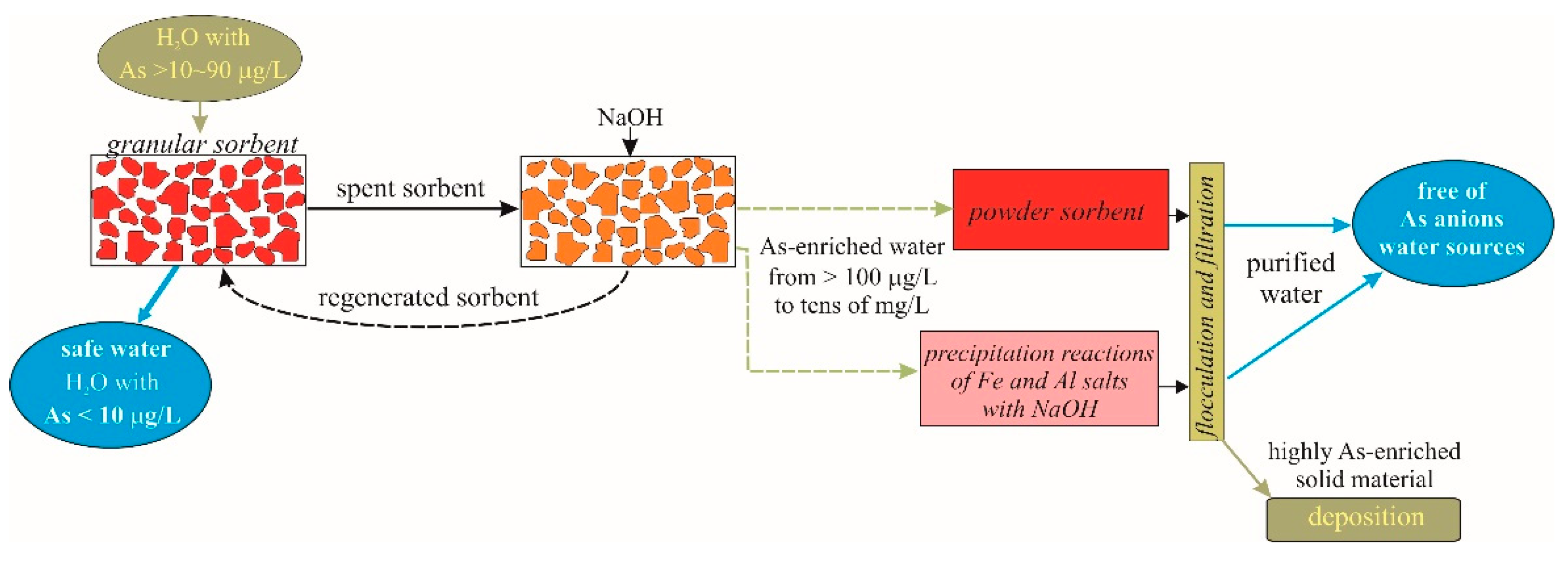
The results of the present study create prerequisites to evaluate the possibilities of using all tested methods. A possible scheme of the technological process is presented in
Figure 9.
Author Contributions
Conceptualization, T.S.; methodology, T.S. and I.K.; validation, T.S. and I.K.; formal analysis, T.S. and I.K.; writing—original draft preparation, T.S.; writing—review and editing, I.K.; visualization, T.S.; project administration, T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant № BG05M2OP001-1.002-0019: "Clean Technologies for Sustainable Environment - Waters, Waste, Energy for a Circular Economy", financed by the Science and Education for Smart Growth Operational Program (2014-2020) and co-financed by the EU through the ESIF.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Day, P.A. Chemistry and mineralogy of arsenic. Elements 2006, 2, 77–83. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Cullen, W.R.; Reimer, K.J. Arsenic speciation in the environment. Chem. Rev. 1989, 89, 713–764. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality; World Health Organization: Geneva, 1993. [Google Scholar]
- Vaclavikova, M.; Gallios, G.P.; Hredzak, S.; Jakabsky, S. Removal of arsenic from water streams: an overview of available techniques. Clean Technologies and Environmental Policy 2008, 10, 89–95. [Google Scholar] [CrossRef]
- Gulledge, J.H.; O’Connor, J.T. Removal of arsenic (V) from water by adsorption on aluminum and ferric hydroxides. J. Am. Water Works Assoc. 1973, 65, 548–552. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, F.; Yang, X. Treatment of As containing wastewater by lime-polyferric sulphate coagulating process. Gongye Shuichuli 2000, 20, 27–29. [Google Scholar]
- Han, B.; Runnells, T.; Zimbron, J.; Wickramasinghe, R. Arsenic removal from drinking water by flocculation and microfiltration. Desalination, 2002; 145, 293–298. [Google Scholar]
- McNeill, L.S.; Edwards, M.A. Soluble arsenic removal at water treatment plants. J. Am. Water Works Assoc. 1995, 105–113. [Google Scholar] [CrossRef]
- Cheng, R.C.; Liang, S.; Wang, H.-C.; Beuhler, J. Enhanced coagulation for arsenic. J. Am. Water Works Assoc. 1994, 86, 79–90. [Google Scholar] [CrossRef]
- Edwards, M.A. Chemistry of Arsenic Removal during coagulation and Fe-Mn oxidation. J. Am. Water Works Assoc. 1994, 4, 64–77. [Google Scholar] [CrossRef]
- Hering, J.G.; Chen, P.; Wilkie, J.A.; Elimelech, M.; Liang, S. Arsenic removal by ferric chloride. , J. Am. Water Works Assoc. 1996, 88, 155–167. [Google Scholar] [CrossRef]
- Logsdon, G.S.; Sorg, T.J.; Symons, J.M. Removal of heavy metals by conventional methods. Proc. 16th Water Qual. Conf., Urbana, Illinois, 1974, USA, P. 111.
- Sorg, T.J.; Logsdon, G.S. Treatment technology to meet the interim primary drinking water regulations for inorganics: Part 2. J. Am. Water Works Assoc., 1978, 7, 379–392. [Google Scholar] [CrossRef]
- Sposito, G. The surface chemistry of soils. Oxford University Press, 1984, 234.
- Gu, B.; Schultz, R.K. Anion retention in soil: Possible application to reduce migration of buried technetium and iodine: A review. US Nuclear Regulatory Comission, 1991, 32.
- Langmuir, D. Aqueous environmental geochemistry. Prentice Hall, 1997, 600.
- Stumm, W.; Morgan, J.J. Aquatic chemistry, Chemical Equilibria and Rates in Natural Waters, Third edition, 1996.
- Perry, R.H.; Green, D.W. Perry’s Chemical engineers Handbook, 7th ed., New York, NY, USA, McGraw-Hill, 1997, 542.
- Seader, J.D.; Henley, E.J. Separation process principles. New York, NY, USA: Wiley 1998, 886.
- Swedlund, P.J.; Webster, J.G. Adsorption and polymerization of silicic acid on ferrihydrite, and its effect on arsenic adsorption. Water Research, 1999, 33, 3413–3422. [Google Scholar] [CrossRef]
- Buekens, A.; Zyaykina, N.N. Adsorbents and adsorption processes for pollution control. Pollution control technologies, 2000, II, ISBN: 978-1-84826-567-7, 504.
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K.S. Removal of arsenic in drinking water by iron oxide-coated sand and ferrihydrite — Batch Studies Water Qual. Res. J. Canada, 2001, 36, 55–70. [Google Scholar]
- Bang, S.; Meng, X. A review of Arsenic interactions with anions and iron hydroxides. Environ. Eng. Res., 2004, 9, 184–192. [Google Scholar] [CrossRef]
- Payne, K.B.; Abdel-Fattah, T.M. Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: effects of pH, temperature, and ionic strength. Journal of Environmental Science and Health, 2005, 40, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Smith, E.; Alston, A.M. Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. J Environ Qual. 2002, 31, 557–63. [Google Scholar]
- Haron, M.J.; Masdan, S.A.; Hussein. M.Z.; Zainal, Z.; Kassim, A. Kinetics and thermodynamic for sorption of arsenate by lanthanum-exchanged zeolite. Malaysian Journal of Analytical Sciences, 2007, 11, 219–228. [Google Scholar]
- Margeta, K.; Zabukovec, N.; Mario Šiljeg, L.; Farkaš, A. Arsenic removal from water/ wastewater using adsorbents—A critical review. Journal of Hazardous Materials, 2007, 142, 1–53. [Google Scholar]
- Andrews, J.R. Arsenic removal using iron-modified zeolites, PhD thesis, 2009, New Mexico Institute of mining and technology, Socorro, New Mexico.
- Jiménez-Cedillo, M.J.; Olguin, M.T.; Fall, Ch. Colin, A. Adsorption capacity of iron- or iron–manganese-modified zeolite-rich tuffs for As(III) and As(V) water pollutants. Applied Clay Science, 2011, 54, 206–216. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng., 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in adsorption processes: State of the art and future prospects. Chemical Reviews 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Gonzalez, M.P.; Mattusch, J.; Einicke, W.D.; Wennrich, R. Sorption on natural solids for arsenic removal. Chem. Eng. J. 2001, 81, 187–195. [Google Scholar] [CrossRef]
- Elizalde-Gonzalez, M.P.; Mattusch, J.; Wennrich, R. Application of natural zeolites for preconcentration of arsenic species in water samples. J. Environ. Monitor. 2001, 3, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Gonzalez, M.P.; Mattusch, J.; Wennrich, R.; Morgenstern, P. Uptake of arsenite and arsenate by clinoptilolite-rich tuffs. Micropor. Mesopor. Mater. 2001, 46, 277–286. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Franus, W.; Panek, R.; Sobczyk, M.; Rusiniak, P.; Szerement, J.; Jarosz, R.; Marcińska-Mazur, L.; Bajda, T.; Mierzwa-Hersztek, M. Zeolite composite materials from fly ash: an assessment of physicochemical and adsorption properties. Materials 2023, 16, 2142. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Rives, V.; Ulibarri, M.A. Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometallates. Coord. Chem. Reviews, 1999, 181, 61–120. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).