Submitted:
13 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Graphene Property
2.1. Electronic Property
2.2. Mechanical Properties
2.3. Magnetic Properties
2.4. Optical and Luminescence Properties
3. Experimental Synthesis
3.1. Brodie's Oxidation Method
3.2. Staudenmaier Method
3.3. Hofmann Method
3.4. Hummer’s Method
3.5. Improved Hummers Method
3.6. Tour Method
4. Reduction of Graphene Oxide
4.1. Chemical Reduction of Graphene Oxide
4.2. Thermal Reduction of GO
4.3. Exfoliated Reduction
4.4. Catalytic Reduction
4.5. Green Synthesis Route for Reduced GO
5. Characterization
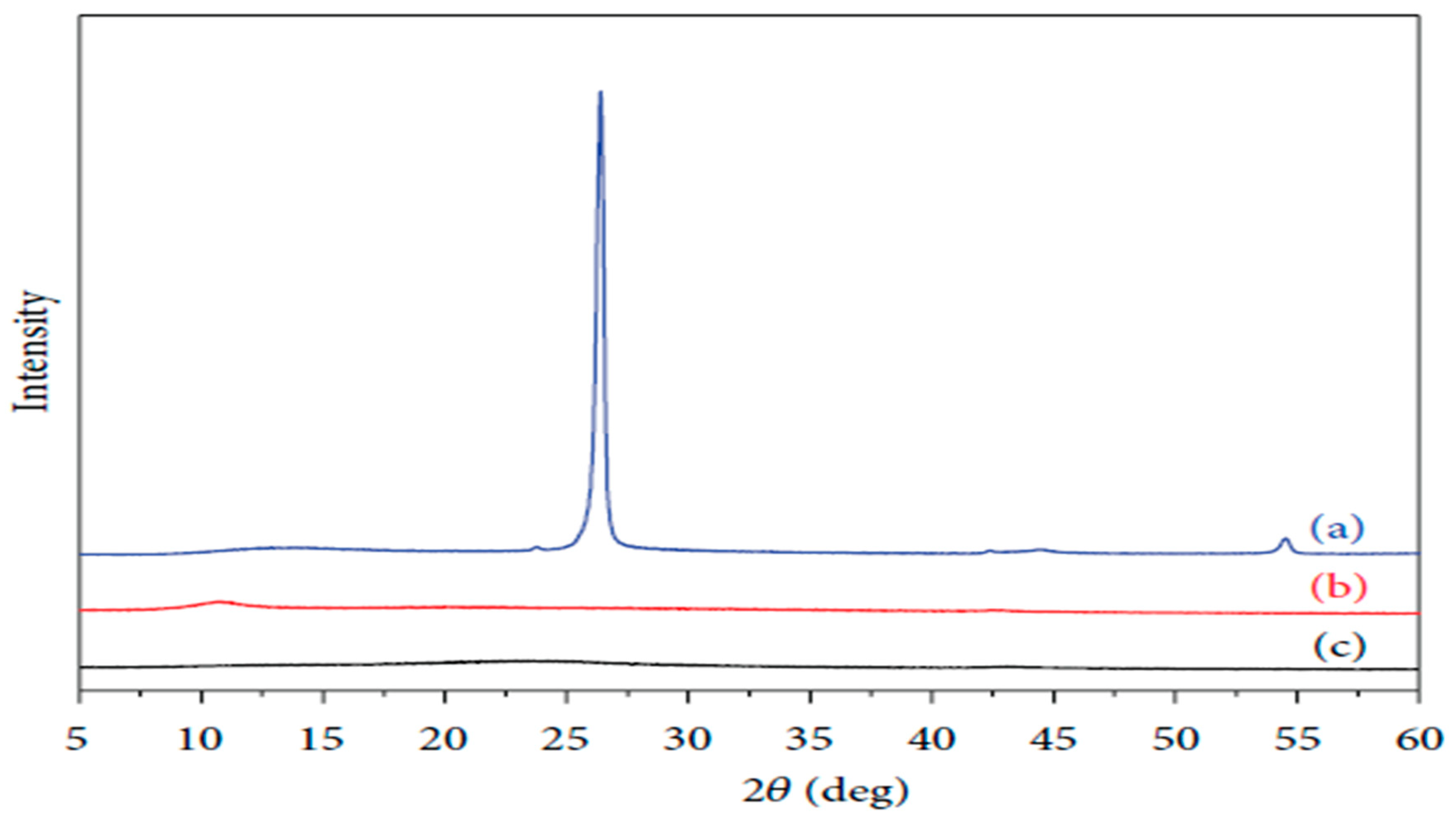
5.1. X-ray Diffraction (XRD)
5.2. Fourier Transform Infra-Red (FTIR)
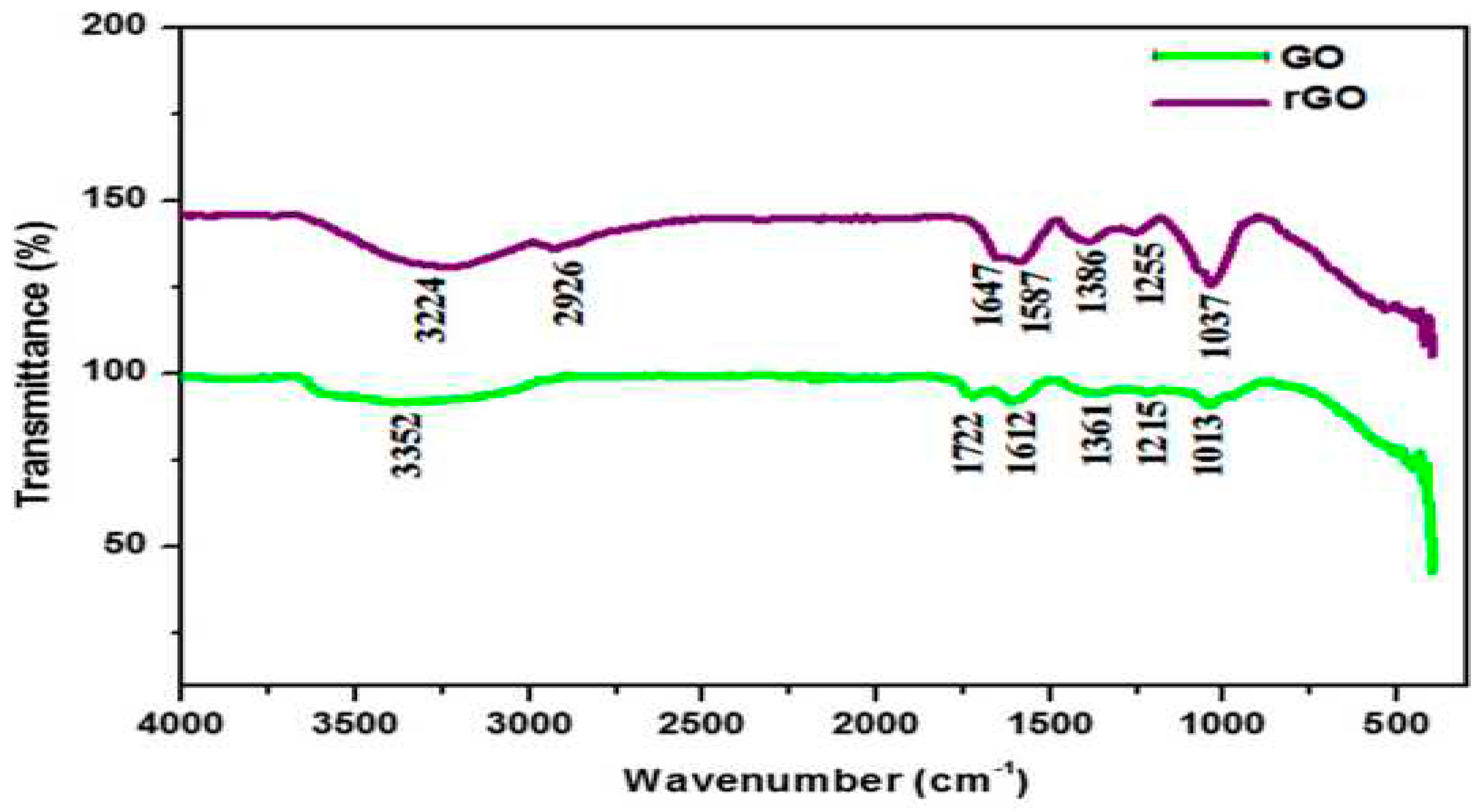
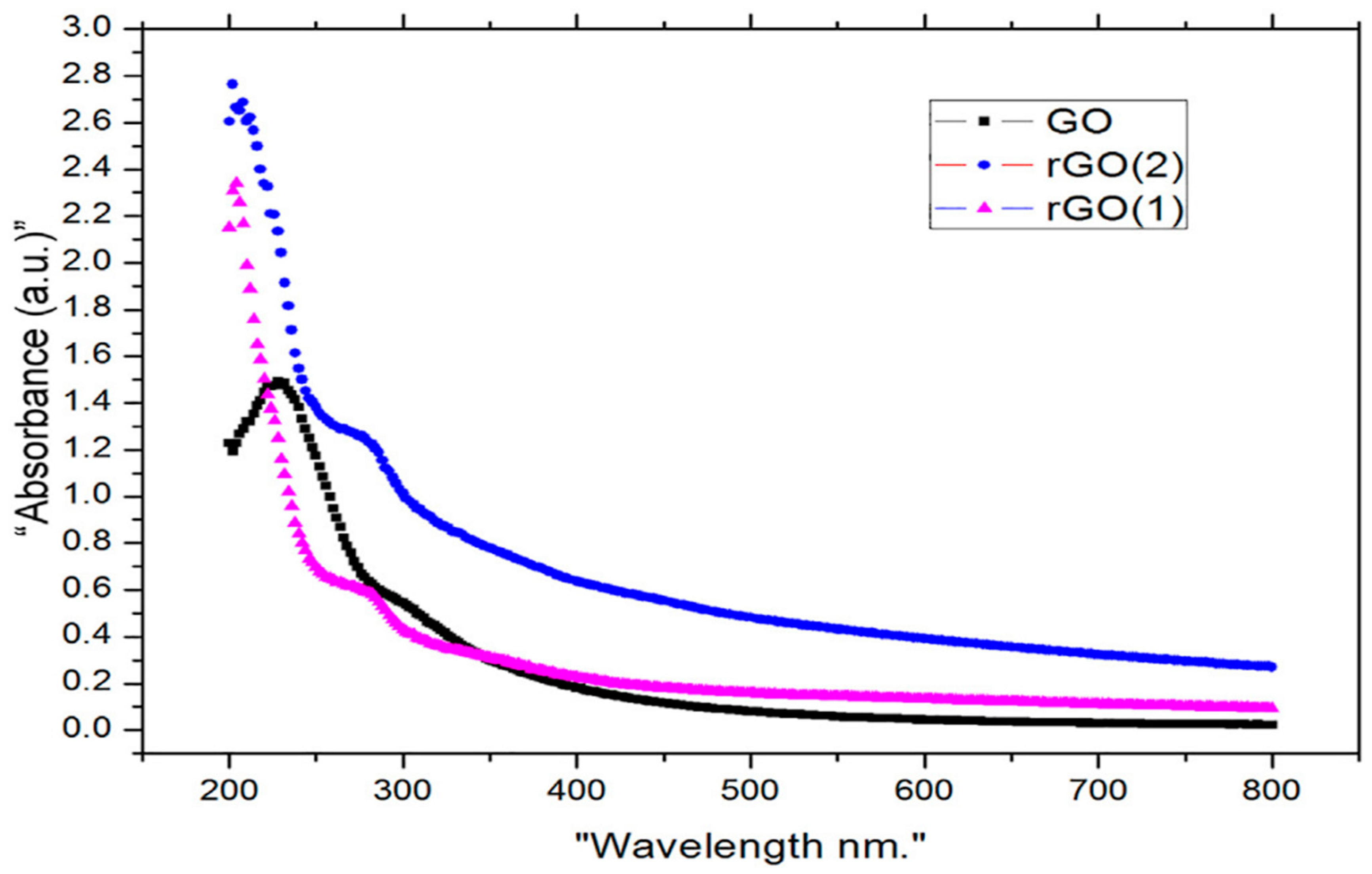
5.3. UV Visible Spectroscopy
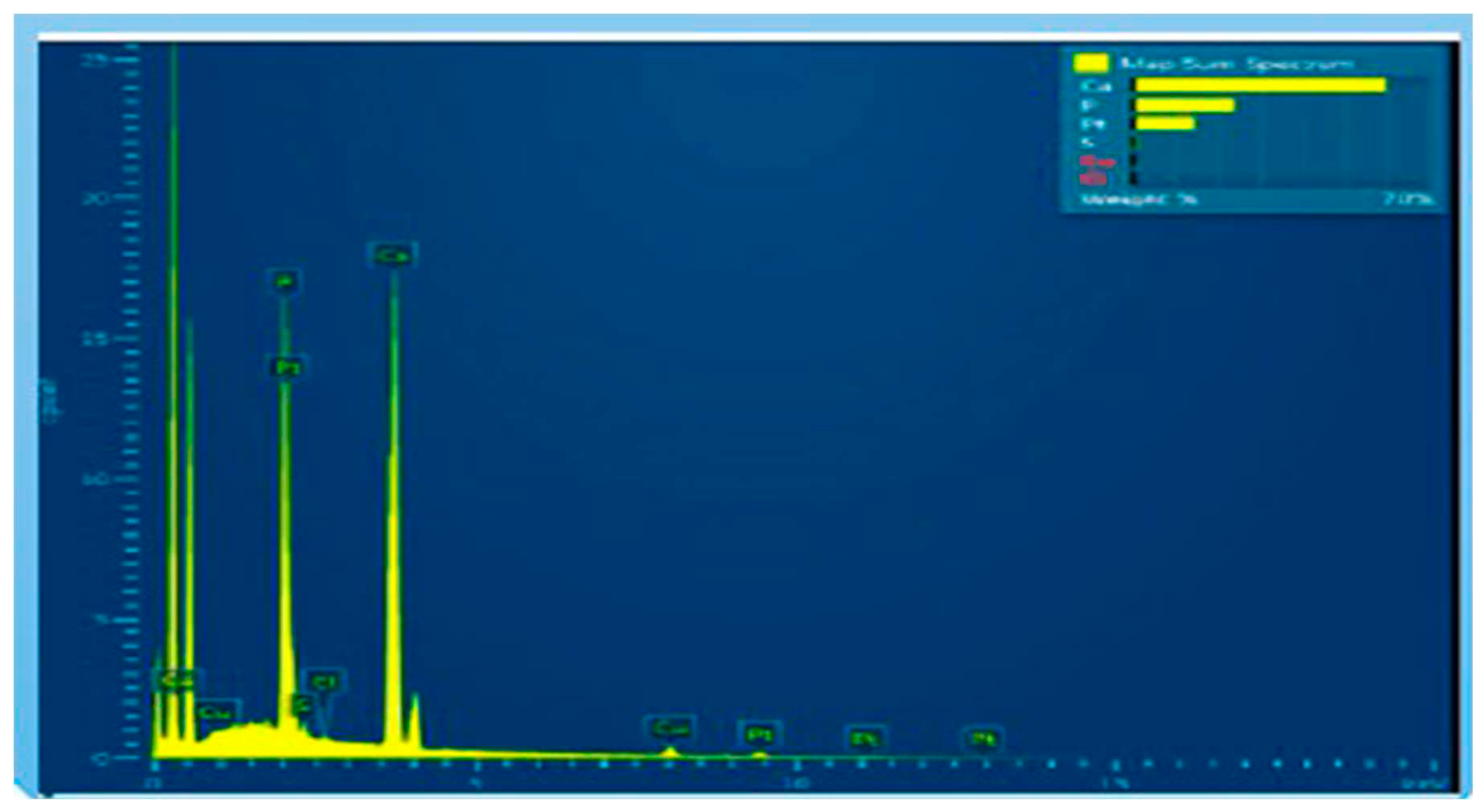
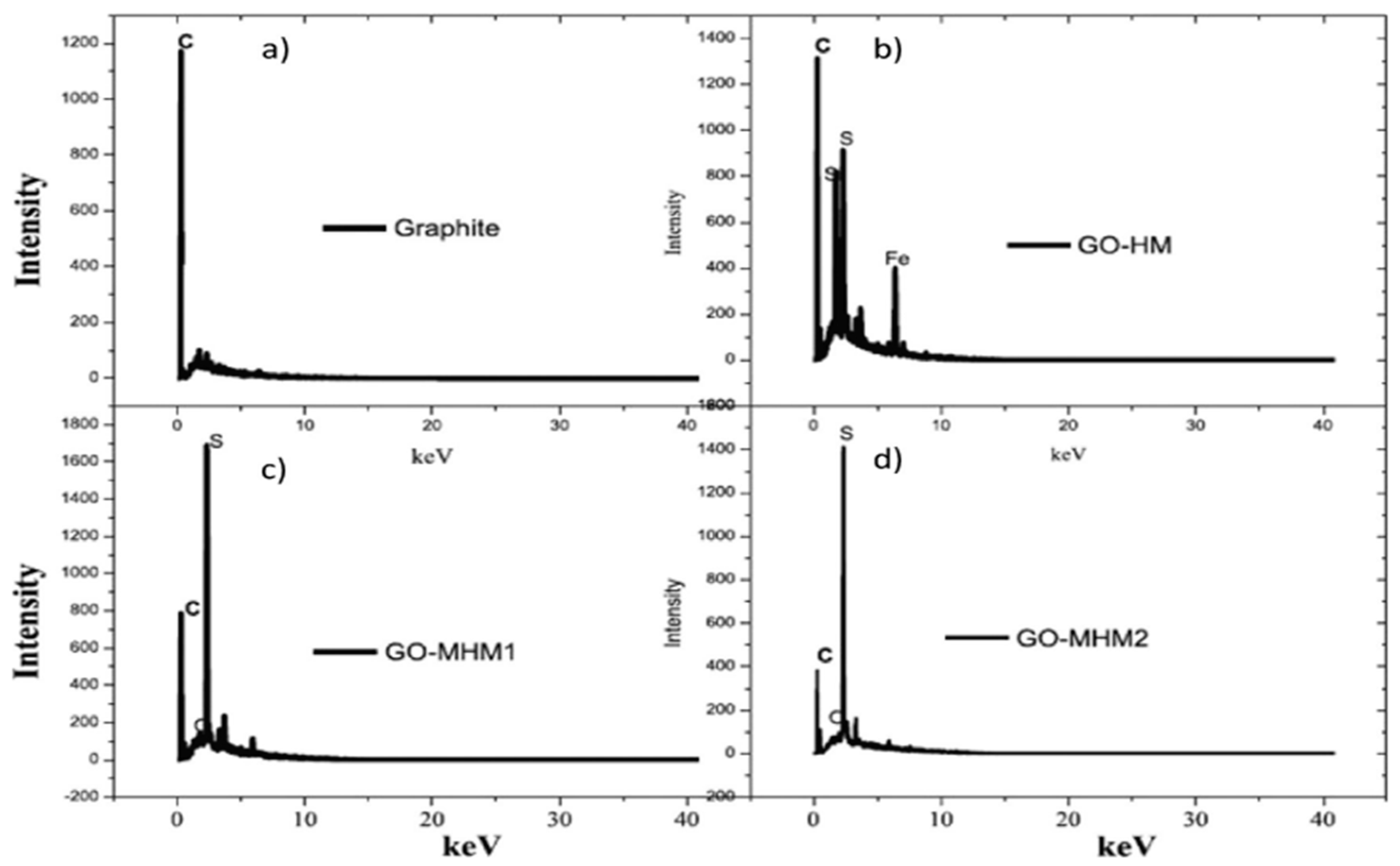
5.4. Energy Dispersive X-ray (EDX)
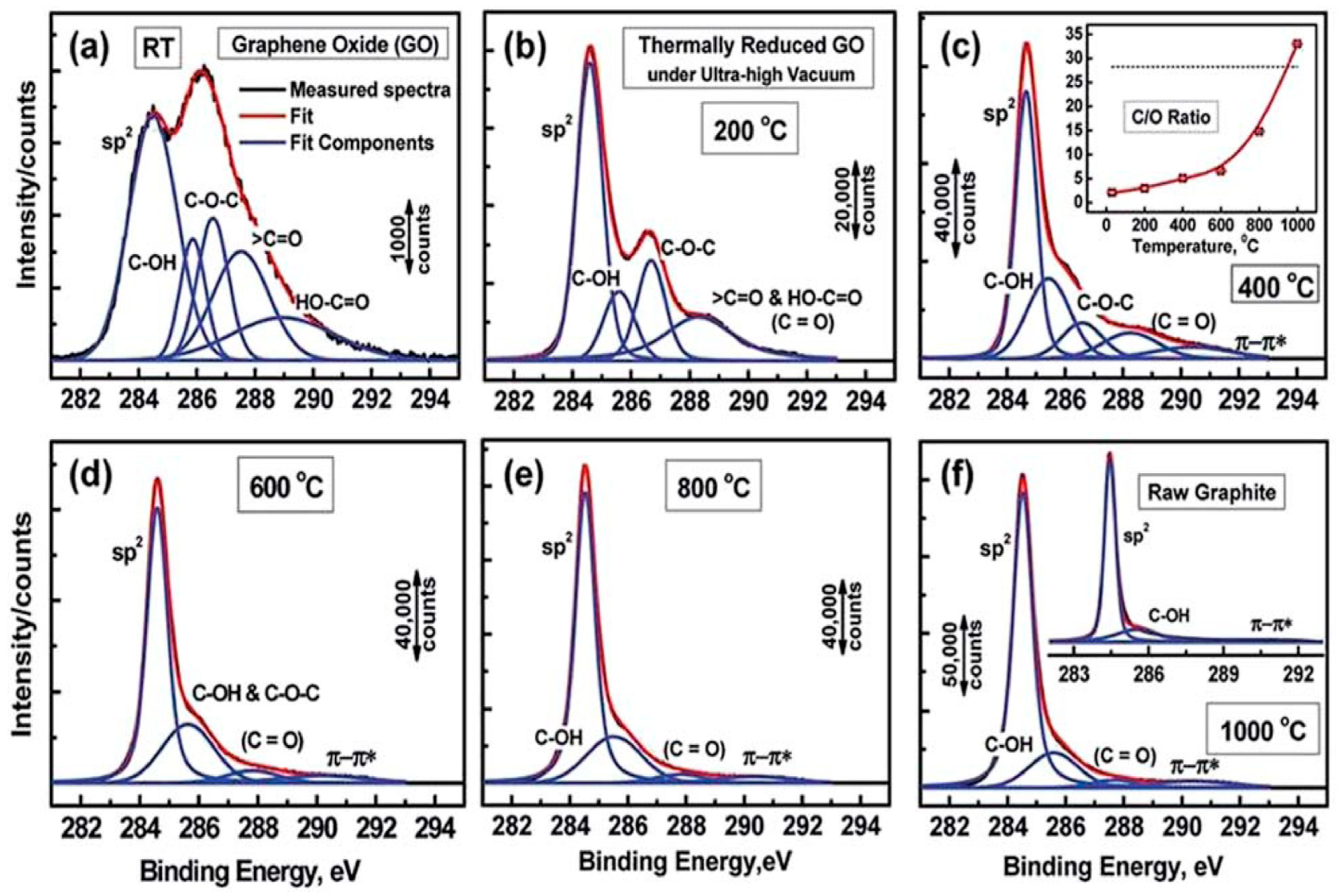
5.5. X-ray Photoelectron Spectroscopy (XPS)
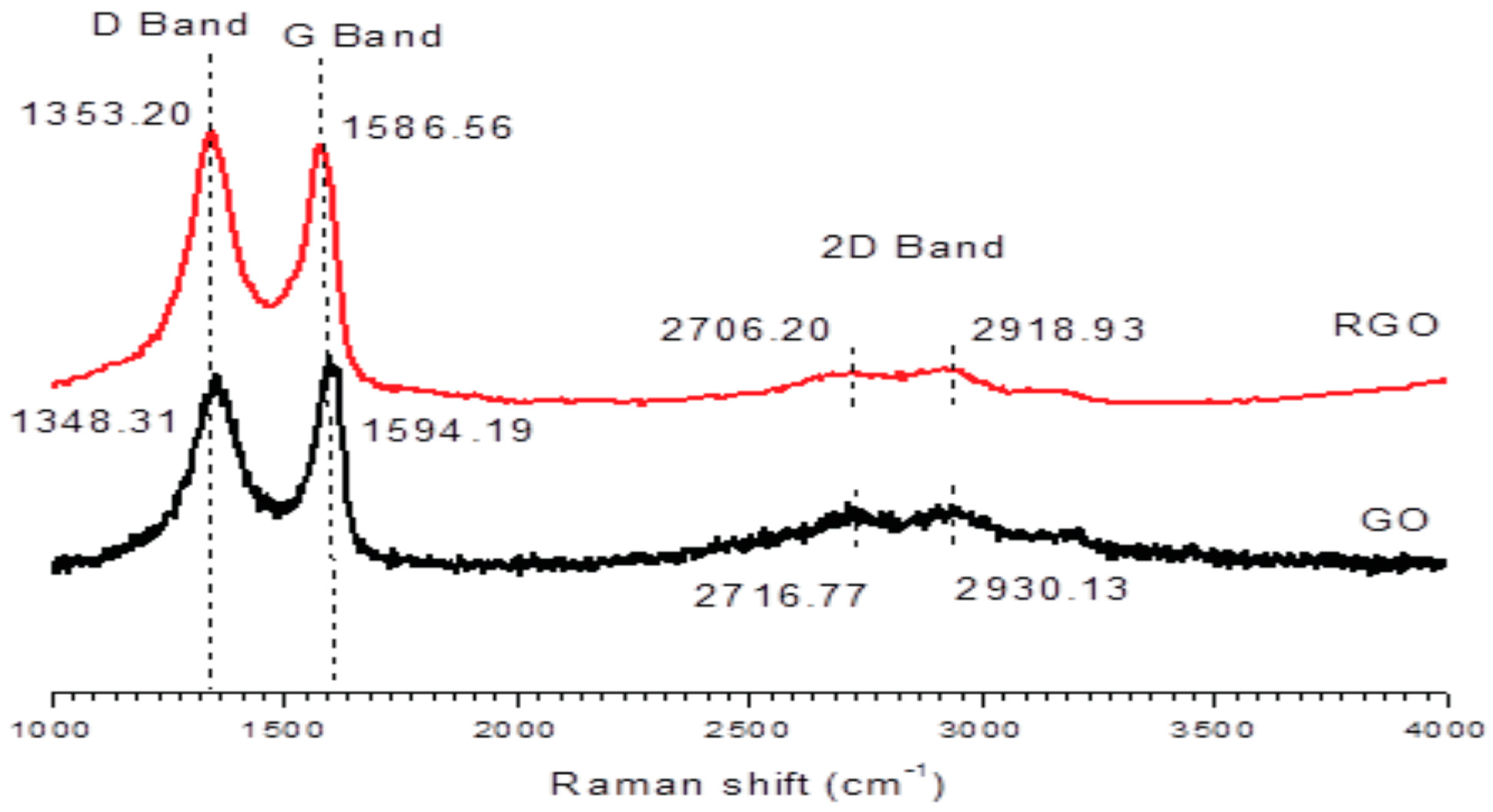
5.6. Raman Spectroscopy
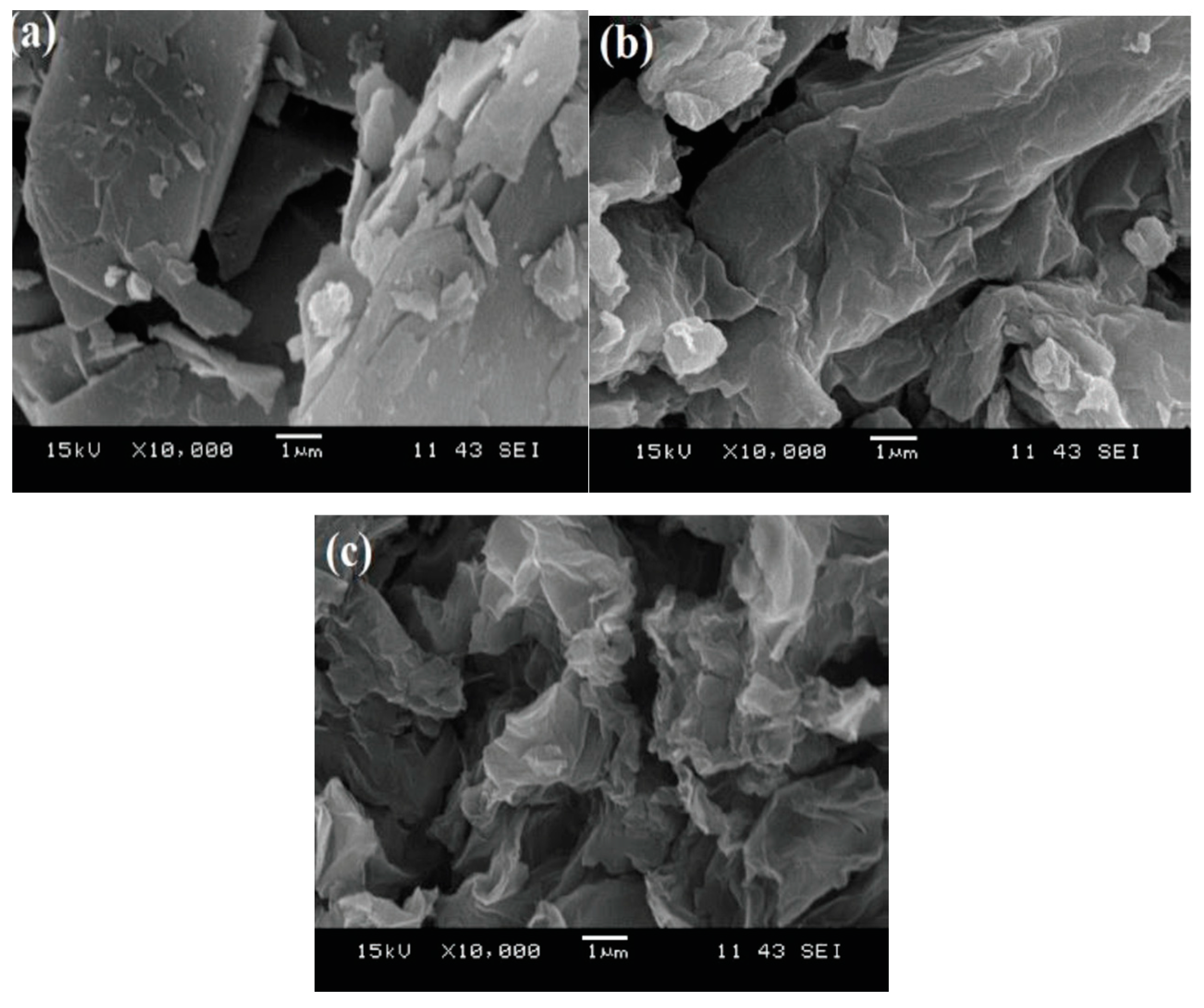
5.7. Scanning Electron Microscopy (SEM)
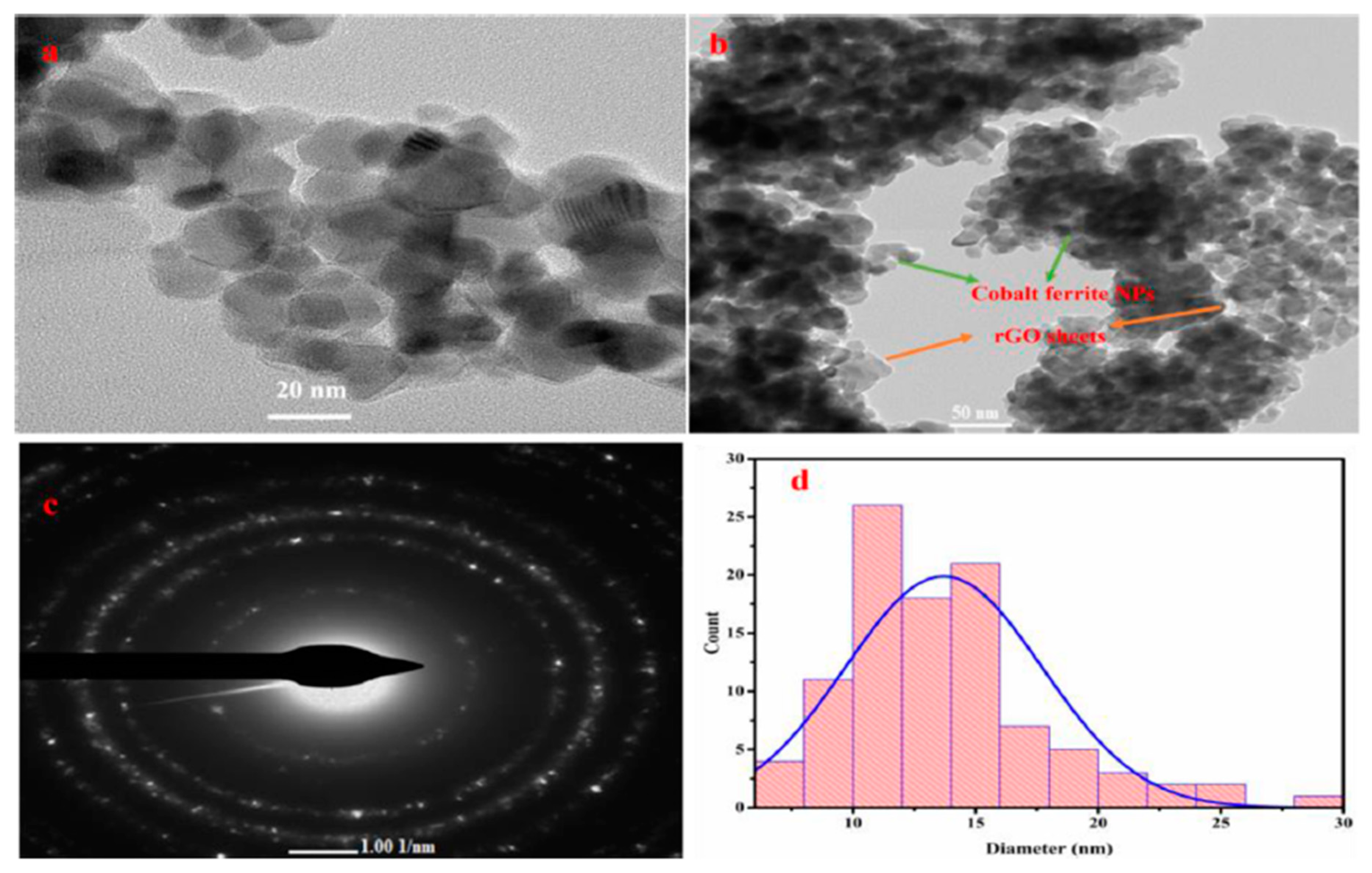
5.8. Transition Electron Microscope (TEM)
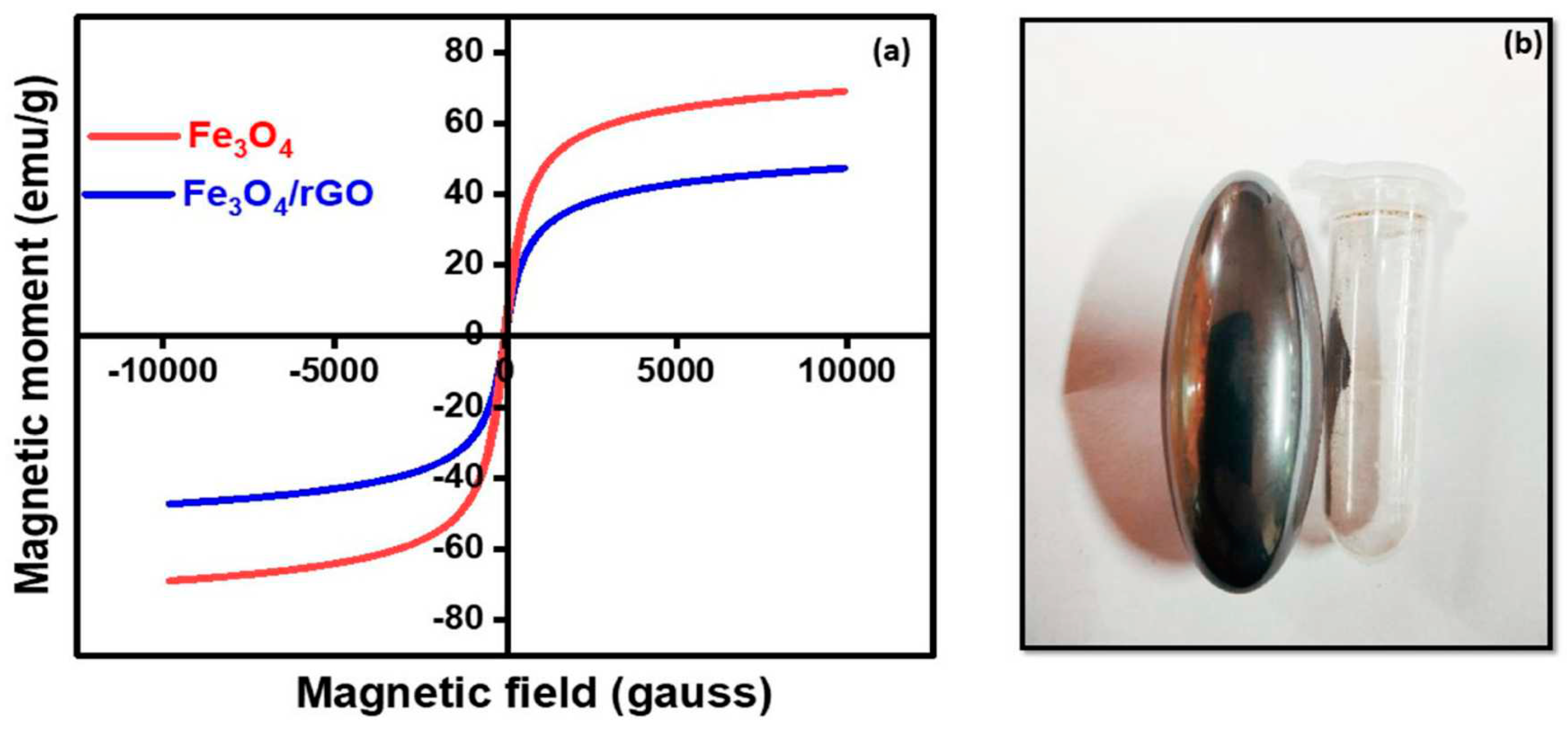
5.9. Vibrating Sample Magnetometer (VSM)
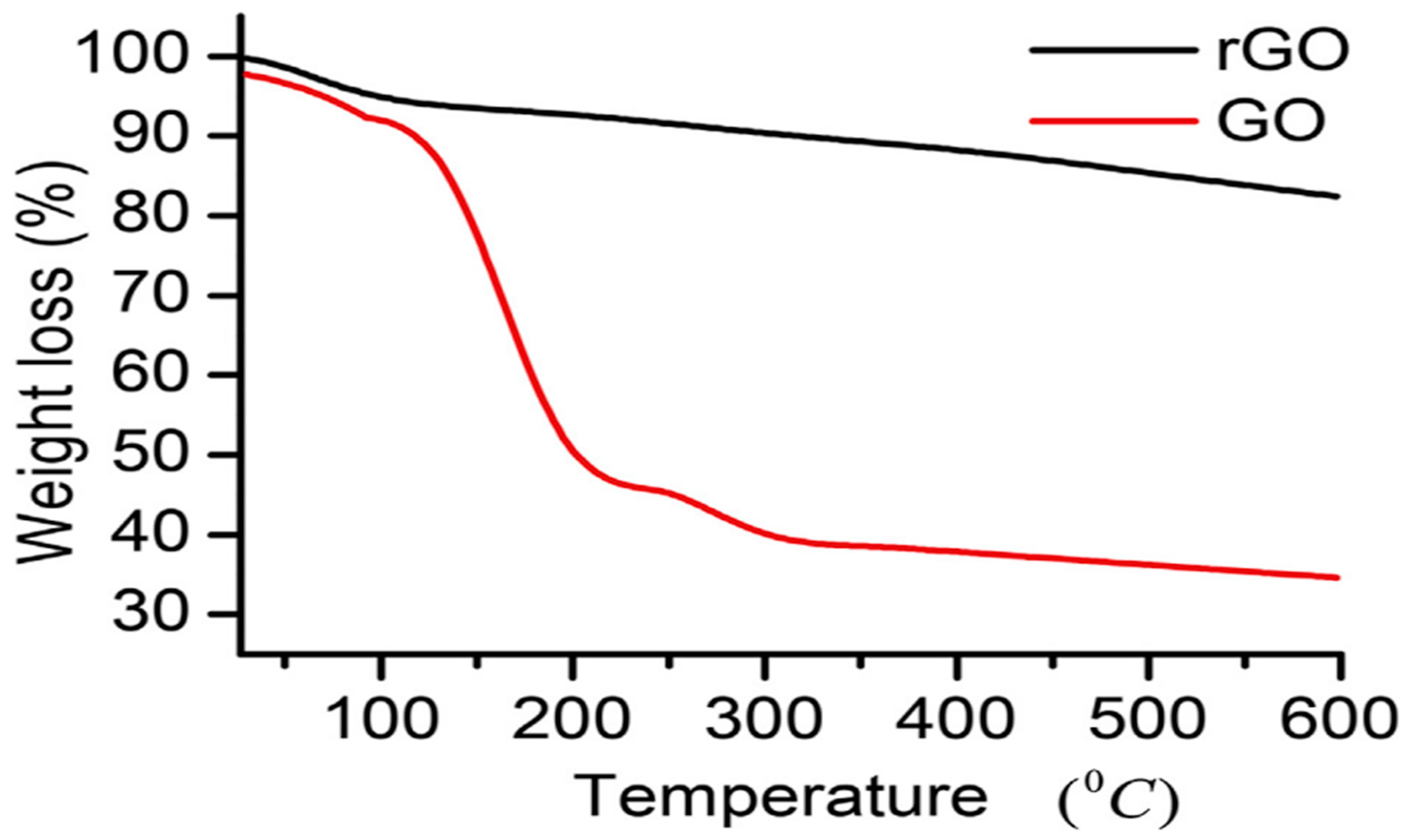
5.10. Thermogravimetric Analysis
6. Applications
6.1. Solar Cell Applications of Organic Photovoltaic
6.2. Graphene Supercapacitor
6.3. Graphene Sensor
6.4. Graphene Battery
6.5. Hydro Voltaic Generators
6.6. Graphene in OLED Screen
6.7. Graphene Shielding
7. Conclusion
References
- S. Alipour, M. Hassani, S. M. H. Hosseini, S. M. Mousavi-Khoshdel, Facile preparation of covalently functionalized graphene with 2,4-dinitrophenylhydrazine and investigation of its characteristics. RSC Adv. 2023, 13, 558. [CrossRef] [PubMed]
- Z. Hong, Z. Xin, W. Lei, B. Wang, Z. Xu, B. Ren, Y. Xiaodong, Synthesis of a Lignin-Enhanced Graphene Aerogel for Lipase Immobilization. ACS Omega. 2023, 8, 2435–2444. [CrossRef] [PubMed]
- Qian Wang, a Zhenjun Song, b Junhui Tao,a Haiqin Jin,a Sha Li,a Yuran Wang,a Xuejuan Liuc and Lin Zhang,; Interface contact and modulated electronic properties by in-plain strains in a graphene–MoS2 heterostructure. RSC Adv. 2023, 13, 2903. [CrossRef] [PubMed]
- B. M. Chufa, B.A. Gonfa, T.Y. Anshebo, G.A. Workneh, A Novel and Simplest Green Synthesis Method of Reduced Graphene Oxide Using Methanol Extracted Vernonia Amygdalina: Large-Scale Production. Adv. condens. Matter Phys. 2021, 10. [Google Scholar]
- B. Nattha, R. Chesta, S. Chaval, Synthesis of reduced graphene oxide quantum dots from graphene oxide via hydrothermal process and their structural, luminescence and magnetic properties. J.Taiwan Inst Chem Eng. 2023, 142, 104667. [Google Scholar] [CrossRef]
- B. Cristina, P. Álvarez, P. Blanco, M. Granda, C. Blanco, R.Santamaría, L.J. Romasantab, R.Verdejo, M.A. López-Manchado, R. Menéndez, Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon. 2013, 65, 156–164. [Google Scholar] [CrossRef]
- S. Sali, H.R. Mackey, A.A. Abdala, Effect of Graphene Oxide Synthesis Method on Properties and Performance of Polysulfone-Graphene Oxide Mixed Matrix Membranes. Nanomaterials. 2019, 9, 769. [CrossRef] [PubMed]
- M. Lojka, A. Jirickova, D. Sedmidubsky, O. Jankovsky, Fast Synthesis of Graphite Oxide via Modified Chlorate Route. Thermophysics. 2018, 020025, 1–6.
- M.J. Yoo, H.B. Park, Effect of Hydrogen Peroxide on Properties of Graphene Oxide in Hummers Method. Carbon. 2019, 141, 515–522. [CrossRef]
- N. M. S. Hidayah, W.W. Liu, C.W. Lai, N. Z. Noriman, C.S. Khe, U. Hashim, H.C. Lee, Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and Characterization. AIP 2017, 1892, 150005.
- M.D.P. Lavin-Lopez, A. Romero, J. Garrido, L. Sanchez-Silva, J.L. Valverde, Influence of Different Improved Hummers Method Modifications on the Characteristics of Graphite Oxide in Order to Make a More Easily Scalable Method. Ind. Eng. Chem. Res. 2016, 55, 12836–12847. [CrossRef]
- I. Sengupta, S. Chakraborty, M. Talukdar, Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 2018, 33, 4113–4122.
- I. Sengupta, S.S. Sharat Kumar, S.K. Pal, S. Chakraborty, Characterization of structural transformation of graphene oxide to reduced graphene oxide during thermal annealing. J. Mater. Res., 2020, 34, 1197–1204. [CrossRef]
- W. Liu, G. Speranza, Chemical Reduction of GO: Comparing Hydroiodic Acid and Sodium Borohydride Chemical Approaches by X-ray Photoelectron Spectroscopy. Carbon, 2022, 8, 20.
- R. Itum, K. Sanjeev, Bamboo shoot extract as a novel and efficient reducing agent for graphene oxide and its supercapacitor application. J Mater Sci: Mater Electron. 2023, 11, 127.
- R. Hidayat, S. Wahyuningsih, A.H. Ramelan, Simple synthesis of rGO (reduced graphene oxide) by thermal reduction of GO (graphene oxide). Mater. Sci. Eng. 2020, 858, 012009.
- M. Ikram, A. Raza, M. Imran, A. Ul-Hamid, A. Shahbaz, S. Ali, Hydrothermal Synthesis of Silver Decorated Reduced Graphene Oxide (rGO) Nanoflakes with Effective Photocatalytic Activity for Wastewater Treatment. Nanoscale Research Letters. 2020, 95, 15:95.
- V. Loryuenyong, K. Totepvimarn, P. Eimburanapravat, W. Boonchompoo, A. Buasri, Preparation and Characterization of Reduced Graphene Oxide Sheets via Water-Based Exfoliation and Reduction Methods. Adv Mater Sci Eng. 2013, 5.
- F. Amato, A. Motta, L. Giaccari, R.D. Pasquale, F.A. Scaramuzzo, R. Zanoni, A.G. Marrani, One-pot carboxyl enrichment fosters water dispersibility of reduced graphene oxide: a combined experimental and theoretical assessment. Nanoscale Adv. 2023, 5, 893. [CrossRef] [PubMed]
- L. Pavko, M. Gatalo, M. Finsgar, F. Ruiz-Zepeda, K. Ehelebe, P. Kaiser, M. Geuß, T. Đukic, A.K. Surca, M. Sala, M. Bele, S. Cherevko, B. Genorio, N. Hodnik, M. Gaberscek, Graphene-Derived Carbon Support Boosts Proton Exchange Membrane Fuel Cell Catalyst Stability. ACS Catal. 2022, 12, 9540–9548.
- T. Nathiya, A. Sivakumar, Green Synthesis of Reduced Graphene Oxide Nanosheets Using Leaf Extract of Lantana camara and Its In-Vitro Biological Activities. J. Clust. Sci. 2021, 32, 559–568. [CrossRef]
- S. Rattan, S. Kumar, J.K. Goswamy, Graphene oxide reduction using green chemistry. Mater. Today: Proc. 2020, 26, 3327–3331. [CrossRef]
- S. Yadav, A.P.S. Raman, H. Meena, A.G. Goswami, Bhawna, V. Kumar, P. Jain, G. Kumar, M. Sagar, D.K. Rana, I. Bahadur, P. Singh, An update on graphene oxide application and toxicity. ACS Omega. 2020, 7, 35387–35445.
- L.K.J. Ting, Y. Gao, H. Wang, T. Wang, J. Sun, J. Wang, Lithium Sulfide Batteries: Addressing the Kinetic Barriers and High First Charge Overpotential. ACS Omega. 2022, 7, 40682–40700. [CrossRef] [PubMed]
- B. Karan, S. Jagdeep, A.S. Dhaliwal, Green synthesis and characterization of superparamagnetic nanocomposite based on reduced graphene oxide/Fe3O4 prepared using leaf extract of Azadirachta indica. Inorg and Nano-Met Chem. 2023, 53, 1–10.
- G.G. Gebreegziabher, A.S. Asemahegne, D.W. Ayele, M. Dhakshnamoorthy, A. Kumar, One-step synthesis and characterization of reduced graphene oxide using chemical exfoliation method. Mater. Today Chem. 2019, 12, 233–239. [CrossRef]
- A. Singh, N. Sharma, M. Arif, R.S. Katiyar, Electrically reduced graphene oxide for photovoltaic application. J. Mater. Res. 2019, 34, 652–660. [CrossRef]
- B. Singh, R. Kaur, R. Kaur, K. Singh S. Rana A highly stable solid-state supercapacitor device based on robust layer-by-layer electrodeposited poly-(3, 4-ethylenedioxythiophene)-reduced graphene oxide–molybdenum disulfide nanocomposite electrode. J. Energy Storage. 2022, 56, 105926. [CrossRef]
- J. Zhu, X. Huang, W. Song, Physical and Chemical Sensors on the Basis of Laser-Induced Graphene: Mechanisms, Applications, and Perspectives. ACS Nano. 2021, 15, 18708–18741. [CrossRef] [PubMed]
- H.A. Rahman, M, Rafi, B.R. Putra, W.T. Wahyuni, Electrochemical Sensors Based on a Composite of Electrochemically Reduced Graphene Oxide and PEDOT: PSS for Hydrazine Detection. ACS Omega. 2023, 8, 3258–3269. [CrossRef] [PubMed]
- H. Xua, H. Chena, H. Lai, Z. Li, X. Donga, S. Cai, X. Chua, C. Gao, Capacitive charge storage enables an ultrahigh cathode capacity in aluminum-graphene battery. J. Energy Chem. 2020, 45, 40–44. [CrossRef]
- S. Jingyuan, F. Sunmiao, W. Wendong, W. Zhao, Z. Rui, L. Bingzhi, L. Li, B. Jiang, C. Haina, L. Ruojuan, W. Wang, Y. Xiaoqin, G. Wenyue, H.R. Mark, G. Wanlin, S. Jingyu, L. Zhongfan, Copper acetate-facilitated transfer-free growth of high-quality graphene for hydrovoltaic generators. Natl Sci Rev, 2022, 9, 1–9.
- S. Reena, K. Ramsingh, S. Sushama, K.G. Kallol, Facile and scalable synthesis of un-doped, doped and co-doped graphene quantum dots: a comparative study on their impact for environmental applications. RSC Adv. 2023, 13, 701. [CrossRef] [PubMed]
- K. Ayesha, Nanocarbon Nanocomposites of Polyaniline and Pyrrole for Electromagnetic Interference Shielding: Design and Effectiveness. Polym Plast Technol Mater. 2022, 61, 1988–2000.
- L.D. Yun, J.Z. Xiao, Y.W. Bian, Y.D. Qiu, Facile synthesis of polypyrrole nanoparticles with tunable conductivity for efficient electromagnetic wave absorption and shielding performance. CrystEngComm, 2022, 24.
- A.T.Smith,A.M.Lachance,S.Zeng,B.Liu,L.Sun. Synthesis, properties, and applications of graphene oxide/reduced grapheneoxide and their nanocomposites. NMS 2019.
- S.Singh, M.R.Hasan, P.Sharma, J.Narang, Graphene nanomaterials: The wondering material from synthesisto applications. Sens.Int. 2022, 3.
- Z. Iqbal, M.S. Tanweer, M. Alam, Reduced Graphene Oxide-Modified Spinel Cobalt Ferrite Nanocomposite: Synthesis, Characterization, and Its Superior Adsorption Performance for Dyes and Heavy Metals. ACS Omega 2023, 8, 6376–6390. [CrossRef]
- I. Bychko, A. Abakumov, O. Didenko, M. Chen, J. Tang, P. Strizhak, Differences in the structure and functionalities of graphene oxide and reduced graphene oxide obtained from graphite with various degrees of graphitization. Journal of Physics and Chemistry of Solids 2022, 164, 110614. [CrossRef]
- V.C. Fernandes, V.F. Domingues, M. S. Nunes, R. Matos, I. Kuźniarska-Biernacka, D.M. Fernandes, A. Guerrero-Ruiz, I.R. Ramos, C. Freire, C. Delerue-Matos, Graphene-Type Materials for the Dispersive Solid-Phase Extraction Step in the QuEChERS Method for the Extraction of Brominated Flame Retardants from Capsicum Cultivars. J. Agric. Food Chem. 2023, 71, 3898–3905.
- H. Kang, D.Y. Kim, J. Cho, Top-Down Fabrication of Luminescent Graphene Quantum Dots Using Self-Assembled Au Nanoparticles. ACS Omega 2023, 8, 5885–5892. [CrossRef] [PubMed]
- B.A. Hussein, A. Abebaw. T.B.G. Shiferac. A.M. Taddessed, A sensitive non-enzymatic electrochemical glucose sensor based on a ZnO/Co3O4/reduced graphene oxide nanocomposite. RSC Adv. 2023.
- M.S. Abubakar, Y. Shalu, R. Pushpesh, K. Raju, K. Firoz, K. Ashok, B. Debasis, Highly Sensitive Electrochemical Immunosensor Platforms for Dual Detection of SARS-CoV-2 Antigen and Antibody-based on Gold Nanoparticle Functionalized Graphene Oxide nanocomposites. ACS Appl. Bio Mater. 2022, 5, 2421–2430.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




