Submitted:
13 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Calculation methods
3. Results and discussions
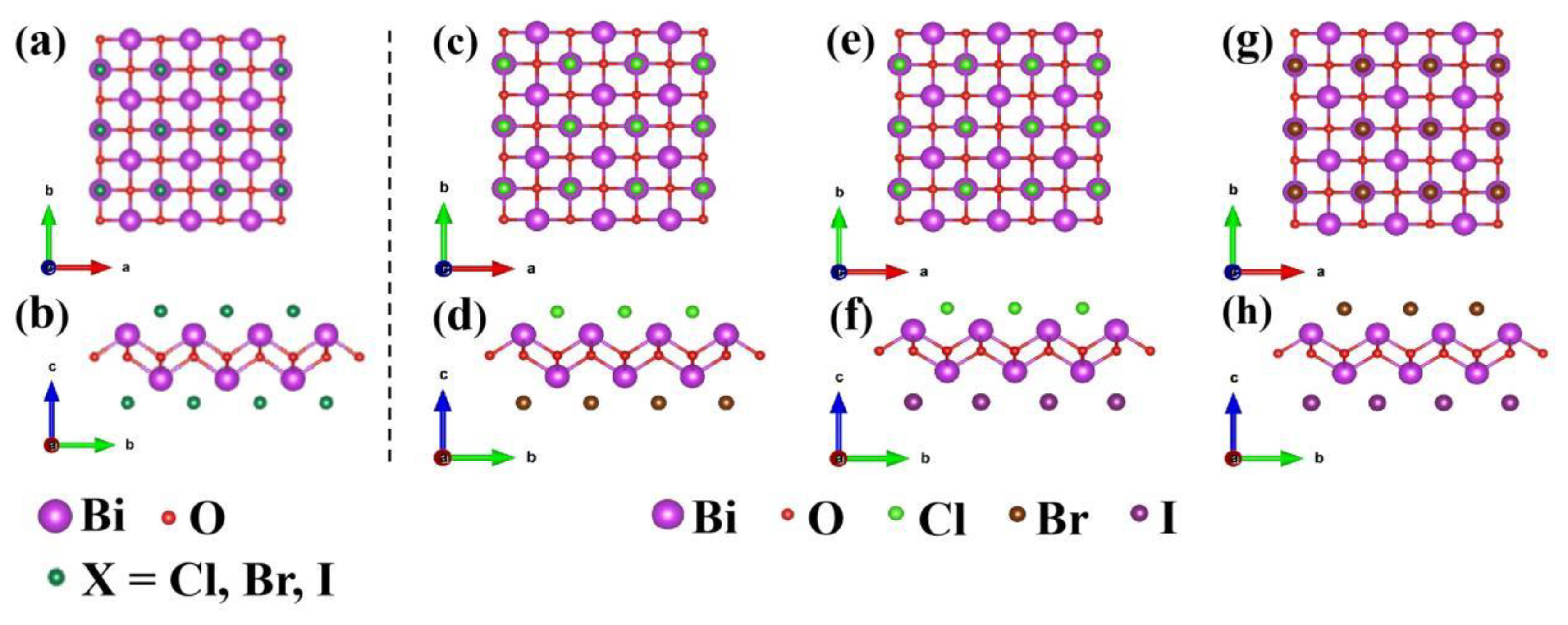
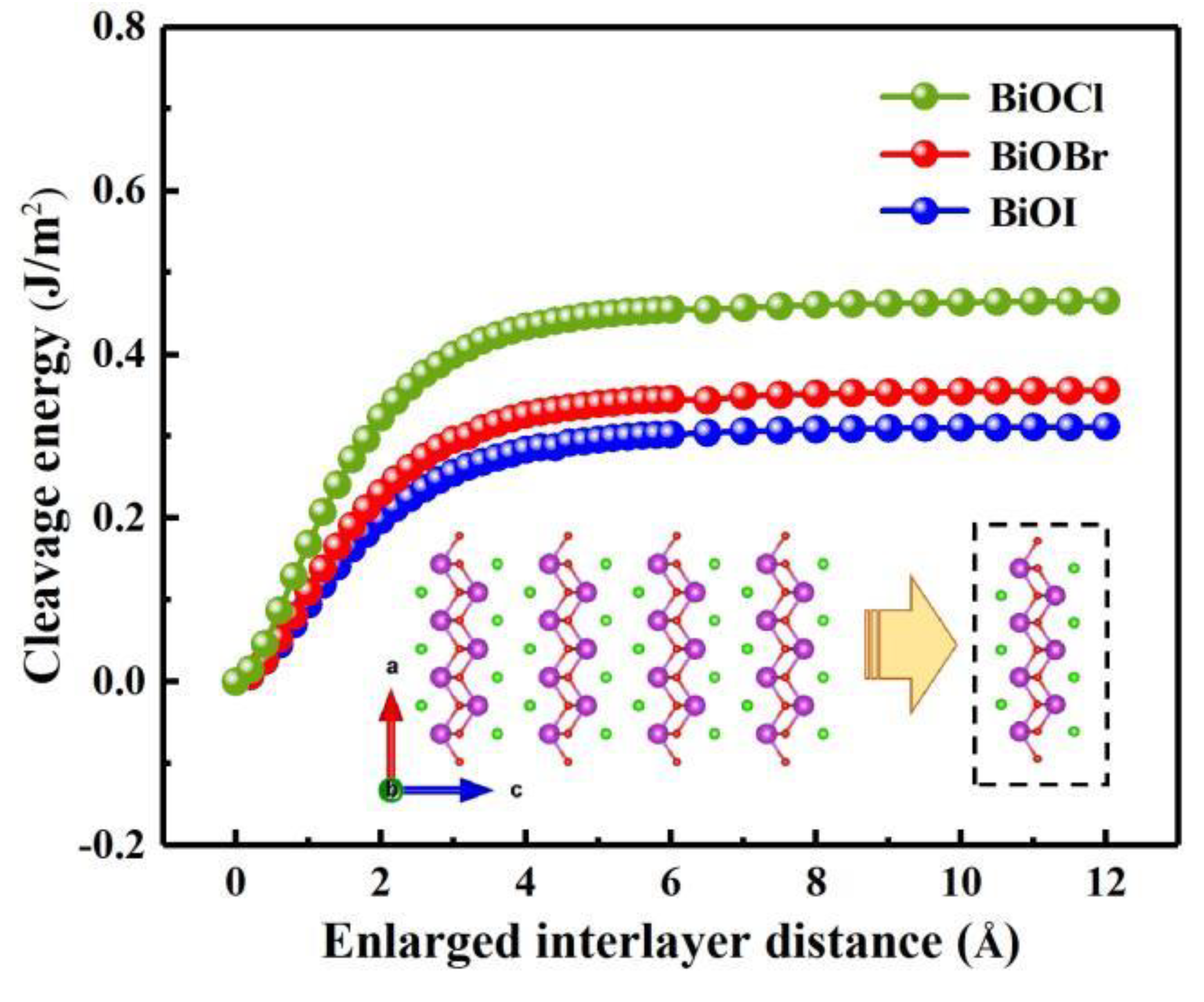
3.1. Crystal structure and stability
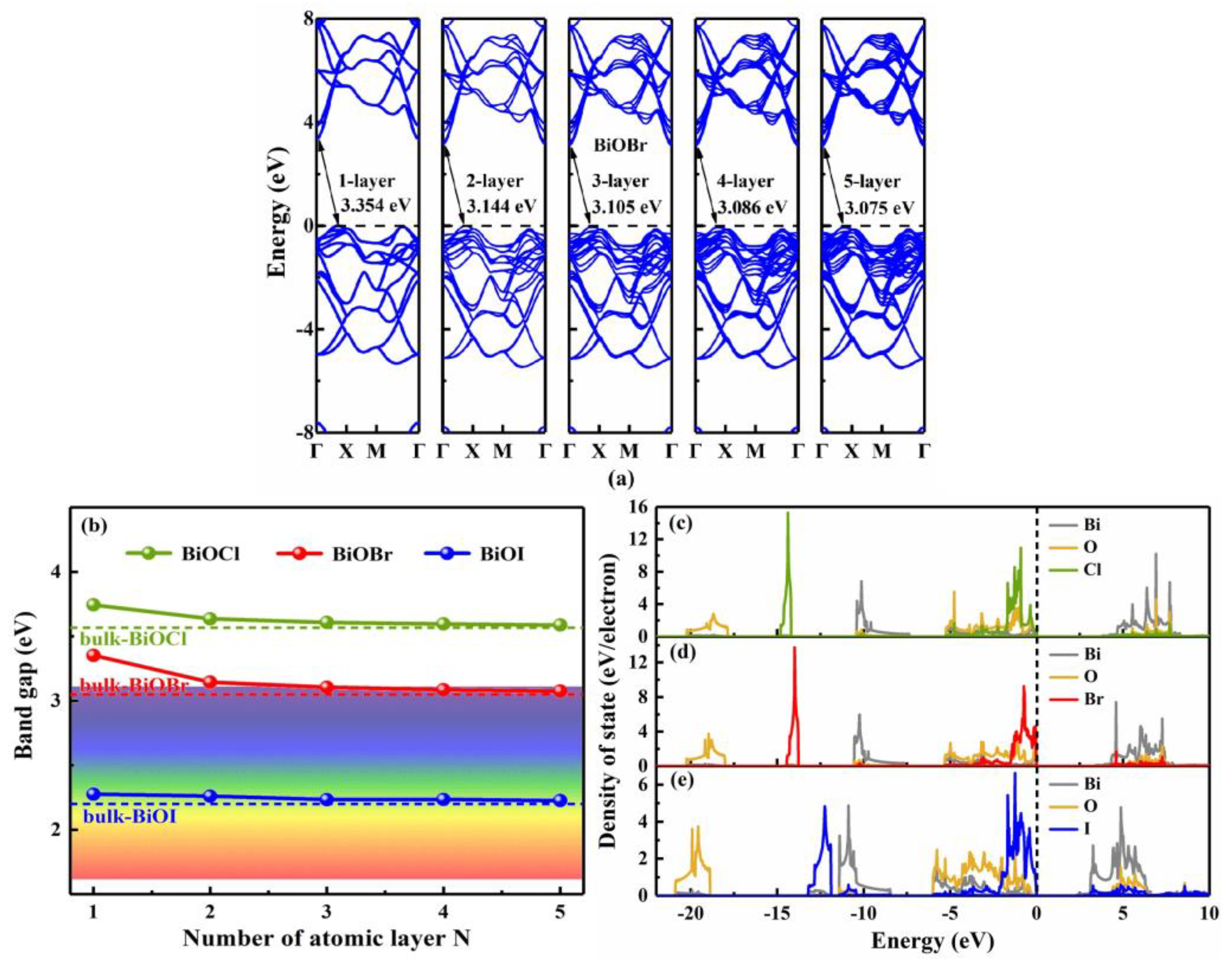
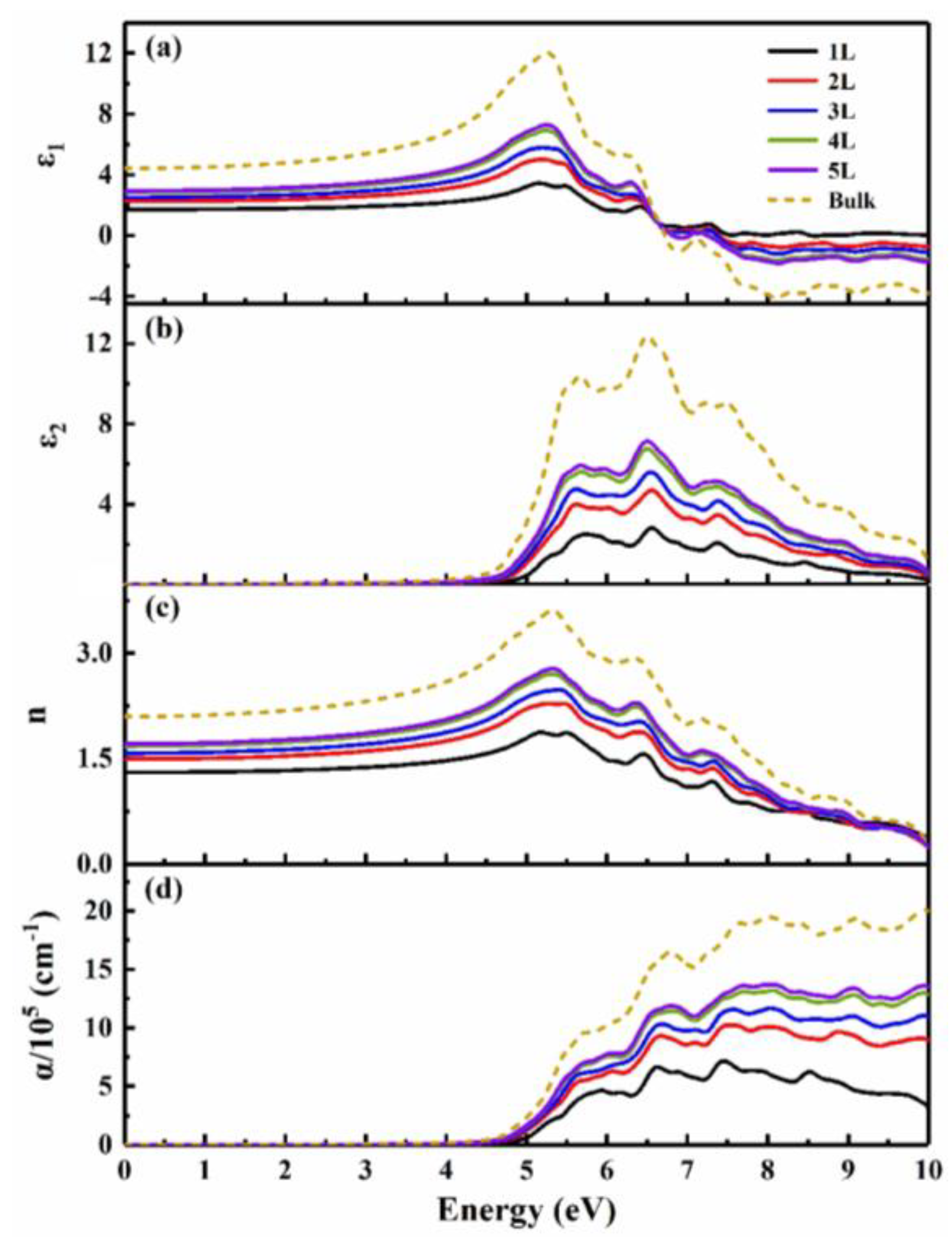
3.2. Thickness-dependent behavior
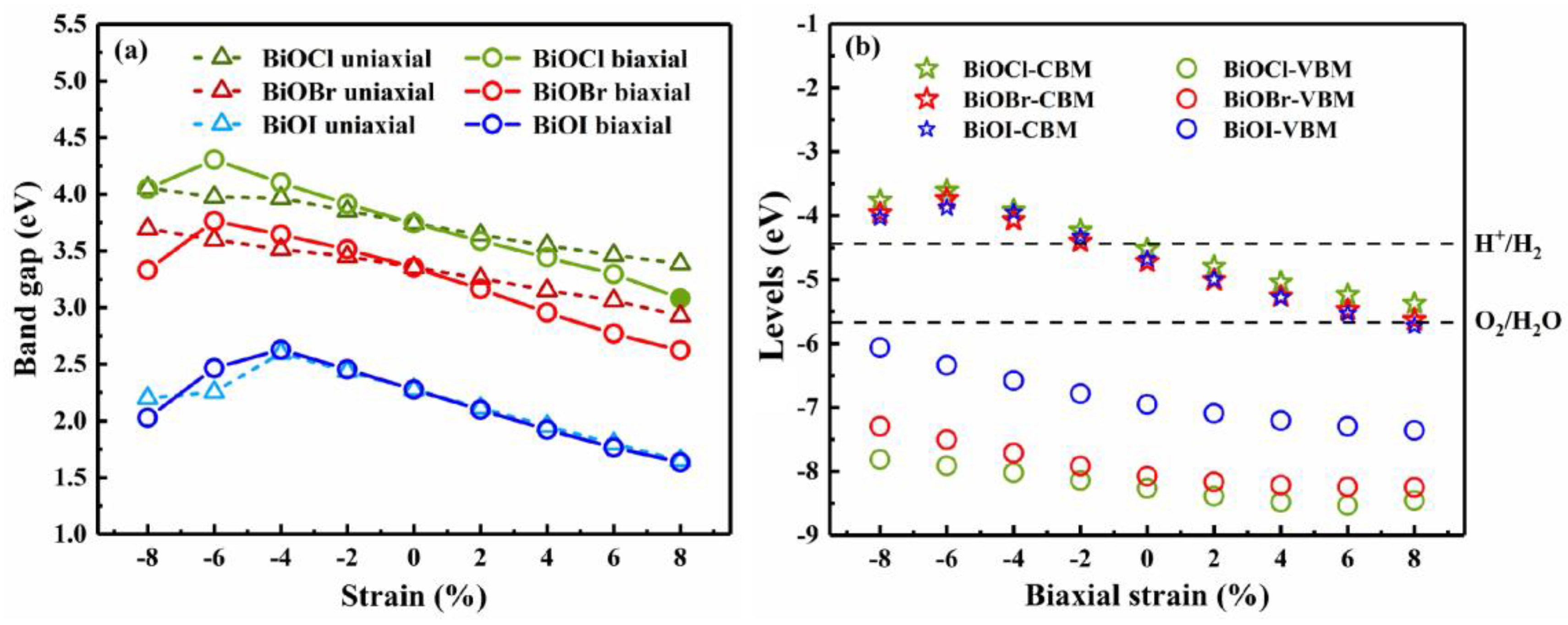
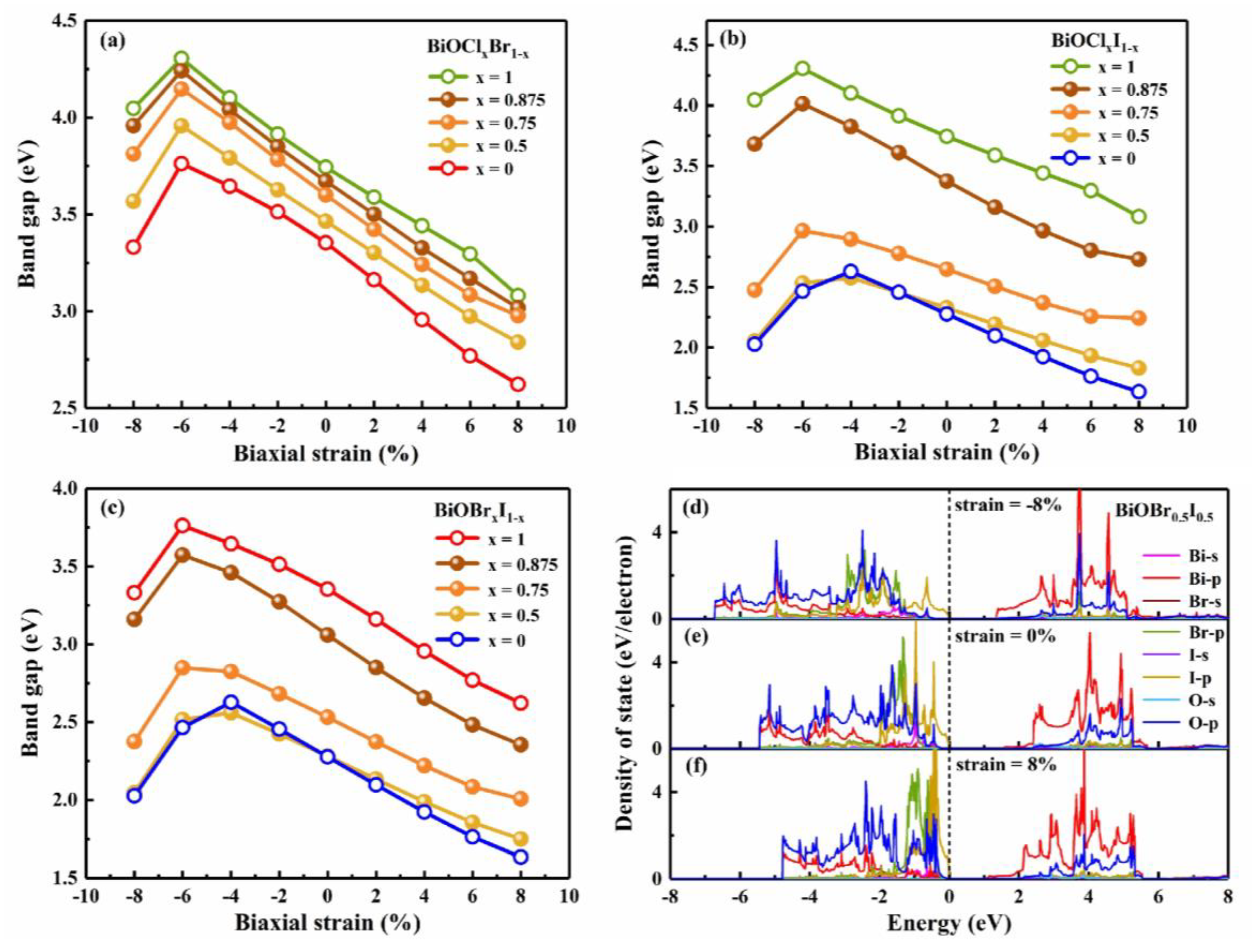
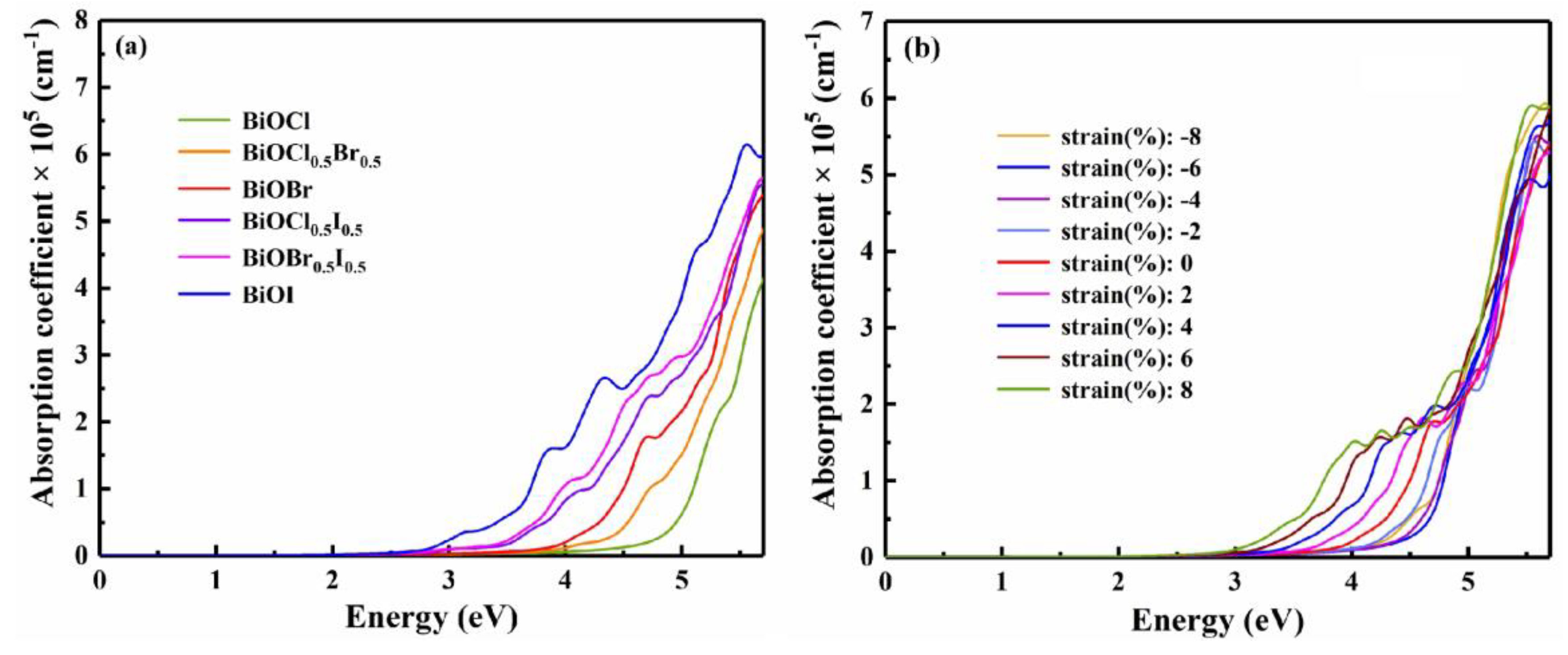
3.3. Strain engineering and composition tuning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K. S.; Geim, A. K.; Morozov, S. V.; Jiang, D.; Zhang, Y.; Dubonos, S. V.; Grigorieva, I. V.; Firsov, A. A. Electric field effect in atomically thin carbon films. Science 2004, 306(5659), 666–669. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A. T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P. D. Phosphorene: an unexplored 2D semiconductor with a high hole mobility. ACS nano 2014, 8(4), 4033–4041. [Google Scholar] [CrossRef]
- Mannix, A. J.; Zhou, X. F.; Kiraly, B.; Wood, J. D.; Alducin, D.; Myers, B. D.; Liu, X.; Fisher, B. L.; Santiago, U.; Guest, J. R.; Yacaman, M. J.; Ponce, A.; Oganov, A. R.; Hersam, M. C.; Guisinger, N.P. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350(6267), 1513–1516. [Google Scholar] [CrossRef]
- Jacoby, M. 2-D materials go beyond graphene. Chemical & Engineering News 2017, 95(22), 36. [Google Scholar]
- Huang, X.; Zhuo, Z.; Yan, L.; Wang, Y.; Xu, N.; Song, H. Z.; Zhou, L. Single-Layer Zirconium Dihalides ZrX2 (X = Cl, Br, and I) with Abnormal Ferroelastic Behavior and Strong Anisotropic Light Absorption Ability. The journal of physical chemistry letters 2021, 12(32), 7726–7732. [Google Scholar] [CrossRef]
- Sahin, H.; Cahangirov, S.; Topsakal, M.; Bekaroglu, E.; Akturk, E.; Senger, R. T.; Ciraci, S. Monolayer honeycomb structures of group-IV elements and III-V binary compounds: First-principles calculations. Physical Review B 2009, 80(15), 155453. [Google Scholar] [CrossRef]
- Butler, S. Z.; Hollen, S. M.; Cao, L.; Cui, Y.; Gupta, J. A.; Gutiérrez, H. R.; Heinz, T. F.; Hong, S. S.; Huang, J.; Ismach, A. F.; Johnston-Halperin, E.; Kuno, M.; Plashnitsa, V. V.; Robinson, R. D.; Ruoff, R. S.; Salahuddin, S.; Shan, J.; Shi, L.; Spencer, M. G.; Terrones, M.; Windl, W.; Goldberger, J. E. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS nano 2013, 7(4), 2898–2926. [Google Scholar] [CrossRef]
- Bao, Q.; Loh, K. P. Graphene photonics, plasmonics, and broadband optoelectronic devices. ACS nano 2012, 6(5), 3677–3694. [Google Scholar] [CrossRef]
- Koppens, F. H.; Mueller, T.; Avouris, P.; Ferrari, A. C.; Vitiello, M. S.; Polini, M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat Nanotechnol 2014, 9(10), 780–793. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Chen, Y.; Lin, J.; Khan, Q.; Zhang, Y.; Li, Y.; Zhang, H.; Xie, H. Recent Progress of Two-Dimensional Thermoelectric Materials. Nano-micro letters 2020, 12(1), 535–549. [Google Scholar]
- Li, F.; Shen, T.; Wang, C.; Zhang, Y.; Qi, J.; Zhang, H. Recent Advances in Strain-Induced Piezoelectric and Piezoresistive Effect-Engineered 2D Semiconductors for Adaptive Electronics and Optoelectronics. Nano-micro letters 2020, 12(1), 106. [Google Scholar]
- Li, X.; Yang, J. First-principles design of spintronics materials. National Science Review 2016, 3(3), 365–381. [Google Scholar] [CrossRef]
- Lightcap, I.; T. Kosel, P.V. Kamat, Anchoring semiconductor and metal nanoparticles on a two-dimensional catalyst mat. Storing and shuttling electrons with reduced graphene oxide. Nano Letters 2010, 10(2), 577–583. [Google Scholar] [CrossRef]
- Wu, S.; Wu, D. First principle calculations of photocatalytic properties of bismuth oxyhalides considering van der Waals correction. 2016 IEEE International Conference on Electro Information Technology (EIT), Grand Forks, ND, USA 2016, 0452-0457.
- Ye, L.; Wang, L.; Xie, H.; Su, Y.; Jin, X.; Zhang, C. Two-Dimensional Layered BiOX (X=Cl, Br) Compounds as Anode Materials for Lithium-Ion Batteries. Energy Technol 2015, 3(11), 1115–1120. [Google Scholar] [CrossRef]
- Xu, K.; Wang, L.; Xu, X.; Dou, S. X.; Hao, W.; Du, Y. Two-dimensional bismuth-based layered materials for energy-related applications. Energy Storage Materials 2019, 19, 446–463. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Li, H.; Guo, S.; Dai, S. Bismuth oxyhalide layered materials for energy and environmental applications. Nano Energy 2017, 41, 172–192. [Google Scholar] [CrossRef]
- Kang, L.; Yu, X.; Zhao, X.; Ouyang, Q.; Di, J.; Xu, M.; Tian, D.; Gan, W.; Ang, C. C. I.; Ning, S.; Fu, Q.; Zhou, J.; Kutty, R. G.; Deng, Y.; Song, P.; Zeng, Q.; Pennycook, S. J.; Shen, J.; Yong, K. T.; Liu, Z. Space-confined microwave synthesis of ternary-layered BiOCl crystals with high-performance ultraviolet photodetection. InfoMat 2020, 2(3), 593–600. [Google Scholar] [CrossRef]
- Zeng, W.; Li, J.; Feng, L.; Pan, H.; Zhang, X.; Sun, H.; Liu, Z. Synthesis of large-area atomically thin BiOI crystals with highly sensitive and controllable photodetection. Advanced Functional Materials 2019, 29(16), 1900129. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Feng, L.; Sun, Y.; Sun, H.; Xiong, W.; Xia, C.; Wang, Z.; Liu, Z. Controllable preparation of ultrathin 2D BiOBr crystals for high-performance ultraviolet photodetector. Science China Materials 2021, 64(1), 189–197. [Google Scholar] [CrossRef]
- Li, J. Q.; Cheng, C.; Duan, M. Y. The electronic and optical properties of multi-layer Bi2O2X (X = S, Se, Te) by first-principles calculations. Applied Surface Science 2023, 618, 156541. [Google Scholar] [CrossRef]
- Wang, G.; Luo, X.; Huang, Y.; Kuang, A.; Yuan, H.; Chen, H. BiOX/BiOY (X, Y = F, Cl, Br, I) superlattices for visible light photocatalysis applications. RSC advances, 2016, 6, 91508–91516. [Google Scholar]
- Zhao, A.; Zhang, L.; Guo, Y.; Li, H.; Ruan, S.; Zeng, Y. J. Emerging members of two-dimensional materials: bismuth-based ternary compounds. 2D Materials 2021, 8(1), 012004. [Google Scholar] [CrossRef]
- Bhachu, D. S.; Moniz, S. J. A.; Sathasivam, S.; Scanlon, D. O.; Walsh, A.; Bawaked, S. M.; Mokhtar, M.; Obaid, A. Y.; Parkin, I. P.; Tang, J.; Carmalt, C. J. Bismuth oxyhalides: synthesis, structure and photoelectrochemical activity. Chem Sci. 2016, 7(8), 4832–4841. [Google Scholar] [CrossRef]
- Ye, L.; Su, Y.; Jin, X.; Xie, H. Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: synthesis, modification, facet effects and mechanisms. Environmental Science: Nano 2014, 1(2), 90–112. [Google Scholar]
- Zhao, Z. Y.; Dai, W. W. Structural, Electronic, and Optical Properties of Eu-Doped BiOX (X = F, Cl, Br, I): A DFT+U Study. Inorganic Chemistry 2014, 53(24), 13001–13011. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Meng, J.; Li, Q.; Yang, J. Single- and few-layer BiOI as promising photocatalysts for solar water splitting. RSC Advances 2017, 7(39), 24446–24452. [Google Scholar] [CrossRef]
- Kong, T.; Wei, X.; Zhu, G.; Huang, Y. Electronic structure and optical properties of BiOI {001} monolayer under biaxial strain. Journal of Materials Science 2018, 53(1), 708–715. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. Journal of computational chemistry 2008, 29(13), 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Kürti, J.; Rajczy, P.; Kertesz, M.; Hafner, J.; Kresse, G. Performance of the Vienna ab initio simulation package (VASP) in chemical applications. Journal of Molecular Structure: THEOCHEM 2003, 624(1-3), 37–45. [Google Scholar]
- Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Physical Review Letters 1996, 77(18), 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. Journal of computational chemistry 2006, 27(15), 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Kerber, T.; Sierka, M.; Sauer, J. Application of semiempirical long-range dispersion corrections to periodic systems in density functional theory. Journal of computational chemistry 2008, 29(13), 2088–2097. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G. E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. The journal of chemical physics 2003, 118(18), 8207–8215. [Google Scholar] [CrossRef]
- Huang, X.; Yan, L.; Zhou, Y.; Wang, Y.; Song, H. Z.; Zhou, L. Group 11 Transition-Metal Halide Monolayers: High Promises for Photocatalysis and Quantum Cutting. The journal of physical chemistry letters 2021, 12(1), 525–531. [Google Scholar] [CrossRef]
- Wang, N.; Li, M.; Xiao, H. Y.; Gao, Z.; Liu, Z. J.; Zu, X.; Li, S.; Qiao, L. Band degeneracy enhanced thermoelectric performance in layered oxyselenides by first-principles calculations. npj Computational Materials 2021, 7(1), 18. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J. C.; Tang, G.; Geng, W. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Computer Physics Communications 2021, 267, 108033. [Google Scholar] [CrossRef]
- Zhang, C. G.; Ji, W. X.; Li, S. S.; Li, P.; Zhang, C. W.; Wang, P. J. 2D ternary nitrides XNY (X=Ti, Zr, Hf; Y=F, Cl, Br) with applications as photoelectric and photocatalytic materials featuring mechanical and optical anisotropy: A DFT study. Journal of Solid State Chemistry 2021, 303(366), 122517. [Google Scholar] [CrossRef]
- Qiao, J.; Kong, X.; Hu, Z. X.; Yang, F.; Ji, W. High-mobility transport anisotropy and linear dichroism in few-layer black phosphorus. Nature communications 2014, 5, 4475. [Google Scholar] [CrossRef]
- Bannister, F. A. The Crystal-Structure of the Bismuth Oxyhalides. Mineralogical Magazine 1935, 24(149), 49–58. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, J.; Yuan, Y.; Zhang, Q.; Gao, Z.; Liu, L. M.; Chen, L. The stabilities and electronic structures of single-layer bismuth oxyhalides for photocatalytic water splitting. Physical Chemistry Chemical Physics 2014, 16(47), 25854–25861. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, R.; Ulbricht, H.; Hertel, T. Interlayer cohesive energy of graphite from thermal desorption of polyaromatic hydrocarbons. Physical Review B 2004, 69(15), 155406. [Google Scholar] [CrossRef]
- Ghosh, B.; Puri, S.; Agarwal, A.; Bhowmick, S. SnP3: A Previously Unexplored Two-Dimensional Material. The Journal of Physical Chemistry C 2018, 122(31), 18185–18191. [Google Scholar]
- Yi, W.; Chen, X.; Wang, Z.; Ding, Y.; Yang, B.; Liu, X. A novel two-dimensional δ-InP3 monolayer with high stability, tunable bandgap, high carrier mobility, and gas sensing of NO2. Journal of Materials Chemistry C 2019, 7(24), 7352–7359. [Google Scholar] [CrossRef]
- Liu, J.; Shen, Y.; Lv, L.; Gao, X.; Meng, X.; Zhou, M.; Yang, X.; Zhang, Y.; Zheng, Y.; Zhou, Z. Mechanical, electronic and photocatalytic properties of binary Ge-based materials GeX2 (X = B, C, N) with a pentagonal structure. Journal of Materials Chemistry C 2022, 10(27), 10147–10156. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Y.; Chen, Z. Be2C Monolayer with Quasi-Planar Hexacoordinate Carbons: A Global Minimum Structure. Angewandte Chemie International Edition 2014, 53(28), 7248–7252. [Google Scholar]
- Liu, C. H.; Li, Z.; Mak, K. F.; Cappelluti, E.; Heinz, T. F. Observation of an electrically tunable band gap in trilayer graphene. Nature Physics 2011, 7(12), 944–947. [Google Scholar]
- Dai, J.; Zeng, X. C. Bilayer Phosphorene: Effect of Stacking Order on Bandgap and Its Potential Applications in Thin-Film Solar Cells. The journal of physical chemistry letters 2014, 5(7), 1289–1293. [Google Scholar] [CrossRef]
- Hu, C. W.; Yang, Y.; Hou, C.; Liang, T. X. Thickness- and strain-tunable electronic structures of two-dimensional Bi2O2Se. Computational Materials Science 2021, 194, 110424. [Google Scholar] [CrossRef]
- Kumar, A.; Ahluwalia, P. K. A first principle comparative study of electronic and optical properties of 1H-MoS2 and 2H-MoS2. Materials Chemistry and Physics 2012, 135(2-3), 755–761. [Google Scholar]
- Fei, R.; Yang, L. Strain-engineering the anisotropic electrical conductance of few-layer black phosphorus. Nano Letters 2014, 14(5), 2884–2889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Wu, D.; Jing, Y.; Zhou, Z. MnPSe3 monolayer: a promising 2D visible-light photohydrolytic catalyst with high carrier mobility. Advanced Science 2016, 3(10), 1600062. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Rao, C. N. R. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, J.; Hou, J.; Lin, Z.; Hu, Z.; Chang, J.; Zhang, J.; Hao, Y. Potential applications of halide double perovskite Cs2AgInX6 (X = Cl, Br) in flexible optoelectronics: unusual effects of uniaxial strains. The journal of physical chemistry letters 2019, 10(5), 1120–1125. [Google Scholar] [CrossRef]
| System | BiOCl | BiOBr | BiOI |
| a (Å) | |||
| 1 Layer | 3.876 | 3.926 | 4.015 |
| 2 Layers | 3.891 | 3.934 | 4.016 |
| 3 Layers | 3.896 | 3.937 | 4.017 |
| 4 Layers | 3.899 | 3.939 | 4.018 |
| 5 Layers | 3.900 | 3.940 | 4.018 |
| Bulk | 3.907 | 3.943 | 4.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





