1. Introduction
The ascomycetous yeast
Geotrichum candidum is a common, dimorphic member of the Saccharomycetes class [
1]. It is known to infect plants and cause post-harvest rot in a variety of fruit and vegetable crops [
2]. It supports the growth of
Pencillium camemberti through the metabolism of lactate, suppresses the growth of undesired organisms, and hence is used by the commercial cheesemaking industry as a ripening culture [
1]. Moreover, it is a commensal parasite able to infect the human skin, respiratory tract, and gastrointestinal tract [
3,
4].
Mycoviruses, viruses that infect and replicate in fungi, have been reported in several members belonging to all phyla of true fungi [
5] as well as oomycetes [
6]. Although mycovirus infections are mostly associated with symptomless infections, they may impact their fungal hosts in several ways [
7,
8,
9,
10]. Mycoviruses with three genome types (single-stranded RNA (ssRNA), double-stranded RNA (dsRNA) and ssDNA) have been reported with ssDNA mycoviruses being less common [
5,
7,
11,
12]. DsRNA mycoviruses are classified within families
Spinareoviridae,
Totiviridae,
Chrysoviridae,
Megabirnaviridae,
Quadriviridae,
Partitiviridae and
Amalgaviridae, and the genus
Botybirnavirus [
13]. Members of
Totiviridae family are classified into five genera:
Giardiavirus,
Trichomonasvirus,
Leishmaniavirus,
Victorivirus, and
Totivirus [
14]. The host range of totiviridae members and totivirus-like genomes has expanded over recent years with the increased utilization of next-generation sequencing technologies to include insects, arthropods, fish, crab and plants [
15,
16,
17,
18,
19]. Historically, virus-like particles (VLPs) and dsRNAs have been detected in
G. candidum without further biological or molecular identification of these associated VLPs or dsRNAs [
20,
21]. Recently, four totiviruses were detected and characterized from
G. candidum isolates from Pakistan [
22]. The current study reports the characterization of two mycoviruses from a different isolate of the same fungus. Interestingly, the two mycoviruses were also classified into
Totiviridae family indicating that totiviruses are common in
G. candidum.
2. Materials and Methods
2.1. Isolation, maintenance, and identification of isolate Gc6
Isolate Gc6 was isolated among other fungal isolates from a soil sample, collected from Amberely, New Zealand, on potato dextrose agar (PDA) using soil-plate method [
23]. Pure culture was obtained, and DNA extracted using a Zymo DNA Fungal/Bacterial Miniprep Kit as described by the manufacturer. The identity of isolate Gc6 was determined by amplifying and sequencing the non-coding internal transcribed spacer (ITS) region of fungal ribosomal DNA (rDNA) using the primer pair ITS4/ITS5 [
24]. Throughout the course of this study, isolate Gc6 was maintained and cultured on PDA plates.
2.2. Purification and visualization of dsRNA
Potato dextrose broth (PDB) media was inoculated with freshly grown fungal mycelial plugs and incubated at 25°C for 5 days. Two grams of fungal mycelia were harvested and used to survey for the presence of dsRNA using a method based on the selective binding of dsRNA to cellulose powder in the presence of 16.5% ethanol as previously described [
25]. Presumably purified dsRNA was treated with DNase and RNase at high salt buffer [
26], separated on ethidium bromide-prestained 1% (w/v) agarose gel in Tris-acetate EDTA (TAE) buffer and visualized on a UV transilluminator.
2.3. Sequencing, bioinformatics, and sequence and phylogenetic analyses of dsRNA
Purified dsRNA fraction was used as a template for reverse transcription polymerase chain reaction (RT-PCR) to generate random cDNA as described by Khalifa and Pearson [
27]. RT-PCR products were purified using a Gel/PCR DNA purification kit (Geneaid Biotech Ltd., Taiwan) and sequenced using Illumina HiSeq2000 at Macrogen Inc. (Seoul, South Korea). Illumina short reads were quality checked using FastQC and based on the quality check reports, Illumina reads were filtered and quality trimmed. Reads were then
de novo assembled using Geneious Prime software (Biomatters, New Zealand) set to medium sensitivity and default parameters. Assembled contigs were identified using Blastx against the GenBank non-redundant (nr) database. To determine the sequence of dsRNA termini and obtain full-length sequences of viral contigs, T4L adapter (5′-PO
4-CCCGTCGTTTGCTGGCTCTTT-NH
2-3′) was ligated to the 3′ end of the dsRNAs using T4 RNA ligase enzyme, T4L-ligated dsRNAs purified and used as a template for RT-PCR amplification of the terminal sequences using T4LC primer (5′-AAAGAGCCAGCAAACGACGGG-3′) together with dsRNA specific primers designed based on the sequences obtained by Illumina sequencing (
Supplementary Materials,
Table S1). RT-PCR products corresponding to the terminal sequences were purified and sequenced by Macrogen Inc. (Seoul, South Korea).
Open reading frames (ORFs) were detected using ORF finder tool (
https://www.ncbi.nlm.nih.gov/orffinder/). Viral protein sequences were aligned using MAFFT [
28] in order to identify conserved motifs. For phylogenetic analysis, related protein sequences were retrieved from NCBI database (accessed in April, 2023) and aligned using MAFFT. Based on the obtained alignments, MEGA-X software [
29] was used to construct maximum-likelihood (ML) phylogenetic trees using the best fit models. Le Gascuel model with gamma distribution and some sites to be evolutionary invariable (LG+
G+
I) and General Reverse Transcriptase + Freq with gamma distribution (rtREV+
G+
F) model with 1000 bootstrap replicates were used for RNA-dependent RNA polymerase (RdRp) and capsid protein (CP) ML phylogenetic trees construction, respectively.
2.4. Small RNA (sRNA) purification and sequencing
Isolate Gc6 was grown on cellophane-covered PDA Petri-dishes for 5 days at 25°C. The sRNA fraction was purified from grown mycelia using mirPremier™ microRNA Isolation Kit (Sigma-Aldrich, USA). Libraries of sRNA were prepared and sequenced using Illumina sequencing at Macrogen Inc. (Seoul, South Korea). Obtained Illumina reads were mapped against the full sequences of dsRNAs obtained from isolate Gc6 using Bowtie2 with default parameters [
30].
3. Results and discussion
Fungal viruses, those able to replicate in fungi, are widespread in several species from the four major taxa of fungi as well as oomycetes [
5,
6]. Their taxonomy has extensively grown in the past few years due to the increasing interest in mycoviruses and associated effects on the one hand, and with the practicability of high throughput sequencing (HGS) technologies and bioinformatic software on another hand [
31]. Currently known mycoviruses have genomes belonging to three nucleic acid types: ssRNA, dsRNA and ssDNA. According to the International Committee on Taxonomy of Viruses (ICTV), mycoviruses with dsRNA genomes are classified into six established families named
Chrysoviridae,
Megabirnaviridae,
Partitiviridae,
Quadriviridae,
Reoviridae and
Totiviridae, and three proposed families:
Alternaviridae,
Botybirnaviridae, and
Fusagraviridae [
32]. Below, we report the molecular characteristics of two dsRNA mycoviruses from the ascomycete
G. candidum.
3.1. Identity of the host fungus
Soil is a rich source of different types of microorganisms that may be infected with viruses. Since these viruses affect the metabolic activity of their hosts, they play a pivotal role in the functionality of the soil ecosystems [
33]. Fungi is one of the main soil microbial components that can harbor a wide range of viruses causing variable effects on their hosts starting from symptomless to severely debilitating infections [
5]. Isolate Gc6 was isolated from a soil sample on PDA using standard isolation methods. A pure culture was obtained, and fungal identity determined by sequencing the ITS region. Nucleotide sequence of the amplified fragment shared nucleotide (nt) sequence identities greater than 98% with the corresponding ITS regions of several
G. candidum isolates. Therefore, Gc6 was considered as an isolate of
G. candidum fungus.
3.2. Presence and sequencing of dsRNA associated with G. candidum isolate Gc6
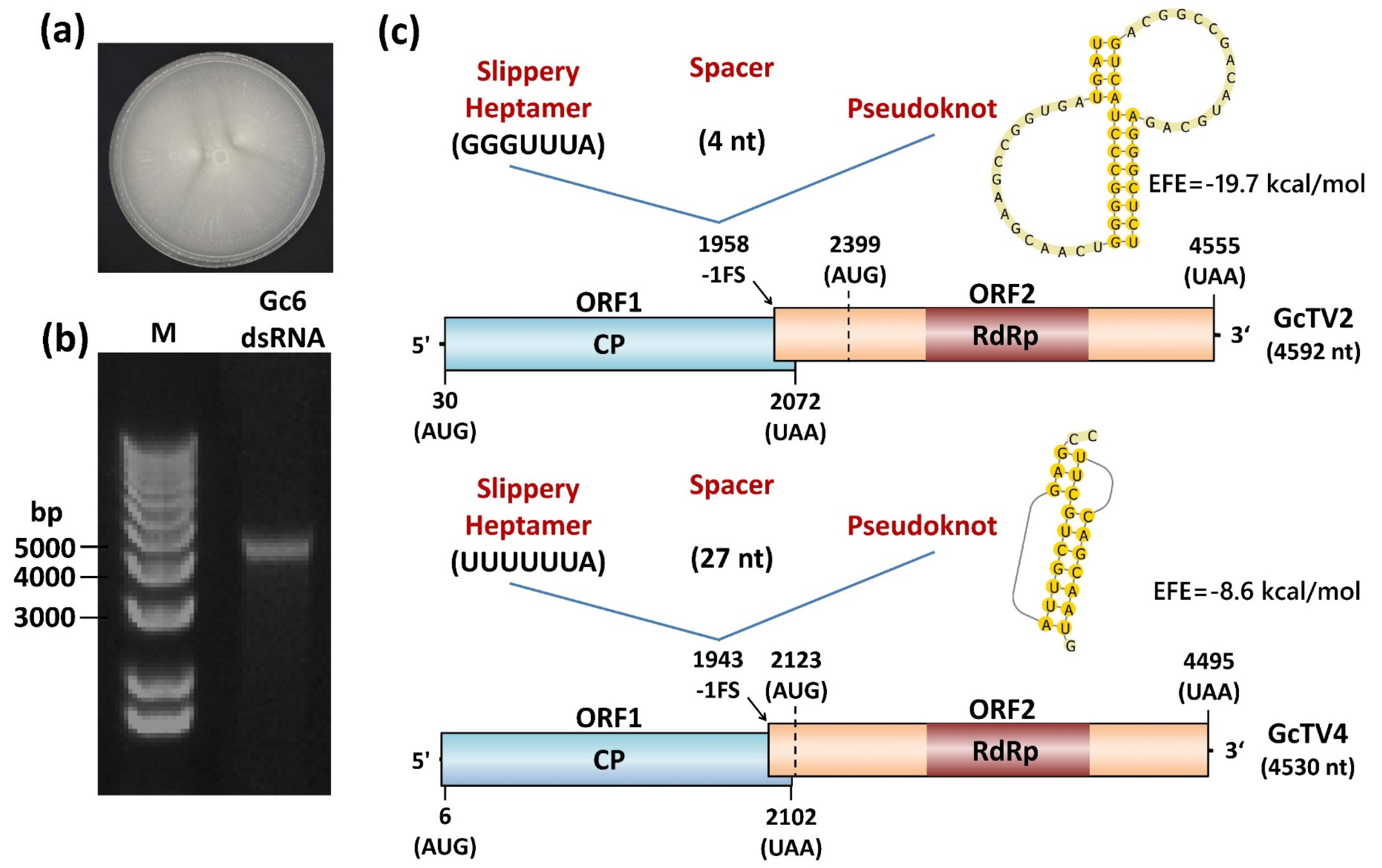
Electrophoretic analysis of dsRNA purified from isolate Gc6 revealed the presence of a visually single nucleic acid band with an estimated molecular weight of about 4.5 kb (
Figure 1a). This band resisted DNase treatment and RNase treatment in high salt buffer, confirming its double-stranded nature. Yeast fungi have been long known as hosts of mycoviruses since the discovery of a killer phenotype associated with
Saccharomyces-virus killer systems [
34]. In
G. candidum, uncharacterized dsRNAs and virus-like particles have been discovered in the past [
20,
35]. Recently, three species belonging to the family
Totiviridae have been characterized at the molecular level [
22].
The composition and identity of this purified dsRNA from
G. candidum isolate Gc6 were determined by sequencing after random cDNA synthesis using purified dsRNA as a template.
De novo assembly of Illumina short reads resulted in the creation of three long contiguous sequences of 4519, 2447 and 743 nt that shared identities with totiviral sequences based upon initial Blastx analysis. The assembly was completed, validated and full-length sequence of Gc6-dsRNAs was obtained using RACE-sequencing. The Gc6-dsRNAs were found to be 4592 (dsRNA1: contig 1) and 4530 (dsRNA2: contigs 2 and 3) nt in length. Blastx searches of dsRNA1 and dsRNA2 full-length sequences revealed up to 91.66 and 89.24% identities to RdRp and 95.29 and 91.69% identities to CP sequences of viruses in the family
Totiviridae, respectively. Therefore, the apparently single dsRNA segment purified from isolate Gc6 consists of two co-migrating segments representing the genomes of two totiviruses. The closest fungal virus to dsRNA1 was Geotrichum candidum totivirus 2 (GcTV2) and therefore, dsRNA1 was considered as an isolate of the same virus (GcTV2-Gc6). DsRNA2 shared the highest identity with an insect totivirus, and the closest fungal totivirus shared RdRp and CP aa sequence identities of 48.05 and 41.86% with dsRNA2. Hence, dsRNA2 was given a new tentative name, Geotrichum candidum totivirus 4 (GcTV4-Gc6). Sequences of GcTV2-Gc6 and GcTV4-Gc6 were deposited to GenBank under accession numbers OR250782 and OR250783, respectively. Infection of a single fungal isolate with multiple related or unrelated mycoviruses has been previously reported in several fungal species[
22,
27,
36,
37,
38,
39,
40,
41,
42,
43]. Mycoviruses previously discovered from
G. candidum as well as those described in the current study were found to cause co-infections in one host isolate.
3.3. Genome organization and analysis of Geotrichum candidum totiviruses
The
Totiviridae family accommodates viruses with single linear, uncapped dsRNA genome segment of approximately 4.6–7.0 kilobase pair (kbp) in length [
14]. The genomes of its members consist of two large, sometime overlapping, ORFs with potential to encode major CPs of about 70-100 kilodalton (kDa) (5′ ORF) and an RdRp (3′ ORF). The GcTV2-Gc6 genome has a GC content of 45.5% and has the potential to code for two long open ORFs that are separated by a 326 nt long intergenic region (IR). ORF1 in frame three is 2043 nt long and located following a 29 nt long 5’ untranslated region (UTR). It starts at nt position 30, ends at an UAA termination codon at nt position 2072 and encodes a 680 amino acid (aa) long protein with an estimated molecular mass of 76.6 kDa. ORF2 in frame two precedes a 37 nt long 3´ UTR and has a length of 2157 nt. It starts at an AUG codon (nt position 2399), terminates at an UAA codon (nt position 4555) and encodes a 718 aa long protein with an estimated molecular weight of 82.06 kDa.
GcTV4-Gc6 genome has a GC content of 42.6% and consists of 2097 (ORF1) and 2373 (ORF2) nt long ORFs with the potential to encode proteins of 698 and 790 aa and estimated molecular weights of 78.6 and 90.11 kDa, respectively. ORF1 and ORF2 start at nt positions 6 and 2123 with an AUG codon and end at nt positions 2102 and 4495, respectively. The two ORFs are separated by a 20 nt long IR and the genome has 5 and 35 nt long UTRs at the 5′ and 3′ termini, respectively. Genome organization of GcTV2-Gc6 and GcTV4-Gc6 is similar to genomes of other
G. candidum totiviruses [
22] and is typical for those of yeast totiviruses coding for two ORFs [
44].
Blastp searches of putative proteins translated from ORF1 and ORF2 (for both GcTV2-Gc6 and GcTV4-Gc6) against the nr protein sequences database returned several hits for CP and RdRp of totiviruses, respectively. In most totiviruses, ORF1 and ORF2 are expressed as a single fusion protein due to the existence of a -1 ribosomal frameshifting mechanism. A slippery site where frameshifting occurs is required near the stop codon of ORF1 and represented by the canonical sequence XXXYYYZ where X is A/G/C/U, Y is A/U and Z is A/C/U [
45]. A pseudoknot secondary structure preceding the slippery site is required to pause the ribosome and increase the efficiency of frameshifting [
46]. Putative slippery sites were found at the 3′ terminus of ORF1 in both viruses. This was represented by the nt stretches GGGUUUA (nt positions 1958-1964) and UUUUUUA (nt positions 1943-1949) for GcTV2-Gc6 and GcTV4-Gc6, respectively. The slippery site in GcTV2-Gc6 is identical to that of Saccharomyces cerevisiae virus L-A (ScV-L-A) [
47]. In GcTV2-Gc6, the slippery site and an H-type pseudoknot at nt positions 1969-2023 are separated by a 4 nt spacer (
Figure 1c). Similarly, the slippery site of GcTV4-Gc6 was followed by a 27 nt long spacer and an H-type pseudoknot at nt positions 1977-1997 (
Figure 1c). Although the presence of -1 frameshifting signature does not necessarily reflect its functionality [
48], the CP and RdRp genes of GcTV2-Gc6 and GcTV4-Gc6 could be expressed as fusion proteins of 1508 and 1496 aa, respectively.
The RNA molecules synthesized by totiviral RdRps are not capped at their 5′ termini which makes them unrecognizable by their host translation machinery [
49,
50]. Therefore, it is established that totiviruses such as ScV-L-A and ScV-L-BC can de-cap their host’s mRNAs and transfer their caps to the totiviral transcripts using a unique cap-snatching mechanism that requires a certain histidine aa residue in the capsid protein [
51,
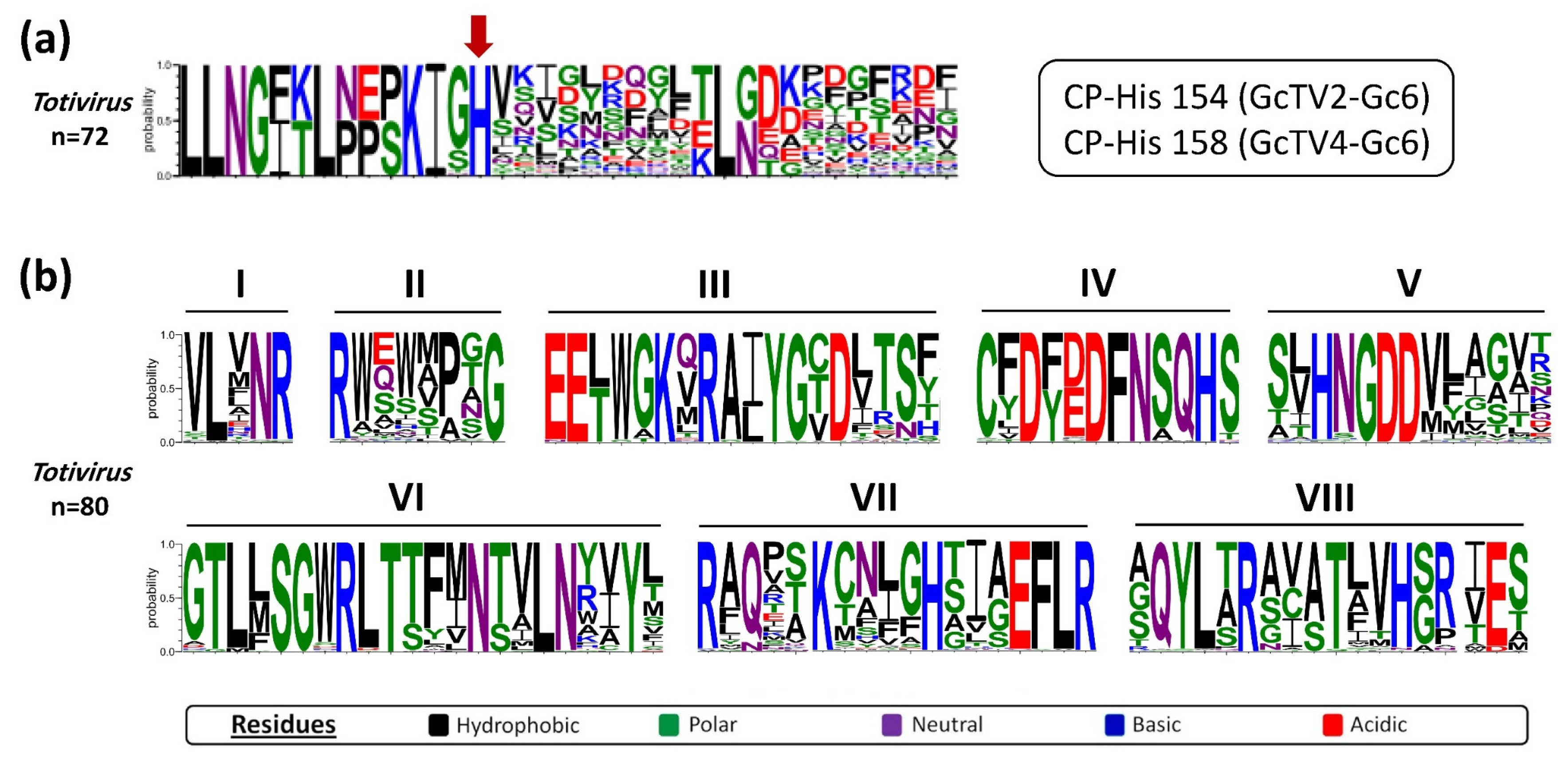
52]. Multiple aa sequence alignments of GcTV2-Gc6 and GcTV4-Gc6 CPs with other totiviral CP sequences revealed the presence of the conserved histidine aa at position 154 and 158 of the translated protein (
Figure 2a), respectively. This is analogous to histidine residues found at aa positions 154, 156 and 159 of CPs of ScV-L-A [
51], ScV-L-BC [
52], red clover powdery mildew-associated totiviruses (RPaTVs) [
53], and Trichoderma koningiopsis totivirus 1 (TkTV1) [
54], respectively. Moreover, aa sequence alignment between RdRps of GcTV2-Gc6, GcTV4-Gc6 and other totiviruses verified the presence of an RT-like superfamily domain (pfam02123) with eight conserved motifs (
Figure 2b) including the GDD motif which is highly conserved among RdRp sequences of dsRNA viruses [
55].
3.4. Phylogenetic analysis
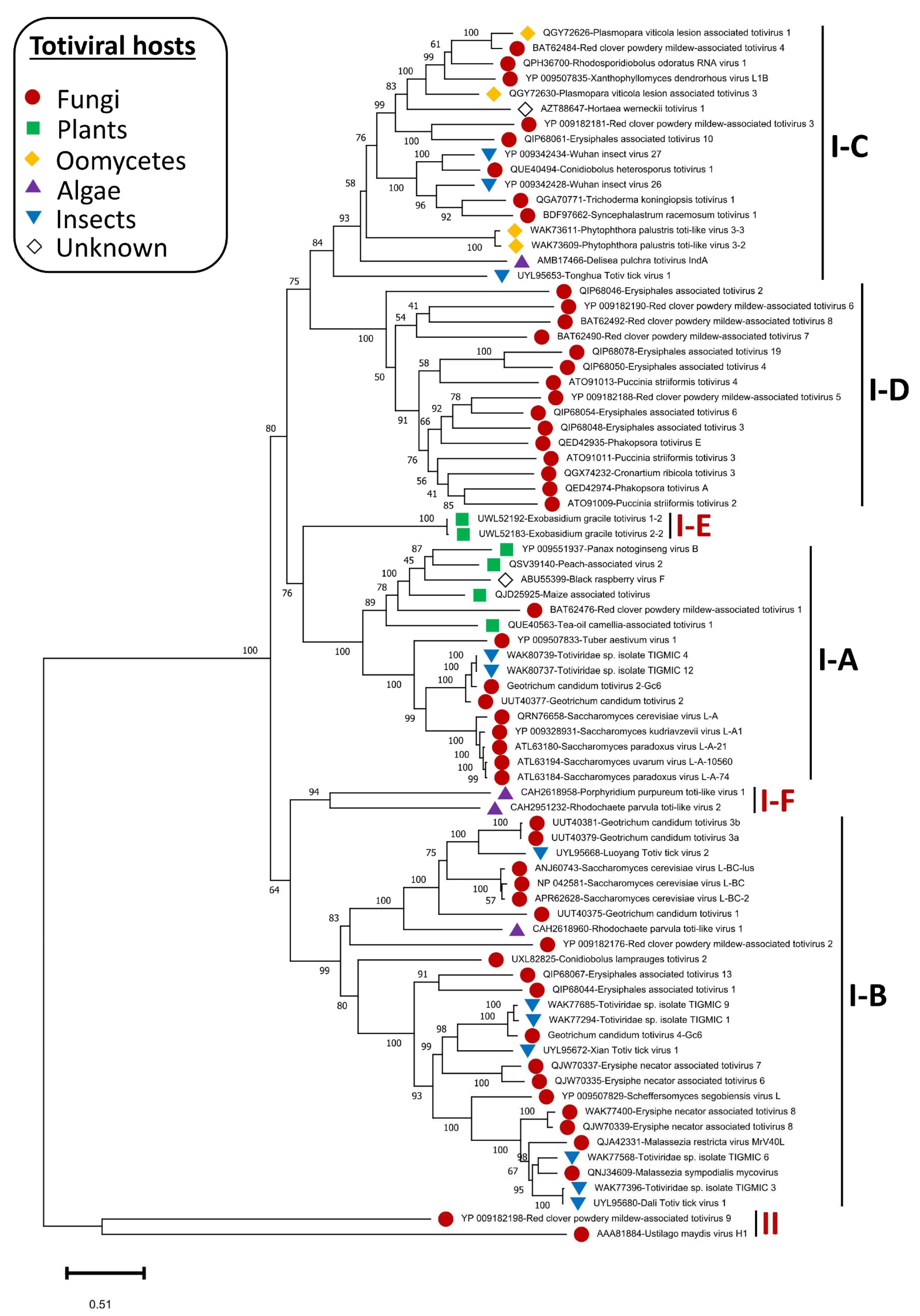
The phylogenetic structure of the genus
Totivirus was determined, and members were found distributed in two major groups (group I and II). Members in the first clade (group I) are further separated into four sister clades (I-A to I-D) [
53]. The current phylogenetic status of the
Totivirus genus has been re-determined in this study based on the increasing diversity of discovered totiviral genomes, partially due to the advent of HGS. The phylogenetic trees constructed based on multiple alignments of RdRp and CP genes (
Figure 3 and Supplementary materials,
Figure S1) revealed that two additional sister clades (I-E and I-F) were formed in group I. The appearance of these two sister clades is taxonomically sensible since subclade I-E accommodates plant totiviruses exclusively and the sister clade I-A comprises totiviruses from a range of hosts including plant totiviruses. Moreover, subclade I-F that hosts totiviral members from algae is more related to subclade I-B where a related algal totivirus is found. Fungal totiviruses are distributed among subclades I-A to I-D among which subclade I-D has no totiviruses from other hosts.
G. candidum totiviruses are distributed among subclades I-A and I-B [
22] which have totiviruses from multiple hosts including insects.
GcTV2-Gc6 was placed together with GcTV2-E1 [
22] in subclade I-A and is most closely related to yeast and insect totiviruses. GcTV4-Gc6 closest viruses were isolated from insects (accession numbers ON812606 and ON812795). The existence of related fungal and insect totiviruses has previously been reported reflecting the existence of common ancestors and possible horizontal virus transmission [
53,
54]. Previous reports suggested that unknown totiviruses from a wide range of different hosts may exist [
53] and this was supported by the presence of closely related fungal and insect totiviruses such as TkTV1 and Wuhan insect virus 26 (WIV26) and WIV27 [
54]. As shown in
Figure 3, this suggestion was confirmed, and it is believed that the diversity of totiviruses will further expand.
3.5. Are G. candidum totiviruses targets for the host’s RNA interference (RNAi)?
Viral infections are repelled by their host defense machinery through RNAi which is a sequence-specific post-transcriptional gene silencing induced by dsRNA [
56]. Viral dsRNA is common during the replication of all viral genome types. It is the replicative form during replication of ssRNA viruses or duplex regions in complementary stretches of their genomes as well as in the transcripts of DNA viruses. Since the nature of dsRNA viral genomes is double-stranded, it is likely that the viral genome itself has the ability to trigger RNAi [
56,
57,
58]. Therefore, the accumulation of
G. candidum totiviruses-derived short interfering RNAs (siRNAs) within isolate Gc6 implies that the host’s RNAi machinery is actively attempting to suppress viral replication and accumulation. A total small RNA (sRNA) fraction was purified from isolate Gc6, sequenced, and mapped against the genomes of GcTV2-Gc6 and GcTV4-Gc6. Populations of 94,045 and 111,336 short reads were mapped to GcTV2 and GcTV4 genomes from isolate Gc6, respectively. A minority of the assembled short reads perfectly matched the corresponding sequences in totiviral genomes. Mostly, the reads had a single mismatch to the genomes of either virus. Some reports indicate that mismatches in siRNA prevent gene silencing while others report that this can be tolerated [
59,
60]. The profile of siRNA mutations against three plant viruses was revealed and found to be due to viral or cellular RdRps [
61].
Diverse types of sRNAs have been identified in RNA-virus infected hosts such as siRNAs, unusually small RNAs (usRNAs), and Piwi-interacting RNAs (piRNAs) [
62,
63]. Virus-derived usRNAs range in length from 13 to 19 nt and were abundantly identified among sRNA populations of human and viral origin [
64,
65]. UsRNAs shorter than the canonical length of functional miRNAs are considered neglected intermediate degradation molecules. However, previous reports indicate that usRNAs derived from miRNA and tRNA could probably be functional in gene regulation [
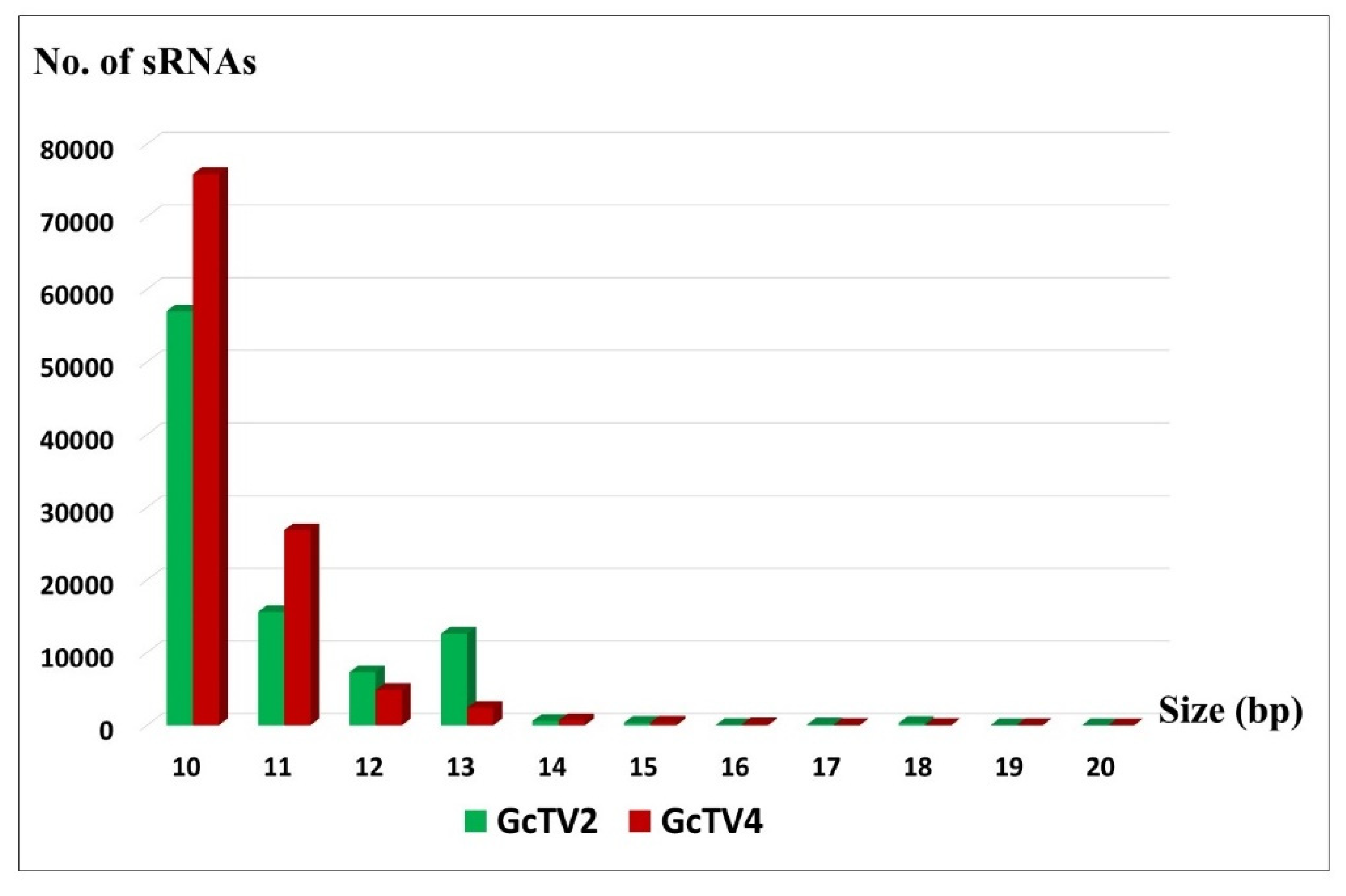
65]. Among detected totivirus-derived small RNAs from isolate Gc6, reads with common siRNA length (21-24 nt) were not abundant. The longest sRNA segment detected was 20 nt in length, mapped to GcTV2-Gc6, and represented by only 10 sequences. The rest of sequenced sRNAs were shorter, ranging from 10 to 19 nt for either virus (
Figure 4). Therefore, the sequenced totivirus-derived sRNAs from isolate Gc6 may represent populations of usRNAs, mostly with a single mismatch in their sequence.
Inclusively, the inability to detect siRNAs, abundance of usRNAs with mismatches and the successful accumulation of totiviral genomes as shown by dsRNA and electrophoresis suggests that the host-defense machinery is not fully able to suppress the replication of
G. candidum viruses and/or the totiviruses manage to counteract the RNAi mechanisms by expressing viral suppressors of RNAi (VSRs). Although VSRs expression has previously been reported for several mycoviruses [
58], our speculations regarding the ability of
G. candidum viruses to express VSRs and the functional viability of their usRNAs are yet to be confirmed in further studies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Primers used for the RT-PCR amplifications of terminal sequences of the two dsRNAs (GcTV2-Gc6 and GcTV4-Gc6). Each primer was used in combination with primer T4LC.; Figure S1: Maximum likelihood phylogenetic tree based on multiple alignments of capsid protein (CP) amino acid (aa) sequences of Geotrichum candidum totivirus 2 (GcTV2-Gc6), GcTV4-Gc6 and other members of the genus Totivirus. The phylogenetic tree was constructed using MEGA-X software and rtREV+G+F as the best evolutionary model with 1000 bootstrap replicates.
Author Contributions
Conceptualization, M.E.K. and R.M.M.; methodology, M.E.K. and R.M.M.; validation, M.E.K.; formal analysis, M.E.K.; investigation, M.E.K.; resources, R.M.M.; data curation, M.E.K. and R.M.M.; writing—original draft preparation, M.E.K.; writing—review and editing, R.M.M.; visualization, M.E.K.; supervision, R.M.M.; project administration, R.M.M.; funding acquisition, R.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: “This research received no external funding” or “This research was funded by NAME OF FUNDER, grant number XXX” and “The APC was funded by XXX”. Check carefully that the details given are accurate and use the standard spelling of funding agency names at
https://search.crossref.org/funding. Any errors may affect your future funding.
Data Availability Statement
GcTV2 and GcTV4 genome sequences are available in GenBank under the accession numbers OR250782 and OR250783, respectively.
Acknowledgments
The authors thank xxxxxx and xxxxxxxxx for their helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alper, I.; Frenette, M.; Labrie, S. Ribosomal DNA polymorphisms in the yeast Geotrichum candidum. Fungal Biol. 2011, 115, 1259–1269. [CrossRef]
- Kim, Y.-K.; Kim, T.-S.; Shim, H.-S.; Park, K.-S.; Yeh, W.-H.; Hong, S.-J.; Shim, C.-K.; Kim, J.-S.; Park, J.-H.; Han, E.-J.; et al. First Report of Sour Rot on Post-harvest Oriental Melon, Tomato, Cucumber, Potato, Pumpkin and Carrot Caused by Geotrichum candidum. Res. Plant Dis. 2011, 17, 232–234. [CrossRef]
- Bonifaz, A.; Vázquez-González, D.; Macías, B.; Paredes-Farrera, F.; Hernández, M.A.; Araiza, J.; Ponce, R.M. Oral geotrichosis: report of 12 cases. J. Oral Sci. 2010, 52, 477–483. [CrossRef]
- Vasei, M.; Imanieh, M.H. Duodenal colonization by Geotrichum candidum in a child with transient low serum levels of IgA and IgM. APMIS 1999, 107, 681–684. [CrossRef]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [CrossRef]
- Xu, Z.; Khalifa, M.E.; Frampton, R.A.; Smith, G.R.; McDougal, R.L.; MacDiarmid, R.M.; Kalamorz, F. Characterization of a Novel Double-Stranded RNA Virus from Phytophthora pluvialis in New Zealand. Viruses 2022, 14, 247. [CrossRef]
- Khalifa, M.E.; MacDiarmid, R.M. A Mechanically Transmitted DNA Mycovirus Is Targeted by the Defence Machinery of Its Host, Botrytis cinerea. Viruses 2021, 13, 1315. [CrossRef]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal–plant interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [CrossRef]
- Tipper, D.J.; Schmitt, M.J. Yeast dsRNA viruses: replication and killer phenotypes. Mol. Microbiol. 1991, 5, 2331–2338. [CrossRef]
- Márquez, L.M.; Redman, R.S.; Rodriguez, R.J.; Roossinck, M.J. A Virus in a Fungus in a Plant: Three-Way Symbiosis Required for Thermal Tolerance. Science (80-. ). 2007, 315, 513–515. [CrossRef]
- Li, P.; Wang, S.; Zhang, L.; Qiu, D.; Zhou, X.; Guo, L. A tripartite ssDNA mycovirus from a plant pathogenic fungus is infectious as cloned DNA and purified virions. Sci. Adv. 2020, 6. [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Natl. Acad. Sci. 2010, 107, 8387–8392. [CrossRef]
- Kondo, H.; Botella, L.; Suzuki, N. Mycovirus Diversity and Evolution Revealed/Inferred from Recent Studies. Annu. Rev. Phytopathol. 2022, 60, 307–336. [CrossRef]
- Wickner, R.B.; Ghabrial, S.A.; Nibert, M.L.; Patterson, J.L.; Wang, C.C. Family Totiviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowits, E.J., Eds.; Elsevier Academic Press: London, 2012; pp. 639–650.
- Alvarez-Quinto, R.A.; Espinoza-Lozano, R.F.; Mora-Pinargote, C.A.; Quito-Avila, D.F. Complete genome sequence of a variant of maize-associated totivirus from Ecuador. Arch. Virol. 2017, 162, 1083–1087. [CrossRef]
- Colmant, A.M.G.; Etebari, K.; Webb, C.E.; Ritchie, S.A.; Jansen, C.C.; van den Hurk, A.F.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Asgari, S.; Hall, R.A. Discovery of new orbiviruses and totivirus from Anopheles mosquitoes in Eastern Australia. Arch. Virol. 2017, 162, 3529–3534. [CrossRef]
- Huang, Y.; Guo, X.; Zhang, S.; Zhao, Q.; Sun, Q.; Zhou, H.; Zhang, J.; Tong, Y. Discovery of two novel totiviruses from Culex tritaeniorhynchus classifiable in a distinct clade with arthropod-infecting viruses within the family Totiviridae. Arch. Virol. 2018, 163, 2899–2902. [CrossRef]
- Mor, S.K.; Phelps, N.B.D. Molecular detection of a novel totivirus from golden shiner (Notemigonus crysoleucas) baitfish in the USA. Arch. Virol. 2016, 161, 2227–2234. [CrossRef]
- Zhao, M.; Xu, L.; Bowers, H.; Schott, E.J. Characterization of Two Novel Toti-Like Viruses Co-infecting the Atlantic Blue Crab, Callinectes sapidus, in Its Northern Range of the United States. Front. Microbiol. 2022, 13. [CrossRef]
- Mor, H.; Steinlauf, R.; Barash, I. Viruslike Particles and Double-Stranded RNA in Geotrichum candidum, the Causal Agent of Citrus Sour Rot. Phytopathology 1984, 74, 921. [CrossRef]
- Matte, O.; Chabalier, C.; Ratomahenina, R.; Bossy, J.P.; Galzy, P. Isolation of a double-stranded RNA and a virus-like particle from Geotrichum candidum. J. Basic Microbiol. 1991, 31, 447–452. [CrossRef]
- Khan, H.A.; Kondo, H.; Shahi, S.; Bhatti, M.F.; Suzuki, N. Identification of novel totiviruses from the ascomycetous fungus Geotrichum candidum. Arch. Virol. 2022, 167, 2833–2838. [CrossRef]
- Warcup, J.H. The Soil-Plate Method for Isolation of Fungi from Soil. Nature 1950, 166, 117–118. [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Elsevier, 1990; pp. 315–322.
- Valverde, R.A.; Nameth, S.T.; Jordan, R.L. Analysis of double-stranded RNA for plant virus diagnosis. Plant Dis. 1990, 74, 255–258. [CrossRef]
- Howitt, R.L.J.; Beever, R.E.; Pearson, M.N.; Forster, R.L.S. Presence of double-stranded RNA and virus-like particles in Botrytis cinerea. Mycol. Res. 1995, 99, 1472–1478. [CrossRef]
- Khalifa, M.E.; Pearson, M.N. Molecular characterization of three mitoviruses co-infecting a hypovirulent isolate of Sclerotinia sclerotiorum fungus. Virology 2013, 441, 22–30. [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [CrossRef]
- Dolja, V. V.; Koonin, E. V. Metagenomics reshapes the concepts of RNA virus evolution by revealing extensive horizontal virus transfer. Virus Res. 2018, 244, 36–52. [CrossRef]
- Wan, X.; Zhao, Y.; Zhang, Y.; Wei, C.; Du, H.; Zhang, H.; Chen, J.; Yang, L.; Zang, R.; Wen, C. Molecular characterization of a novel partitivirus isolated from the phytopathogenic fungus Aplosporella javeedii. Arch. Virol. 2021, 166, 1237–1240. [CrossRef]
- Sutela, S.; Poimala, A.; Vainio, E.J. Viruses of fungi and oomycetes in the soil environment. FEMS Microbiol. Ecol. 2019, 95. [CrossRef]
- Bevan, E.A.; Herring, A.J.; Mitchell, D.J. Preliminary Characterization of Two Species of dsRNA in Yeast and their Relationship to the “Killer” Character. Nature 1973, 245, 81–86. [CrossRef]
- Matte, O.; Chabalier, C.; Ratomahenina, R.; Bossy, J.P.; Galzy, P. Isolation of a double-stranded RNA and a virus-like particle fromGeotrichum candidum. J. Basic Microbiol. 1991, 31, 447–452. [CrossRef]
- Baeza, M.; Bravo, N.; Sanhueza, M.; Flores, O.; Villarreal, P.; Cifuentes, V. Molecular characterization of totiviruses in Xanthophyllomyces dendrorhous. Virol. J. 2012, 9, 140. [CrossRef]
- Herrero, N.; Zabalgogeazcoa, I. Mycoviruses infecting the endophytic and entomopathogenic fungus Tolypocladium cylindrosporum. Virus Res. 2011, 160, 409–413. [CrossRef]
- Khalifa, M.E.; Pearson, M.N. Molecular characterisation of an endornavirus infecting the phytopathogen Sclerotinia sclerotiorum. Virus Res. 2014, 189, 303–309. [CrossRef]
- Khalifa, M.E.; Pearson, M.N. Molecular characterisation of novel mitoviruses associated with Sclerotinia sclerotiorum. Arch. Virol. 2014, 159, 3157–3160. [CrossRef]
- Kim, J.W.; Choi, E.Y.; Lee, J.I. Genome Organization and Expression of the Penicillium stoloniferum Virus F. Virus Genes 2005, 31, 175–183. [CrossRef]
- Osaki, H.; Nomura, K.; Matsumoto, N.; Ohtsu, Y. Characterization of double-stranded RNA elements in the violet root rot fungus Helicobasidium mompa. Mycol. Res. 2004, 108, 635–640. [CrossRef]
- Park, Y.; James, D.; Punja, Z.K. Co-infection by two distinct totivirus-like double-stranded RNA elements in Chalara elegans (Thielaviopsis basicola). Virus Res. 2005, 109, 71–85. [CrossRef]
- Preisig, O.; Wingfield, B.D.; Wingfield, M.J. Coinfection of a Fungal Pathogen by Two Distinct Double-Stranded RNA Viruses. Virology 1998, 252, 399–406. [CrossRef]
- Icho, T.; Wickner, R.B. The Double-stranded RNA Genome of Yeast Virus L-A Encodes Its Own Putative RNA Polymerase by Fusing Two Open Reading Frames. J. Biol. Chem. 1989, 264, 6716–6723. [CrossRef]
- Bekaert, M.; Rousset, J.-P. An Extended Signal Involved in Eukaryotic −1 Frameshifting Operates through Modification of the E Site tRNA. Mol. Cell 2005, 17, 61–68. [CrossRef]
- Rice, N.R.; Stephens, R.M.; Burny, A.; Gilden, R. V. The gag and pol genes of bovine leukemia virus: Nucleotide sequence and analysis. Virology 1985, 142, 357–377. [CrossRef]
- Brierley, I. Ribosomal frameshifting on viral RNAs. J. Gen. Virol. 1995, 76, 1885–1892. [CrossRef]
- Jamal, A.; Sato, Y.; Shahi, S.; Shamsi, W.; Kondo, H.; Suzuki, N. Novel Victorivirus from a Pakistani Isolate of Alternaria alternata Lacking a Typical Translational Stop/Restart Sequence Signature. Viruses 2019, 11, 577. [CrossRef]
- Fujimura, T.; Esteban, R. Yeast Double-stranded RNA Virus L-A Deliberately Synthesizes RNA Transcripts with 5′-Diphosphate. J. Biol. Chem. 2010, 285, 22911–22918. [CrossRef]
- Rowley, P.A.; Ho, B.; Bushong, S.; Johnson, A.; Sawyer, S.L. XRN1 Is a Species-Specific Virus Restriction Factor in Yeasts. PLOS Pathog. 2016, 12, e1005890. [CrossRef]
- Fujimura, T.; Esteban, R. Cap-snatching mechanism in yeast L-A double-stranded RNA virus. Proc. Natl. Acad. Sci. 2011, 108, 17667–17671. [CrossRef]
- Fujimura, T.; Esteban, R. Cap Snatching in Yeast L-BC Double-stranded RNA Totivirus. J. Biol. Chem. 2013, 288, 23716–23724. [CrossRef]
- Kondo, H.; Hisano, S.; Chiba, S.; Maruyama, K.; Andika, I.B.; Toyoda, K.; Fujimori, F.; Suzuki, N. Sequence and phylogenetic analyses of novel totivirus-like double-stranded RNAs from field-collected powdery mildew fungi. Virus Res. 2016, 213, 353–364. [CrossRef]
- Khalifa, M.E.; MacDiarmid, R.M. A Novel Totivirus Naturally Occurring in Two Different Fungal Genera. Front. Microbiol. 2019, 10. [CrossRef]
- Routhier, E.; Bruenn, J.A. Functions of Conserved Motifs in the RNA-Dependent RNA Polymerase of a Yeast Double-Stranded RNA Virus. J. Virol. 1998, 72, 4427–4429. [CrossRef]
- Gammon, D.B.; Mello, C.C. RNA interference-mediated antiviral defense in insects. Curr. Opin. Insect Sci. 2015, 8, 111–120. [CrossRef]
- Mongelli, V.; Saleh, M.-C. Bugs Are Not to Be Silenced: Small RNA Pathways and Antiviral Responses in Insects. Annu. Rev. Virol. 2016, 3, 573–589. [CrossRef]
- Rodriguez Coy, L.; Plummer, K.M.; Khalifa, M.E.; MacDiarmid, R.M. Mycovirus-encoded suppressors of RNA silencing: Possible allies or enemies in the use of RNAi to control fungal disease in crops. Front. Fungal Biol. 2022, 3. [CrossRef]
- Zeng, Y.; Cullen, B.R. Sequence requirements for micro RNA processing and function in human cells. RNA 2003, 9, 112–123. [CrossRef]
- Elbashir, S.M.; Martinez, J.; Patkaniowska, A.; Lendeckel, W.; Tuschl, T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001, 20, 6877–6888. [CrossRef]
- Nigam, D.; LaTourrette, K.; Garcia-Ruiz, H. Mutations in virus-derived small RNAs. Sci. Rep. 2020, 10, 9540. [CrossRef]
- Usme-Ciro, J.A.; Campillo-Pedroza, N.; Almazán, F.; Gallego-Gomez, J.C. Cytoplasmic RNA viruses as potential vehicles for the delivery of therapeutic small RNAs. Virol. J. 2013, 10, 185. [CrossRef]
- Kolliopoulou, A.; Santos, D.; Taning, C.N.T.; Wynant, N.; Vanden Broeck, J.; Smagghe, G.; Swevers, L. PIWI pathway against viruses in insects. WIREs RNA 2019, 10. [CrossRef]
- Hess, A.M.; Prasad, A.N.; Ptitsyn, A.; Ebel, G.D.; Olson, K.E.; Barbacioru, C.; Monighetti, C.; Campbell, C.L. Small RNA profiling of Dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC Microbiol. 2011, 11, 45. [CrossRef]
- Li, Z.; Kim, S.W.; Lin, Y.; Moore, P.S.; Chang, Y.; John, B. Characterization of Viral and Human RNAs Smaller than Canonical MicroRNAs. J. Virol. 2009, 83, 12751–12758. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).