1. Introduction
Postural control is a cornerstone of our daily life activities, forming the fundamental basis for a wide range of tasks [
1]. Our ability to maintain postural stability is not only essential for preventing falls but also for facilitating efficient and coordinated movements [
2]. This complex process relies on several mechanisms, with a significant emphasis on the strength of the ankle's mobilizing muscles and the coordination of neuromuscular activation [
1,
3,
4]. The muscles surrounding the ankle joint function as stabilizers, furnishing the requisite force to uphold balance and counter external disturbances effectively [
5]. Concurrently, the neuromuscular system precisely governs the activation and synchronization of these muscles, adapting seamlessly to alterations in terrain or the demands of various movements.
Alterations associated with sarcopenia can have a profound impact on the functioning of the neuromuscular system [
6,
7,
8,
9], potentially resulting in adverse effects on postural control [
10,
11,
12,
13]. This decline in neuromuscular function plays a pivotal role in predisposing older individuals to a heightened risk of falls [
10,
14,
15,
16,
17,
18]. Extensive research has thoroughly explored the influence of aging on postural control, consistently revealing a prevalent trend of increased Center of Pressure (CoP) displacement as individuals advance in age [
14,
15]. To counterbalance the diminishing efficiency of the musculotendinous system that naturally occurs with aging, there is a corresponding surge in the activity of postural muscles, particularly those encompassing the ankle joint during upright stance [
1,
16,
17,
18,
19]. This heightened muscle engagement aims to bolster overall ankle stiffness through co-contraction, primarily involving the plantar and dorsal flexor muscles [
20,
21,
22,
23]. While this strategy proves beneficial in responding to transient disturbances, it also comes at the cost of escalated energy expenditure and premature fatigue, potentially jeopardizing postural stability and elevating the risk of falls [
24,
25,
26].
It is well established that obesity is associated with postural control alterations [
2,
3,
5,
6] due to the increased body mass of each body segments [
27,
28,
29]. Postural control alterations in obese individuals could be explained by the anterior position of the center of pressure (CoP) from the axis of rotation (i.e., ankle joint) related to the accumulation of fat body mass in the abdominal area [
30]. Consequently, obese individuals have to generate higher force at the ankle joint to counteract the forward position of CoP in order to avoid falls [
10,
11,
31]. Other explanations of the altered postural control in obese individuals were related to altered proprioception process and increased ankle muscle activities. In fact, obesity is associated with low sensitivity of the plantar mechanoreceptors due to the high-pressure supporting body mass [
32]. In this context, Wa & Madigan et al., [
33] reported that altered postural control parameters in obese individuals were related to decreased sensitivity of plantar mechanoreceptors. Altered postural control may also be related to increased ankle muscle activities [
3]. Thus, higher muscle activities could induce earlier fatigue development in muscle extremities [
34] since obesity is associated with earlier fatigue and delayed muscle recovery [
35].
The combination of obesity and aging could dramatically reduce postural control abilities and capacities to perform daily living activities [
21,
22,
36]. Several authors confirm that an increase in body mass, particularly when combined with age, induces altered postural control parameters (e.g., speed and area of CoP during static postural control testing) due to the addition of mechanisms of alterations related to age and obesity. In this context, Maktouf et al., [
3] demonstrated that altered postural control in obese older adults could be related to an increase in plantar flexors activities and decreased force production capacities relative to their body mass.
To date, only a limited number of studies have explored the effects of combined physical activity program on neuromuscular strategies at the ankle joint during static and proactive postural control specifically to older adults with sarcopenic obesity (SO). The implementation of the Total Mobility Plus Program (TMP), designed to encompass a diverse spectrum of exercises targeting strength, power, endurance, and mobility, is poised to address these intricacies comprehensively. One notable study by Maktouf et al. [
19] focused on the impact of their intervention on balance parameters. However, their research primarily concentrated on balance-related outcomes and lacked a comprehensive evaluation of the crucial neuromuscular elements essential for improving balance, such as muscle activation. Recognizing these gaps in the existing literature, it becomes increasingly apparent that a comprehensive investigation encompassing postural control regulation, coupled with a detailed exploration of the potential underlying neuromuscular factors driving improvements, is imperative.
This study protocol seeks to comprehensively assess the efficacy of the TMP program on postural control parameters and neuromuscular capacity of ankle muscles in older adults with SO. Furthermore, this investigation aims to explore the intricate relationship between the enhancements in postural parameters and the concurrent modifications in neuromuscular strategies.
2. Materials and Methods
2.1. Experimental design
This study employed a single-blinded, prospective, controlled, randomized multicenter design, wherein participants were randomly assigned to either the Intervention Group (IG) or the Control Group (CG). The control group underwent pre- and post-evaluation tests without any intervention, while the intervention group engaged in a rigorous 4-month program with tri-weekly sessions. The recruitment phase took place over 3 weeks, including participant recruitment (1 week), screening (1 week), and pre- and post-intervention experimental testing (3 weeks). These assessments encompassed anthropometric measurements, clinical health evaluations, balance tests with electromyography assessment of ankle muscle activation, and maximal voluntary contraction tests of plantar and dorsal flexor muscles.
2.2. Recruitment and randomization
Participants for this study were recruited from four distinguished Tunisian centers, each specializing in diverse aspects of obesity, between March 1st and October 31st, 2022, through a combination of direct clinic recruitment and leaflet distribution. Randomization occurred on the day of inclusion and was overseen by the chief investigator at each center. Each participant was randomly assigned to one of the two groups: the CG or the IG. The specifics of the TMP program were not disclosed to the participants. Instead, a blinded assessor visited participants twice before and after the program, while an unblinded kinesiologist conducted separate visits during the program to provide treatment and exercise sessions. The randomization list had been generated by an independent statistician from the Clinical Research Unit of our laboratory, with no direct involvement in the study. This list was computer-generated and subsequently uploaded into an online case report form. Each study participant received a unique allocation study number in a sequential format (TMP00X). Blinding was maintained until the database was finalized. All necessary data stipulated by the study protocol were diligently entered into the Electronic Data Capture system in real-time as they were acquired to ensure contemporaneous record-keeping. A Clinical Research Associate oversaw the meticulous handling of the study, including the collection, documentation, recording, and reporting of all handwritten data in strict adherence to the principles of Good Clinical Practice. To maintain data integrity and accuracy, a computerized quality control system was implemented, which included mechanisms for detecting missing data and verifying data consistency.
2.3. Intervention program
The TPM program is reported using Template for Intervention Description and Replication Guidelines (TIDieR) and based on the methodology presented in the study of Ferhi et al., [
39] as demonstrated in
Table 1.
Table 1.
Description of the TMP intervention
Table 1.
Description of the TMP intervention
| Intervention Description and Replication Guidelines (TIDieR) |
|---|
| Name |
The TMP program. |
| Why |
To improve static postural control in older adults with SO. |
| What (materials) |
Diverse range of physical materials: chairs, balls, markers, slats, cups, hoops, elastic bands, and weighted bags, foam rollers, balance boards, resistance tubes and bands, exercise mats, medicine balls, step platforms, cones, kettlebells, and step platforms. |
| What (procedures) |
The TMP program spanned 16 weeks, with three 60-minute sessions per week, totaling 48 sessions throughout the intervention period. Each session followed a structured format, starting with a 10-minute warm-up. The core of the session included motor skill exercises and strengthening/posture exercises, with the duration determined by the prescribed training volume. Finally, each session concluded with a 5-minute cooldown phase. |
| Who |
By a kinesiologist specialized in adapted physical activity. |
| How |
In collective, face-to-face sessions. |
| Where |
In the fitness hall of each respective structure. |
| When and how much |
The TMP program commenced and was completed over a 4-month duration. Participants attended three sessions per week, accumulating a total of 48 sessions throughout the intervention period. Each session had a duration of 60 minutes. The design of exercise types within the program was tailored to the training load of each session and was based on predetermined training intensity and volume for individual sessions. Each exercise type regimen consisted of 1 to 5 sets, with repetitions ranging from a minimum of 3 to a maximum of 15 per set. |
| Tailoring |
Adjustments in training load were determined following each session using the Rating of Perceived Exertion (RPE) scale. The RPE scale ranged from 0 (no difficulty) to 10 (extremely difficult), and the group's training load was calculated by multiplying the RPE score of the session by its duration. For instance, if a group had an average RPE score of 6 in a 60-minute session, the training load totaled 360 arbitrary units (a.u.). This approach ensured the effectiveness of the training, particularly concerning the progressive challenge it presented, and facilitated the monitoring of potential overtraining syndrome. When the training load exceeded 300 a.u. (equivalent to 5 × 60 minutes), it was maintained for the subsequent session. If the training load fell below 300, the number of sets and repetitions was increased by 25% in the following session. |
| Modifications |
Modifications were implemented during each session, including an escalation in the number of sets or repetitions according to the training intensity. Furthermore, the level of difficulty was systematically elevated by incorporating obstacles, imposing time limits for task completion, and employing various methods to secure a gradual progression while intensifying the challenge. |
| How well (planned) |
The TMP program consisted of three micro-cycles, each encompassing 16 sessions. During the first micro-cycle, the emphasis was on volume, while the second micro-cycle focused on intensity. The third micro-cycle aimed to achieve a balance between volume and intensity. |
| How well (actual) |
All participants completed the TMP program |
2.5. Outcomes measures
2.5.1. Steady-state and proactive postural control
The evaluation of static steady-state postural control was performed utilizing the Romberg Test (ROM). Participants were directed to maintain an upright position for 30 seconds without wearing shoes. They were instructed to keep their feet close together and extend their arms fully in front of their bodies, with palms facing upwards, while keeping their eyes closed. If participants opened their eyes, made arm or foot movements to regain stability, or needed assistance from the operator, the test was terminated. Each participant completed three trials, with a one-minute rest period between each trial, and the best-recorded result was noted as the standing time in seconds.
To evaluate proactive postural control, the Timed Up and Go Test (TUG) was deployed. Participants were instructed to sit on a chair with a height of 46 cm and position their arms on the armrests. They were then asked to rise from the chair, walk 3 meters at their usual walking pace, turn around, and return to a seated position. Two test trials were conducted, and the best time achieved in seconds was recorded as the outcome measure.
2.5.2. Anthropometric measurement
Participants' height (H) was accurately measured using a digital floor scale. Body mass (BM) and fat body fat (%) were assessed using an impedance-meter (Tanita; SC 240-Class III; Tanita Europe B.V., Amsterdam, The Netherlands). Body mass index (BMI, kg/m²), Fat body mass (FBM, kg) and lean body mass (LBM, kg) were determined through the following formulas:
2.5.3. Maximal voluntary contraction testing measurement
Maximal voluntary contractions (MVC) were collected using a dynamometer (Sauter FL1K; Type: Force Gauge; Sauter GmbH, Balingen, Germany) during isometric contractions of ankle plantar flexors (PF) and dorsal flexors (DF) muscles following instructions explained in the study of Maktouf et al. 2018. For each condition, three trials were performed with a 1 min rest in-between. For both MVC of PF and DF, the mean of the maximal value of the three trials were taken. Relative force (MVC/BM) was calculated.
2.5.4. Postural control evaluation
Postural control during quiet standing was assessed using a force platform (Zebris; FDM S; sampling rate: 100 Hz; Zebris Medical GmbH, Isny, Germany). Participants were instructed to stand barefoot on the platform with their feet together and arms alongside their body. They performed postural trials under three different conditions: (1) Eyes Opened (EO): Participants stood with their eyes open. (2) Eyes Closed (EC): Participants stood with their eyes closed. (3) Tandem Condition (TC): Participants stood in a tandem stance, where one foot was placed directly in front of the other. Each trial lasted for 30 seconds and was followed by a 30-second rest period. Displacements of the Center of Pressure (CoP) were recorded. Two postural control parameters were extracted from the CoP data: Mean Sway Area: This parameter represents the area of the 95% confidence ellipse that encapsulates the sway of the CoP. It is measured in square centimeters (cm²). Mean Velocity of CoP Displacements: This parameter indicates the average velocity at which the CoP moved during the trial. It is measured in millimeters per second (mm/s). These measurements provide insights into how participants' postural control varies under different sensory conditions (EO, EC, and TC). The addition of TC allows for an evaluation of postural stability while standing in a more challenging stance.
2.5.5. Electromyography evaluation
EMG data from the ankle joint muscles are captured during MVC of PF and dorsal DF, during a treadmill walking test and balance tests. The collection process uses the Trigno® Wireless Biofeedback System (Delsys Inc., Natick, MA), with the EMG record-ing synchronized with the platefrom data during postural control trials via a control device that operates based on a synchronization switch system (ON/OFF). The sensors, consisting of two pairs of silver bar contacts with 10 mm interelectrode spacing, are positioned on the gastrocnemius medialis (GM), soleus (SOL), and tibialis anterior (TA) of the dominant leg, in accord-ance with the recommendations of SENIAM. The raw EMG signals are then post-processed using Matlab software (Matlab R2013a, MathWorks, Natick, USA). Data are collected over a 10-second period, beginning at the 10th second of each trial (postural control test). The data are band-pass filtered at 15–500 Hz via a second-order Butterworth digital filter to remove any noise or movement interference. Subsequently, the data are rectified and smoothed through root mean square analysis (RMS) with a 20-ms window [
3]. For the MVC tests, a moving window with a width of 20 ms is employed to identify the peak RMS EMG activity resulting from the three MVC efforts for each type of contraction. Subsequently, all RMS EMG data collected during postural control tests was normalized to peak RMS EMG [
3]. The normalized RMS of the GM (RMS GM), SOL (RMS SOL), and TA (EMG TA) from each postural control test were used on this study.
2.6. Statistical analysis
2.6.1. Sample size
All The sample size is calculated using the freeware G*Power (version 3.1.9.4). The ANOVA test was predefined for power analysis. The estimation was based on predefined control of type I error (alpha = 0.05) and Type II error (beta = 0.60), with a moderate level of estimated effect size (r = 0.35). Under these settings, 40 participants were required as the minimum sample size.
2.6.2. Statistical procedures
Statistical analyses were conducted using Statistica Software 13.0 (Software, Inc., Tul-sa, OK). The initial step involved checking the normality of data distribution through Kol-mogorov-Smirnov tests. For data that exhibited a normal distribution, paired t-tests were employed to compare results within the same group before and after the implementation of the TMP program. Additionally, independent samples t-tests were used to make compari-sons between the IG and CG before and after the TMP program. Furthermore, we examined the relationships between changes in postural parameters, neuromuscular parameters of the DF and PF, and muscle activity of the GM, SOL, and TA using Pearson's correlation analysis. All data were presented as means and standard deviations, with the significance threshold set at p < 0.05 for all results.
3. Results
3.1. Participants
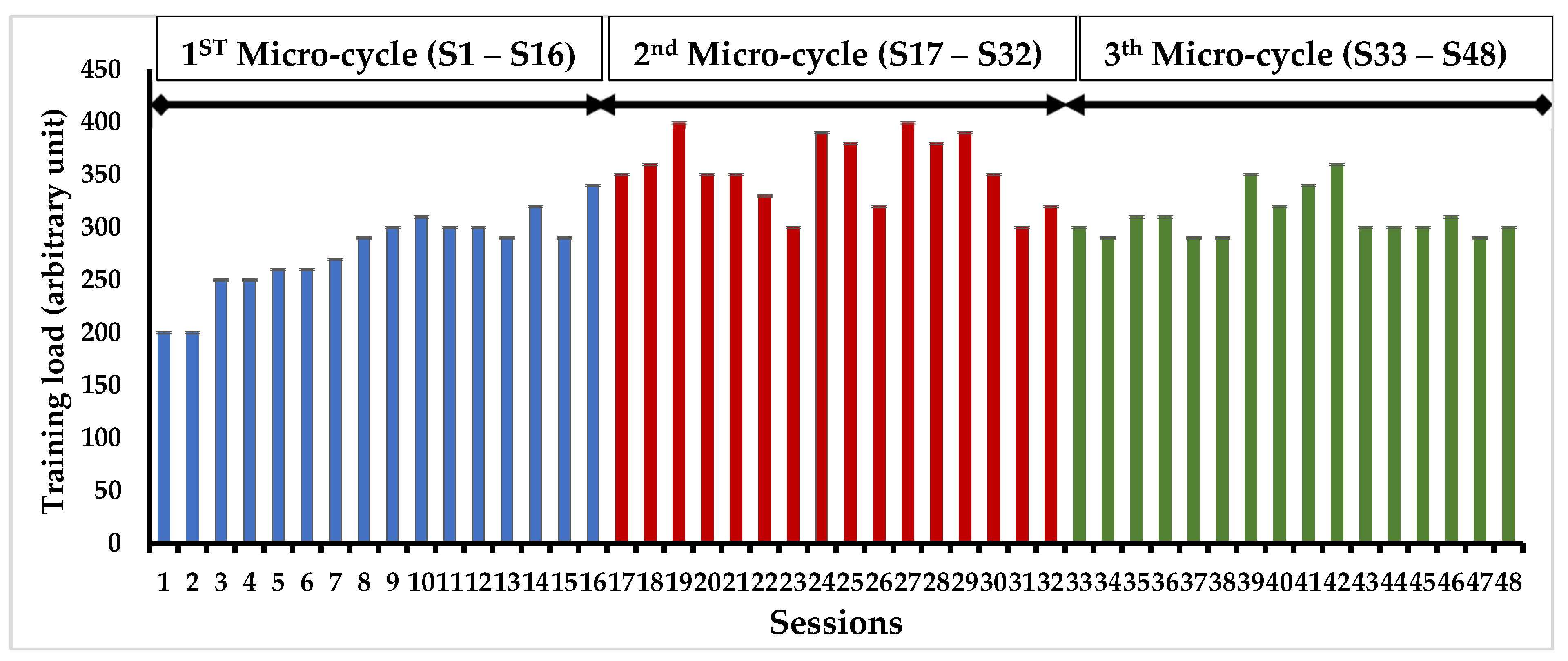
In our study, we initially recruited 80 volunteers, only 65 participants met the eligibility criteria we had set. Among those initially included, 15 individuals did not complete the study as they did not adhere to the study protocol, with 7 from CG and 8 from the IG. Various factors contributed to participant dropout, including the unfortunate passing of 15 participants, 5 individuals relocating to other centers, 6 participants experiencing health-related hospitalizations such as stroke, hip fracture, and ankle sprain, and 4 participants discontinuing their involvement for personal reasons. Eventually, a cohort of 40 participants who successfully completed the entire study were randomly divided into two groups (Table 1): the CG (n=25, mean age = 75.9 ± 5.4 years; mean BMI = 32.9 ± 2.3 kg/m²) and the IG (n=25, mean age = 76.1 ± 3.5 years; mean BMI = 34.4 ± 4.0 kg/m²).The progression of training load during the PMT program was measured in all participants (Figure 1). Analysis of the Ricci and Gagnon questionnaire revealed that both the CG (11.2 ± 2.5) and IG (10.7 ± 3.4) were inactive before the PMT program.
Figure 1.
Longitudinal progression of training load in the intervention group over a four-month timeframe.
Figure 1.
Longitudinal progression of training load in the intervention group over a four-month timeframe.
3.2. Anthropometric parameters
Table 1 presents results of the ROM, TUG and anthropometric parameters at the baseline and after TMP intervention. At the baseline assessment, there were no significant differences in anthropometric, ROM and TUG between the CG and IG. However, after completing the TMP program, IG demonstrated significant decreases of time of standing up (ROM, -34.6%, p<0.05) and time of TUG (-15.6, p<0.05). Moreover, the TG demonstrated a significant increase in LBM (+8.5%, p < 0.05), a decrease in FBM (-17.7%, p < 0.05).
Table 1.
Differential Analysis of balance and anthropometric characteristics between the control and intervention groups at the baseline and after the TMP program.
Table 1.
Differential Analysis of balance and anthropometric characteristics between the control and intervention groups at the baseline and after the TMP program.
| |
CG |
IG |
|
CG |
IG |
|
| |
At Baseline |
p |
After the Intervention |
p |
| Characteristics |
Anthropometric parameters |
| Age (years) |
75.9 ± 5.4 |
76.3 ± 3.5 |
NS |
76.3 ± 5.4 |
76.7 ± 3.5 |
NS |
| Body height (cm) |
163.2 ± 4.2 |
165.7 ± 4.9 |
NS |
163.2 ± 4.2 |
165.7 ± 4.9 |
NS |
| Body mass (kg) |
92.1 ± 6.4 |
94.2 ± 5.1 |
NS |
90.9 ± 5.4 |
92.6 ± 6.4*+ |
NS |
| BMI (kg/m²) |
34.7 ± 2.3 |
34.5 ± 4.0 |
NS |
33.4 ± 2.4 |
31.9 ± 1.4 |
NS |
| Body fat (%) |
40.0 ± 4.3 |
39.0 ± 4.5 |
NS |
40.4 ± 7.1 |
32.6 ± 4.5*+ |
<.01 |
| FBM (kg) |
31.9 ± 3.6 |
36.7 ± 5.5 |
NS |
36.8 ± 3.6 |
30.2 ± 5.5*+ |
<.05 |
| LBM (kg) |
60.1 ± 4.2 |
57.5 ± 6.4 |
NS |
54.2 ± 4.2 |
62.4 ± 3.4*+ |
<.05 |
| Waist circumference (cm) |
94.8 ± 4.9 |
89.3 ± 4.8 |
NS |
94.2 ± 4.1 |
85.4 ± 4.9 |
NS |
| Hip circumference (cm) |
92.0 ± 6.5 |
98.9 ± 4.6 |
NS |
92.4 ± 6.1 |
96.5 ± 6.0 |
NS |
| Hand grip (N) |
13.7 ± 3.2 |
13.0 ± 2.5 |
NS |
13.3 ± 3.0 |
16.3 ± 3.3*+ |
<.05 |
| Maximal gait speed (m/s) |
0.8 ± 0.2 |
0.7 ± 0.3 |
|
0.8 ± 0.2 |
1.1 ± 0.4*+ |
<.05 |
| Tests |
Balance parameters |
| Time Up and Go (s) |
12.9 ± 1.9 |
14.1 ± 2.1 |
NS |
13.1 ± 1.64 |
11.9 ± 1.34 |
P<.05 |
| Romberg test (s) |
13.7 ± 3.2 |
13.3 ± 3.0 |
NS |
14.3 ± 2.37 |
8.7 ± 2.32 |
P<.01 |
3.3. Maximal voluntary contraction testing
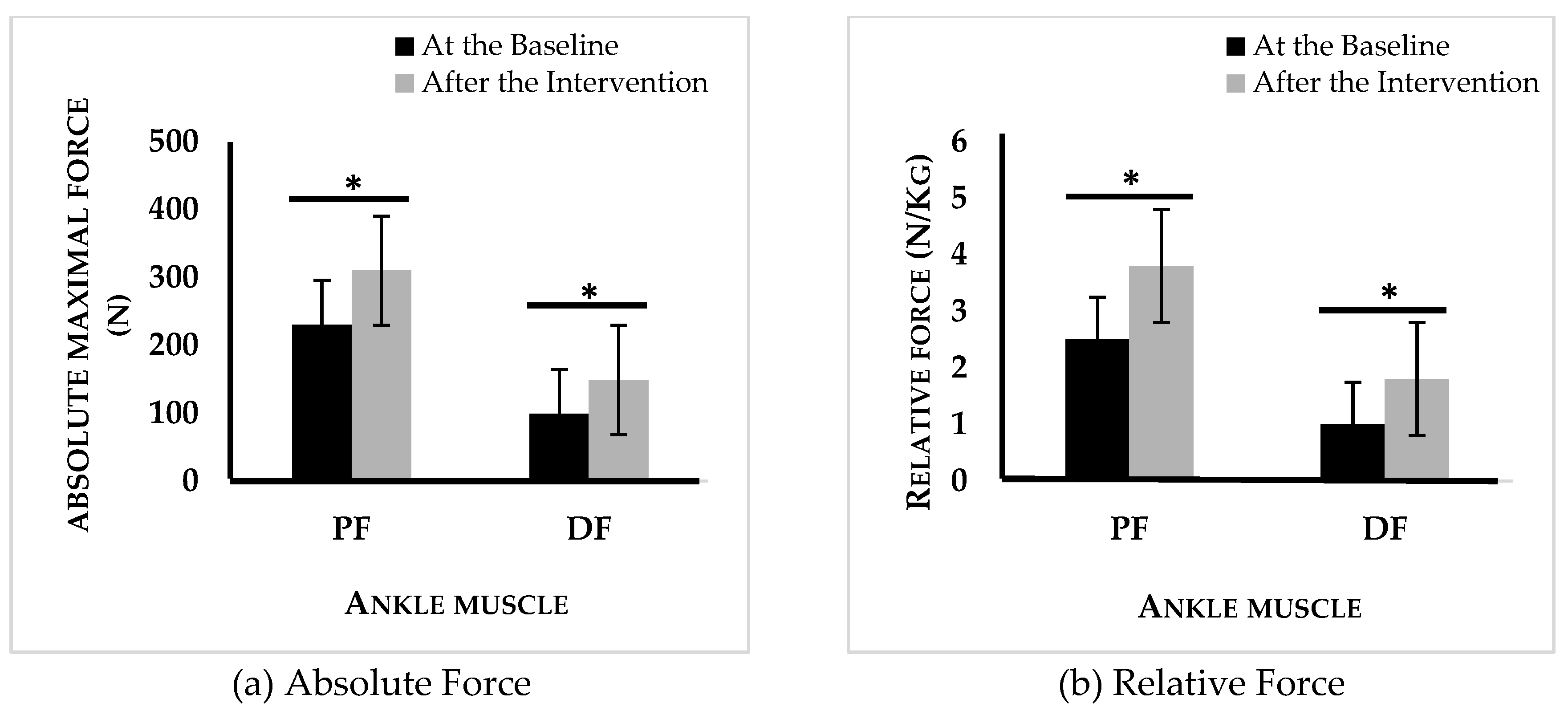
The TMP intervention contributed to notable gains in both absolute and relative peak strength for plantar (+40%, +25%, p<0.05) and dorsiflexor (+51%, +30%, p<0.05) muscles (Figure 2).
Figure 2.
Absolute (a) and relative (b) force of plantar and dorsal flexors of the intervention group at the baseline and after intervention.n PF: plantar flexor, DF: dorsal flexor, *: p < 0,05 difference between before and after physical program activity.
Figure 2.
Absolute (a) and relative (b) force of plantar and dorsal flexors of the intervention group at the baseline and after intervention.n PF: plantar flexor, DF: dorsal flexor, *: p < 0,05 difference between before and after physical program activity.
3.4. Postural control evaluation
Table 2 represents CoP parameters during postural control tests at the baseline and after TMP program. After the BSM intervention, IG showed a reduction in the CoP area in EO condition (-50%; p<0.001), EC condition (-27%; p<0.01), and TC (-34%; p<0.01). A decrease in oscillation velocity was also observed in EO condition (-27%; p<0.01), EC (-19%, p<0.01) and TC (-37%; p<0.01) following the TMP program.
Table 2.
Changes in Center of Pressure (CoP) Parameters in the Intervention Group Before and After 4-month PMT intervention Across Different Conditions.
Table 2.
Changes in Center of Pressure (CoP) Parameters in the Intervention Group Before and After 4-month PMT intervention Across Different Conditions.
| |
|
Intervention group |
| Conditions |
CoP parameters |
Baseline |
After PMT |
Δ (%) |
p |
| EO |
Area (cm²) |
8,4 ± 3.2 |
5.2 ± 2.9* |
-26 |
p<.001 |
| Velocity (mm /s-1) |
24.9 ± 10.6 |
17.0 ± 4.2* |
-31 |
p<.01 |
| EC |
Area (cm²) |
10.7 ± 3.7 |
7.8 ± 3.6* |
-27 |
p<.01 |
| Velocity (mm/s-1) |
32.6 ± 10.2 |
26.5 ± 7.6 |
-19 |
p<.05 |
| TC |
Area (cm²) |
24.8 ± 5.1 |
16.4 ± 4.5* |
-34 |
p<.01 |
| Velocity (mm /s-1) |
54.9 ± 14.1 |
34.4 ± 6.3* |
-37 |
p<.05 |
3.5. Electromyography evaluation.
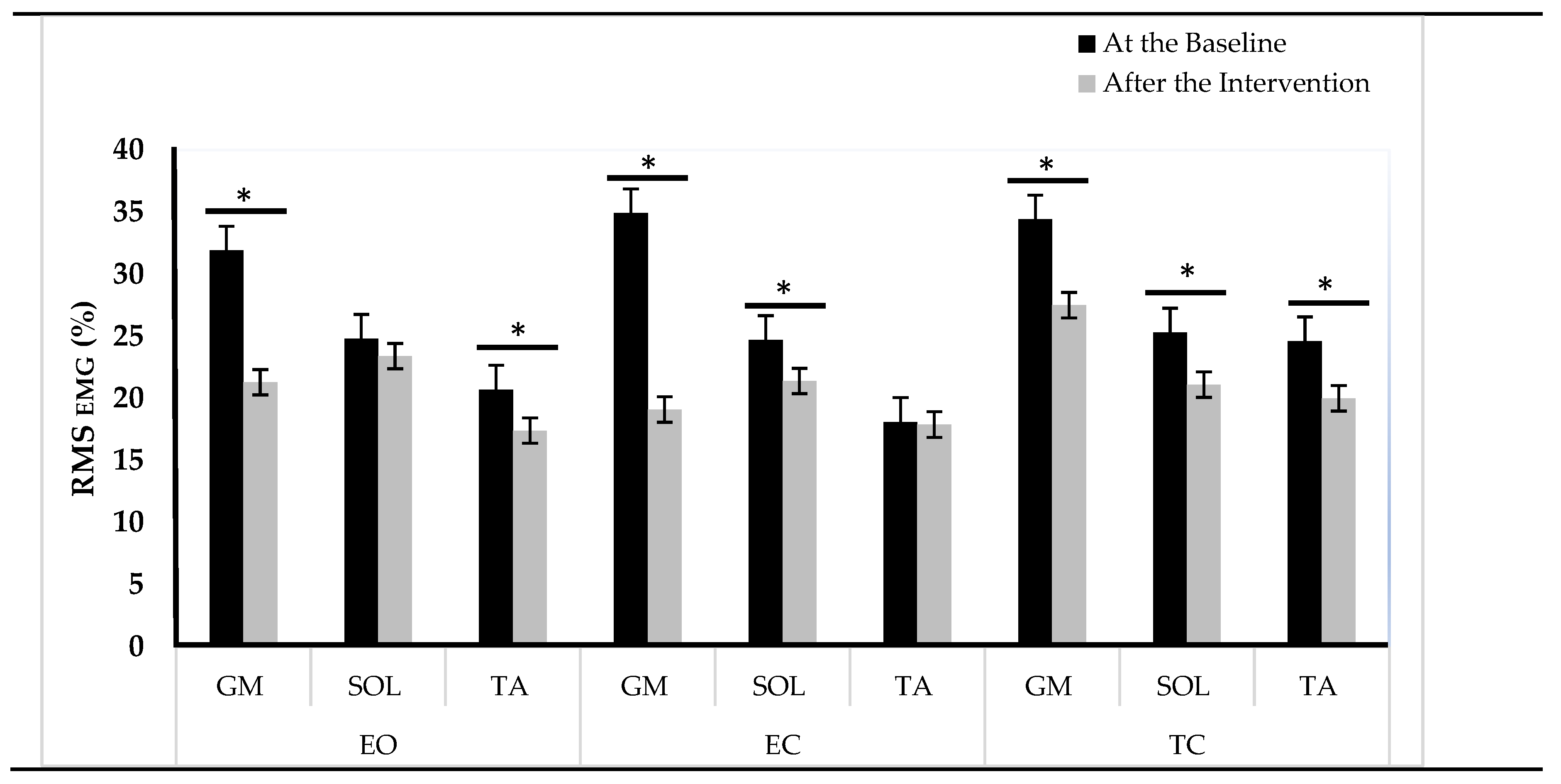
Figure 3 represents ankle muscle activities during postural control tests at the baseline and after TMP program. Following the TMP intervention, the IG showed significant reductions in GM activity in the EO (33.2%, p < 0.001), EC (29.9%, p < 0.01), and TC (20%, p < 0.01) conditions. SOL activity showed a decrease of 16.6% in both EC and TC conditions (p < 0.05). TA activity also manifested a decline in EO condition (15.9%, p < 0.05) and TC (17.4%, p < 0.05) conditions.
3.6. Pearson’s correlation analysis
Table3 presents the correlation coefficients between postural control parameters and relative strength of ankle muscles. Ϫ PF was correlated with Ϫ CoP area and velocity in EO (r = 0.71, r = 0.69, p<0.05, respectively) and EC (r = 0.62, r = 0.55, p<0.05, respectively) conditions. Ϫ DF was correlated with Ϫ CoP area and velocity in EO (r = 0.42, r = 0.54, p<0.05, respectively) and EC (r = 0.51, r = 0.49, p<0.05, respectively) conditions.
Table 3.
Pearson’s correlation analysis.
Table 3.
Pearson’s correlation analysis.
| |
Intervention group |
| EO |
EC |
TC |
| Ϫ Area |
Ϫ Velocity |
Ϫ Area |
Ϫ Velocity |
Ϫ Area |
Ϫ Velocity |
| Ϫ PF relative |
0.71* |
0.69* |
0.62* |
0.55* |
0.37 |
0.39 |
| Ϫ DF relative |
0.42* |
0.54* |
0.51* |
0.49* |
0.39 |
0.42* |
4. Discussion
The aim of this study was to evaluate the effects of a 4-month comprehensive physical activity program on the steady-state and proactive aspects of postural control, as well as on neuromuscular functions in older adults with obesity. The findings demonstrate that the TMP program resulted in enhancements in both static and proactive postural control. These improvements appear to be associated with a decrease in EMG activities and an augmentation in the force production capabilities of muscles responsible for ankle joint mobility.
The results of this study demonstrate significant improvements in both steady-state (-15.6% reduction in standing time) and proactive balance (-34.6% reduction in time to achieve TUG) following participation in the TMP program. These improvements are further supported by notable reductions in various CoP displacement parameters, particularly a decrease in the area (-26% to -34%) and the CoP velocity when transitioning to an upright position in different conditions (-19% to -34%). These findings collectively indicate an overall enhancement in postural control. These observations align with previous research that has highlighted the trainability of postural control functions in both normal-weight older adults [
40,
41,
42,
43,
44], and obese older adults [
19].
It was previously suggested that the cumulative impact of age and obesity on postural control could be associated with synergistic effects arising from both sarcopenia and obesity [
25,
45,
46,
47]. In obese adults, postural control alterations can be attributed to several factors. Firstly, the constant load bearing associated with excess weight often leads to reduced plantar sensitivity due to the hyperactivation of plantar mechanoreceptors [
48,
49]. Secondly, managing the mechanical demands of increased body mass, particularly when distributed away from the rotational axis (as in the ankle joint, resembling an inverted pendulum model), results in an increased gravitational torque. To counteract this torque, which acts along the anteroposterior axis, obese individuals must generate higher muscular torque to maintain an upright posture. These challenges are further compounded by the presence of sarcopenia, a condition characterized by progressive alterations in neuromusculoskeletal, proprioceptive, and visual systems, collectively impairing postural control [
12,
50,
51,
52,
53,
54,
55]. Considering these considerations, we propose two hypotheses to explain the observed improvement in postural control among obese older adults with SO observed in our study. The first hypothesis relates to an increase in musculoskeletal capacities, possibly induced by strength exercises (e.g., plantar, and dorsal flexions, adapted squats) included in the TMP program. The second hypothesis relates to the enhancement of proprioceptive capacities resulting from posture and balance exercises. This hypothesis gains support from the improved postural parameters observed during tasks that require greater utilization of proprioceptive resources, particularly in the tandem position. These findings suggest that the TMP intervention, especially the balance exercises, effectively enhanced the proprioceptive capabilities of the participants. Furthermore, the improvements in postural parameters during this position were not correlated with changes in relative strength, indicating that the enhancement of postural control in older adults with SO likely relies on improved proprioceptive capacities rather than solely on increased strength.
Regarding the first hypothesis, the IG demonstrated a significant increase in relative ankle muscle force, with a 25% improvement in the PF and a 30% enhancement in the DF. The improvement in relative force was positively correlated with a decrease in the CoP parameters, specifically the area (r = 0.71) and velocity (r = 0.69) in open eyes condition. These findings suggest that the enhanced maximal force production capacity of the plantar flexor muscles, achieved through muscle strengthening exercises, contributed partially to the improvement in balance quality among older adults with SO. Obese older adults often struggle to generate sufficient force relative to their body mass during postural control, and this improvement in force production could be attributed to two possibilities: an increase in muscle mass and an improvement in neural mechanisms. In this study, an increase in LBM was observed without a change in BMI, explaining the modification of body composition following the TMP program. The lack of significant changes in body composition can be attributed to the substantial increase in LBM, which replaced fat body mass. This underscores the importance of considering body composition as a crucial indicator of the effectiveness of exercise programs, especially in older adults with SO. Regarding neural implications, a reduction in ankle muscle activity during postural control trials was observed. GM activity decreased (from -20% to -33.2%), SOL activity (-16.6%), TA (from -15.9% to -17.4). Importantly, postural control alterations in older adults with SO were previously attributed to increased ankle muscle activities, which were identified as a neuromuscular strategy to compensate for neuromuscular weakness and proprioceptive system degeneration. However, increased ankle muscle activation leads to higher energy expenditure, increased costs, and early fatigue, ultimately elevating the risk of falls. It is plausible to suggest that the TMP program improved the strategy of controlling ankle muscle activation during postural control, potentially reducing energy costs and, likely, early fatigue.
The impact of obesity on neuromuscular capacities can be elucidated by examining multiple factors, such as motor unit recruitment [
56], intrinsic muscle properties [
51,
52], and systemic inflammation [
47,
53]. Moreover, adipose tissue in obese individuals’ functions as a dynamic endocrine organ, secreting an array of hormones and pro-inflammatory cytokines, like TNF-α, IL-1α, IL-6, and CRP [
59]. On the other hand, obesity can exacerbate the loss of muscle mass—often referred to as sarcopenia—which is already compromised in older adults with sarcopenia. The results observed in this study, including an increase in muscle mass and a reduction in neuromuscular activation, suggest that the TMP program, through its exercises, has a reversible effect on the mechanisms that lead to neuromuscular system alterations associated with the synergistic effects of obesity and sarcopenia. However, it is challenging to determine whether the reduction in ankle muscle activity is directly related to the improvement of the neuromuscular system or to a decreased demand associated with postural regulation in older adults with SO. This necessitates further investigations to better understand the underlying mechanisms of neuromuscular system modification, postural control regulation, and the effects of physical activity on the reversibility and trainability of the neuromuscular system.
Limitations and perspectives
This study acknowledges several limitations that warrant consideration. Firstly, the small sample size utilized in this research may restrict the generalizability of our findings, particularly given the heterogeneity often observed in older adults and obese individuals. These populations can sometimes present a complex interplay of factors, resulting in characteristics that may not be wholly reflective of those in our study sample. Future investigations with larger cohorts are essential to validate our results and enhance statistical power. Additionally, our study primarily focused on the muscles surrounding the ankle joint, omitting potential influences of excess adipose tissue in the regions of the knee and hip joints on EMG signals. To comprehensively assess neuromuscular aspects, incorporating 3D gait analysis that evaluates joint moments and ranges of motion across all three joints (hip, knee, and ankle) would be beneficial. Moreover, we did not monitor participants' dietary intake, but future research could explore the synergistic effects of physical activity and protein consumption on outcomes.
5. Conclusions
This study unveiled significant enhancements in both steady-state and proactive postural control following a comprehensive physical activity program. These improvements were coupled with remarkable reductions in various parameters associated with the CoP displacement, indicating an overall advancement in postural control regulation. These positive changes can be attributed to an increase in ankle muscle force, as well as a decrease in muscle activation, particularly in the plantar flexor and dorsiflexor muscles. Importantly, these improvements were not solely linked to increased muscular strength but also to the development of enhanced proprioceptive capacities, potentially fostered through balance exercises within the program. These findings highlight the reversibility of neuromuscular system alterations associated with the synergistic effects of sarcopenia and obesity, emphasizing the trainability of postural control regulation within this population. By incorporating these insights into clinical practice and public health strategies, we can strive to optimize the health and well-being of older adults dealing with sarcopenic obesity.
Author Contributions
Conceptualization, E.M. and W.M.; methodology, W.M. and S.B; software, E.M.; validation, S.D., S.B. and B.B.; formal analysis, O.G.C.; investigation, M.M.; resources, S.G.S.; data curation, O.G.C.; writing—original draft preparation, E.M.; writing—review and editing, W.M.; visualization, S.G.S; supervision, W.M. and S.D.; project administration, O.G.C; funding acquisition, S.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol is meticulously developed and implemented in unwavering alignment with the principles delineated in the Helsinki Declaration. Furthermore, the study protocol, alongside the patient information letter and informed consent documents, has received approved from the Ethics Committee of the South Ethics Committee for the Protection of Persons in Tunisia, denoted by the reference number C.P.P. SOUTH /No. 0477/2022, on 22 February 2022. The study has also been formally registered with the Pan African Clinical Trials Registry, where it has been assigned the unique registration identifier PACTR202306912191110.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting the reported results of this study will be published on the Pan African Clinical Trials Registry after the publication of the article. Interested parties can access the data by referring to the registry once it becomes available.
Acknowledgments
We would like to extend our sincere appreciation to the medical staff and directors of the institutions where we recruited the participants for their valuable support and facilitation throughout this study. We would also like to express our gratitude to the patients who participated in this study. Their willingness to be involved and their cooperation during the data collection process have been crucial to the success of our research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cattagni, T.; Scaglioni, G.; Laroche, D.; Van Hoecke, J.; Gremeaux, V.; Martin, A. Ankle Muscle Strength Discriminates Fallers from Non-Fallers. Front. Aging Neurosci. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Laughton, C.A.; Slavin, M.; Katdare, K.; Nolan, L.; Bean, J.F.; Kerrigan, D.C.; Phillips, E.; Lipsitz, L.A.; Collins, J.J. Aging, Muscle Activity, and Balance Control: Physiologic Changes Associated with Balance Impairment. Gait Posture 2003, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Combined Effects of Aging and Obesity on Postural Control, Muscle Activity and Maximal Voluntary Force of Muscles Mobilizing Ankle Joint. J. Biomech. 2018, 79, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Billot, M.; Simoneau, E.M.; Hoecke, J. Van; Martin, A. Age-Related Relative Increases in Electromyography Activity and Torque According to the Maximal Capacity during Upright Standing. Eur J Appl Physiol 2010, 109, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Baudry, S.; Lecoeuvre, G.; Duchateau, J. Age-Related Changes in the Behavior of the Muscle-Tendon Unit of the Gastrocnemius Medialis during Upright Stance. 2012, 296–304. [Google Scholar] [CrossRef]
- Duchateau, J.; Nicol, C.; Baudry, S. Le Vieillissement Du Système Neuromusculaire: De La Sarcopénie à La Dynapénie. Kinesitherapie 2014, 14, 45–51. [Google Scholar] [CrossRef]
- Power, G.A.; Dalton, B.H.; Rice, C.L. Human Neuromuscular Structure and Function in Old Age: A Brief Review. J. Sport Heal. Sci. 2013, 2, 215–226. [Google Scholar] [CrossRef]
- Edström, E.; Altun, M.; Bergman, E.; Johnson, H.; Kullberg, S.; Ramírez-León, V.; Ulfhake, B. Factors Contributing to Neuromuscular Impairment and Sarcopenia during Aging. Physiol. Behav. 2007, 92, 129–135. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.; Rantenen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic Obesity-Definition,Etiology and Consequences. Curr Opin Clin Nutr Metab Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Del Porto, H.C.; Pechak, C.M.; Smith, D.R.; Reed-jones, R.J. Biomechanical Effects of Obesity on Balance. Int. J. Exerc. Sci. 2012, 5, 301–320. [Google Scholar]
- Pereira, C.; Silva, R.A.D.; de Oliveira, M.R.; Souza, R.D.N.; Borges, R.J.; Vieira, E.R. Effect of Body Mass Index and Fat Mass on Balance Force Platform Measurements during a One-Legged Stance in Older Adults. Aging Clin. Exp. Res. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- LV, A.; DV, de O.; B, F.; SMMG, B. The Influence of Age and Overweight or Obesity on Foot Sensitivity and Postural Control: A Systematic Review. Australas. J. Ageing 2020, 39, e251–e258. [Google Scholar] [CrossRef]
- Nascimento, M. de M.; Gouveia, É.R.; Gouveia, B.R.; Marques, A.; Martins, F.; Przednowek, K.; França, C.; Peralta, M.; Ihle, A. Associations of Gait Speed, Cadence, Gait Stability Ratio, and Body Balance with Falls in Older Adults. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- Melzer, I.; Oddsson, L.I.E. Altered Characteristics of Balance Control in Obese Older Adults. Obes. Res. Clin. Pract. 2016, 10, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. [Fall Risk and Fracture. Fall Risk Assessment]. Clin. Calcium 2013, 23. [Google Scholar]
- Wu, G. Age-Related Differences in Body Segmental Movement during Perturbed Stance in Humans. Clin. Biomech. 1998, 13, 300–307. [Google Scholar] [CrossRef]
- Waters, D.L.; Hale, L.; Grant, A.M.; Herbison, P.; Goulding, A. Osteoporosis and Gait and Balance Disturbances in Older Sarcopenic Obese New Zealanders. Osteoporos. Int. 2010, 21, 351–357. [Google Scholar] [CrossRef]
- Aibar-Almazán, A.; Martínez-Amat, A.; Cruz-Díaz, D.; Jiménez-García, J.D.; Achalandabaso, A.; Sánchez-Montesinos, I.; de la Torre-Cruz, M.; Hita-Contreras, F. Sarcopenia and Sarcopenic Obesity in Spanish Community-Dwelling Middle-Aged and Older Women: Association with Balance Confidence, Fear of Falling and Fall Risk. Maturitas 2018, 107, 26–32. [Google Scholar] [CrossRef]
- Maktouf, W.; Durand, S.; Beaune, B.; Boyas, S. Influence of Obesity and Impact of a Physical Activity Program on Postural Control and Functional and Physical Capacities in Institutionalized Older Adults: A Pilot Study. J. Phys. Act. Heal. 2019, 17, 169–176. [Google Scholar] [CrossRef]
- Cattagni, T.; Scaglioni, G.; Cornu, C.; Berrut, G.; Martin, A. De La Fonction Neuromusculaire. 2015, 13, 363–380. [Google Scholar] [CrossRef]
- Macie, A.; Matson, T.; Schinkel-Ivy, A. Age Affects the Relationships between Kinematics and Postural Stability during Gait. Gait Posture 2023, 102, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Takahashi, M.; Shinkoda, K. Differences of Muscle Co-Contraction of the Ankle Joint between Young and Elderly Adults during Dynamic Postural Control at Different Speeds. J. Physiol. Anthropol. 2017, 36, 32. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, E.; Martin, A.; Porter, M.M.; Van Hoecke, J.; Orr, R.; De Vos, N.J.; Singh, N.A.; Ross, D.A.; Stavrinos, T.M.; Fiatarone-Singh, M.A.; et al. Static Balance Improvement in Elderly after Dorsiflexors Electrostimulation Training. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 2006, 61, 69–78. [Google Scholar] [CrossRef]
- Bobowik, P.; Wiszomirska, I. The Impact of Obesity and Age on the Risk of Falls in Elderly Women. Acta Bioeng. Biomech. 2021, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of Sarcopenic Obesity in Older Individuals with a History of Falling. Arch. Gerontol. Geriatr. 2016, 65, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-Mediated Reinnervation of Skeletal Muscle in Elderly People: An Update. Eur. J. Transl. Myol. 2022, 32. [Google Scholar] [CrossRef]
- Ponta, M.L.; Gozza, M.; Giacinto, J.; Gradaschi, R.; Adami, G.F. Effects of Obesity on Posture and Walking: Study Prior to and Following Surgically Induced Weight Loss. Obes. Surg. 2014, 24. [Google Scholar] [CrossRef]
- Menegoni, F.; Galli, M.; Tacchini, E.; Vismara, L.; Cavigioli, M.; Capodaglio, P. Gender-Specific Effect of Obesity on Balance. Obesity 2009. [Google Scholar] [CrossRef]
- Teasdale, N.; Hue, O.; Marcotte, J.; Berrigan, F.; Simoneau, M.; Dore, J.; Marceau, P.; Marceau, S.; Tremblay, A. Reducing Weight Increases Postural Stability in Obese and Morbid Obese Men. Int. J. Obes. 2007, 31, 153–160. [Google Scholar] [CrossRef]
- Corbeil, P.; Simoneau, M.; Rancourt, D.; Tremblay, A.; Teasdale, N. Increased Risk for Falling Associated with Obesity: Mathematical Modeling of Postural Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 126–136. [Google Scholar] [CrossRef]
- Debelle, H.; Maganaris, C.N.; O’brien, T.D. Role of Knee and Ankle Extensors’ Muscle-Tendon Properties in Dynamic Balance Recovery from a Simulated Slip. Sensors 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Handrigan, G.A.; Simoneau, M.; Teasdale, N.; Corbeil, P. THE EFFECTS OF ADDED MASS ON PLANTAR SOLE SENSITIVITY IN UPRIGHT STANDING. J. Biomech. 2012, 45, S233. [Google Scholar] [CrossRef]
- Wu, X.; Madigan, M.L. Impaired Plantar Sensitivity among the Obese Is Associated with Increased Postural Sway. Neurosci. Lett. 2014, 583. [Google Scholar] [CrossRef]
- Pajoutan, M.; Mehta, R.K.; Cavuoto, L.A. The Effect of Obesity on Central Activation Failure during Ankle Fatigue: A Pilot Investigation. Fatigue Biomed. Heal. Behav. 2016, 4, 115–126. [Google Scholar] [CrossRef]
- Pajoutan, M.; Ghesmaty Sangachin, M.; Cavuoto, L.A. Central and Peripheral Fatigue Development in the Shoulder Muscle with Obesity during an Isometric Endurance Task. BMC Musculoskelet. Disord. 2017, 18, 314. [Google Scholar] [CrossRef]
- Kong, H.H.; Won, C.W.; Kim, W. Effect of Sarcopenic Obesity on Deterioration of Physical Function in the Elderly. Arch. Gerontol. Geriatr. 2020, 89, 104065. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, A.-A.; Habchi, H.; Habchi, P.; Dembele, I.A.; Andres, E. Physical Activity in the Elderly and Frailty Syndrome: A Retrospective Study in Primary Care. Medicines 2022, 9, 51. [Google Scholar] [CrossRef]
- Smith, T.; Gildeh, N.; Holmes, C. The Montreal Cognitive Assessment: Validity and Utility in a Memory Clinic Setting. Can. J. Psychiatry 2007, 52, 329–332. [Google Scholar] [CrossRef]
- Hamza Ferhi , Sabri Gaied Chortane, Sylvain Durand, Bruno Beaune, S.B. and W.M. Effects of Physical Activity Program on Body Composition, Physical Performance, and Neuromuscular Strategies during Walking in Older Adults with Sarcopenic Obesity : Randomized Controlled Trial. 2023. [Google Scholar]
- Kharsany, A.B.M.; Buthelezi, T.J.; Frohlich, J.A.; Samsunder, N.; Mahlase, G.; Williamson, C.; Travers, A.; Marais, J.C.; Dellar, R.; Karim, S.S.A. КГИ - Главная - Газoжидкoстные Технoлoгии Page 1 of 30. 2011, 1–30. [Google Scholar]
- Gerards, M.H.G.; McCrum, C.; Mansfield, A.; Meijer, K. Perturbation-Based Balance Training for Falls Reduction among Older Adults: Current Evidence and Implications for Clinical Practice. Geriatr. Gerontol. Int. 2017, 17, 2294–2303. [Google Scholar] [CrossRef]
- Lelard, T.; Doutrellot, P.L.; David, P.; Ahmaidi, S. Effects of a 12-Week Tai Chi Chuan Program Versus a Balance Training Program on Postural Control and Walking Ability in Older People. Arch. Phys. Med. Rehabil. 2010, 91, 9–14. [Google Scholar] [CrossRef]
- Lelard, T.; Ahmaidi, S. Effects of Physical Training on Age-Related Balance and Postural Control. Neurophysiol. Clin. 2015, 45, 357–369. [Google Scholar] [CrossRef]
- Lesinski, M.; Hortobágyi, T.; Muehlbauer, T.; Gollhofer, A.; Granacher, U. Effects of Balance Training on Balance Performance in Healthy Older Adults: A Systematic Review and Meta-Analysis. Sport. Med. 2015, 45, 1721–1738. [Google Scholar] [CrossRef]
- Kong, H.H.; Won, C.W.; Kim, W. Effect of Sarcopenic Obesity on Deterioration of Physical Function in the Elderly. Arch. Gerontol. Geriatr. 2020, 89, 104065. [Google Scholar] [CrossRef]
- Scott, D.; Shore-Lorenti, C.; McMillan, L.; Mesinovic, J.; Clark, R.A.; Hayes, A.; Sanders, K.M.; Duque, G.; Ebeling, P.R. Associations of Components of Sarcopenic Obesity with Bone Health and Balance in Older Adults. Arch. Gerontol. Geriatr. 2018, 75, 125–131. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G. The Impact of Obesity on Skeletal Muscle Strength and Structure through Adolescence to Old Age. Biogerontology 2016, 17, 467–483. [Google Scholar] [CrossRef]
- Hue, O.; Berrigan, F.; Simoneau, M.; Marcotte, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Muscle Force and Force Control after Weight Loss in Obese and Morbidly Obese Men. Obes. Surg. 2008, 18, 1112–1118. [Google Scholar] [CrossRef]
- Simoneau, M.; Teasdale, N. Balance Control Impairment in Obese Individuals Is Caused by Larger Balance Motor Commands Variability. Gait Posture 2015, 41, 203–208. [Google Scholar] [CrossRef]
- Jeong, H.; Wayne Johnson, A.; Brent Feland, J.; Petersen, S.R.; Staten, J.M.; Bruening, D.A. Added Body Mass Alters Plantar Shear Stresses, Postural Control, and Gait Kinetics: Implications for Obesity. PLoS One 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Henry, M.; Baudry, S. Age-Related Changes in Leg Proprioception: Implications for Postural Control. J. Neurophysiol. 2019, 122, 525–538. [Google Scholar] [CrossRef]
- Zemková, E.; Kyselovičová, O.; Jeleň, M.; Kováčiková, Z.; Ollé, G.; Štefániková, G.; Vilman, T.; Baláž, M.; Kurdiová, T.; Ukropec, J.; et al. Unilateral Stability and Visual Feedback Body Control Improves After Three-Month Resistance Training in Overweight Individuals. J. Mot. Behav. 2017, 49, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Wearing, S.C.; Hennig, E.M.; Byrne, N.M.; Steele, J.R.; Hills, A.P. The Biomechanics of Restricted Movement in Adult Obesity. Obes. Rev. 2006. [CrossRef]
- Zhang, Z.; Gao, Y.; Wang, J. Effects of Vision and Cognitive Load on Anticipatory and Compensatory Postural Control. Hum. Mov. Sci. 2019. [Google Scholar] [CrossRef]
- Frontera, W.R. Physiologic Changes of the Musculoskeletal System with Aging: A Brief Review. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.G.; Pfeiffer, R.F.; LeDoux, M.S.; Schilling, B.K. Neuromuscular Rate of Force Development Deficit in Parkinson Disease. Clin. Biomech. 2017, 45, 14–18. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambélé-Pearson, G.L. Combined Effects of Body Composition and Ageing on Joint Torque, Muscle Activation and Co-Contraction in Sedentary Women. Age (Dordr). 2014, 36, 9652. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of Force Development: Physiological and Methodological Considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef]
- Erskine, R.M.; Tomlinson, D.J.; Morse, C.I.; Winwood, K.; Hampson, P.; Lord, J.M.; Onambélé, G.L. The Individual and Combined Effects of Obesity- and Ageing-Induced Systemic Inflammation on Human Skeletal Muscle Properties. Int. J. Obes. 2017, 41, 102–111. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).