1. Introduction
The SARS-CoV-2 virus initiated the recent pandemic, probably having originated in the Wuhan region of China [
1,
2]. The pandemic got the medical profession and society by surprise and no medical treatment was known at the time for the novel viral infection. Therefore, intense research was initiated to combat and prevent the disease leading to the discovery of various agents capable of fighting the infection [
3]. In particular, research into the matter of inadequate vitamin D levels which may be related to the severe infection and adverse outcome led to the observation that severe SARS-CoV-2 infection may be related to inadequate vitamin D levels [
4,
5,
6,
7]. Vitamin D is a hormone related to calcium and bone metabolism with significant immune modulating properties [
8]. In particular, it has been shown that vitamin D may act to enhance the innate immune response [
9] and to contribute to the response of the organism to infectious agents, such as mycobacterium tuberculosis [
10] and mycobacterium leprae [
11]. Vitamin D induces the production of cathelicidin thus having an antiviral effect [
12]. Consequently, vitamin D administration was implemented in the management of SARS-CoV-2 infection [
13,
14,
15].
Dexamethasone was applied as first and second line treatment and led to the discovery that dexamethasone may prevent severe disease and may improve adverse outcome in the form of hospitalization within the acute care unit as well as intubation [
16,
17]. Infection from the SARS-CoV-2 virus may manifest as a mild viral illness or may take the form of a severe disease which may lead to adverse outcomes [
18]. In particular, it may necessitate hospitalization or admission to the acute care unit or it may lead to intubation and in severe cases to death [
19].
Thrombosis as well as a tendency to develop thrombosis may accompany a viral infection [
20] and it has been reported in relationship to Covid-19 infection [
21,
22]. In particular, Covid-19 has been proposed to be a thrombogenic virus [
23,
24] and to cause thromboembolic disease and cardiac thrombotic complications [
25]. In accordance, microthrombi have been observed in the lungs at autopsy in patients having died from the SARS-CoV-2 infection [
26]. Thrombotic markers, such as fibrinogen and d-dimers have been shown to increase during Covid-19 infection [
27,
28]. D-dimers may be a predictive factor in identifying thrombosis and high levels may be an index of severity of the infection and death [
23]. Anticoagulation is necessary and heparin is considered the preferred anticoagulant as it may have antiviral action [
23]. In the paper herein we measured d-dimer and fibrinogen levels and we found increased d-dimer and fibrinogen levels which were inversely related to vitamin D levels. In accordance low vitamin D levels have been associated with thromboembolic events [
29] and vitamin D may have an antithrombotic action [
30].
Ferritin ensures the availability of iron for cellular activities while protecting the DNA and proteins from the possible toxic effects of iron [
31]. Elevated levels of ferritin are observed in infections [
32] and are thought to be an inflammatory index and appear to be an important host defense mechanism against bacteria as iron may be cytotoxic to bacteria [
33]. Ferritin levels were shown to increase during the course of SARS-CoV-2 infection and the role of ferritin in the course of the disease was discussed [
28,
34]. In particular, increased ferritin levels may be an index of severity as well as a prognostic factor in SARS-CoV-2 patients [
35]. In our study, we observed high ferritin levels in patients with Covid-19 disease, which were inversely related to vitamin D levels.
The aim was to describe the relationship of vitamin D levels with the outcome of the disease in a cohort of patients who were hospitalized for severe SARS-CoV-2 infection. Low vitamin D levels were observed in patients with severe SARS-CoV-2 infection which were related to the outcome of the infection. Vitamin D levels were inversely related to d-dimer, fibrinogen and ferritin levels.
2. Results
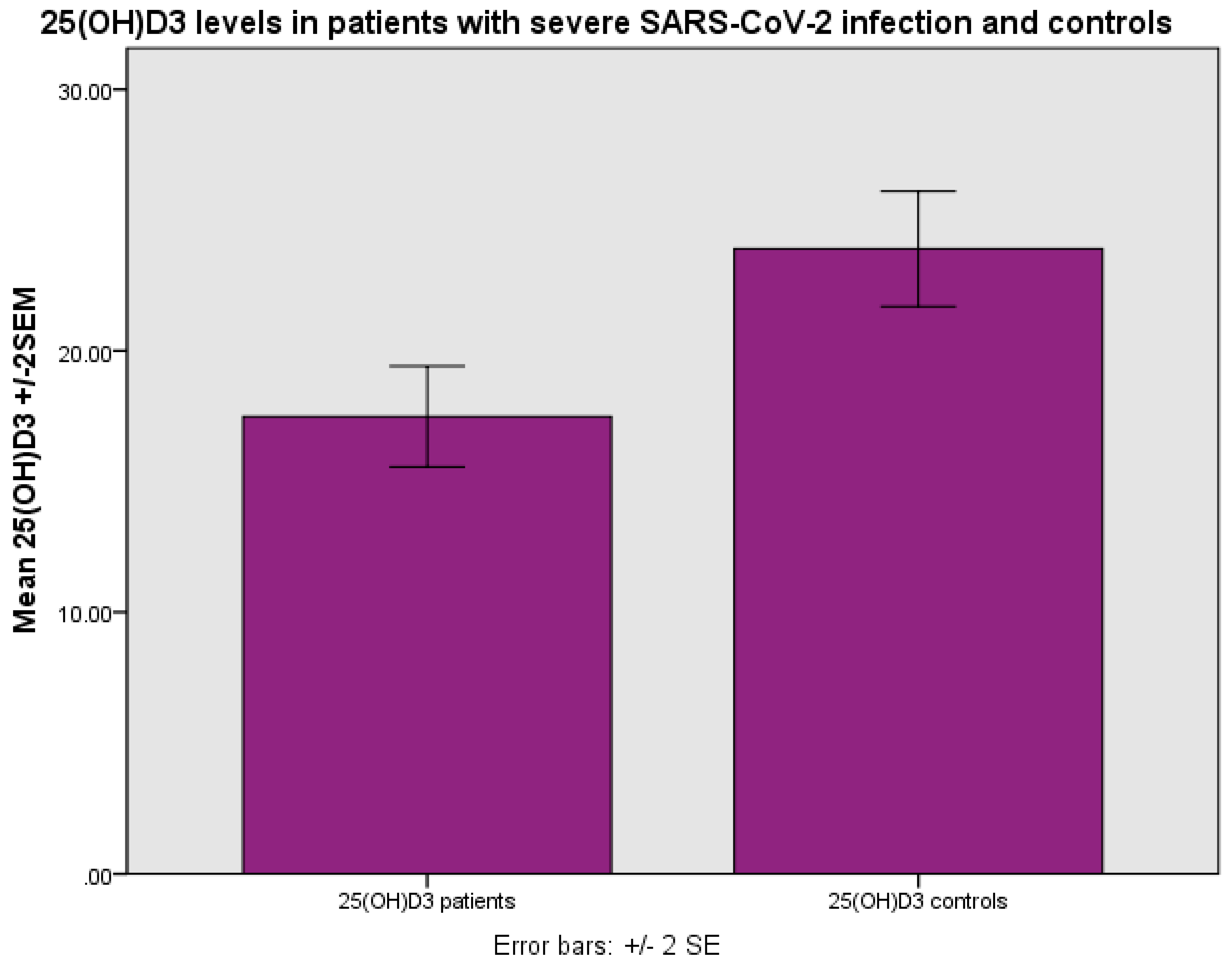
The levels of 25(OH)D
3 were 17.36±8.80 ng/ml (mean±SD) as compared with 24.34±10.34 ng/ml in the control group, p<0.001 (Student’s t test) (
Figure 1). The levels of 25(OH)D
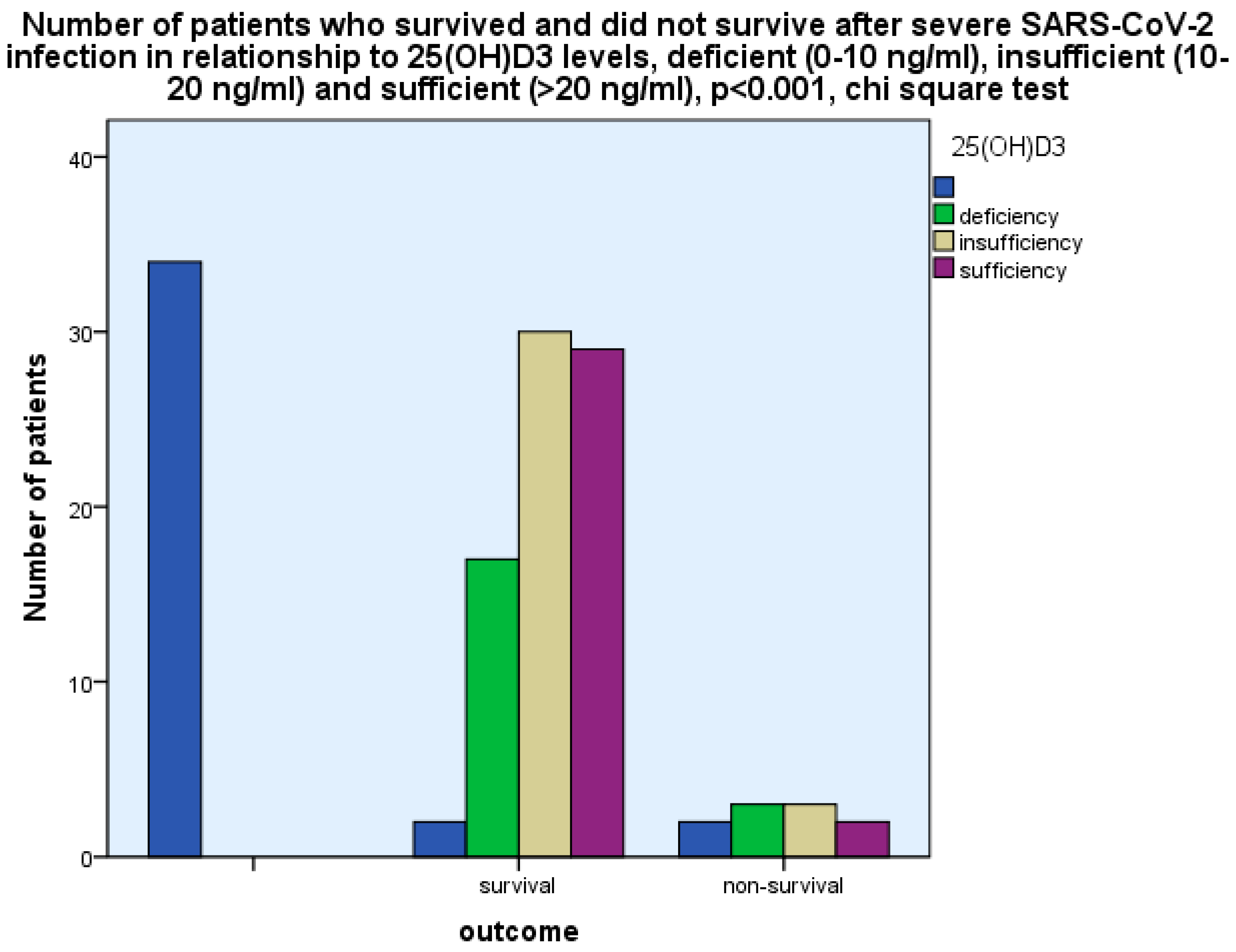
3 were found to be related to the outcome, i.e., survival as opposed to non-survival, as more patients with 25(OH)D
3 deficiency (0-10 ng/ml) and insufficiency (10-20 ng/ml) had a fatal outcome as compared with those with 25(OH)D
3 sufficiency (>20 ng/ml), p<0.001, chi-square test, p<0.001, Fischer’s exact test (
Figure 2).
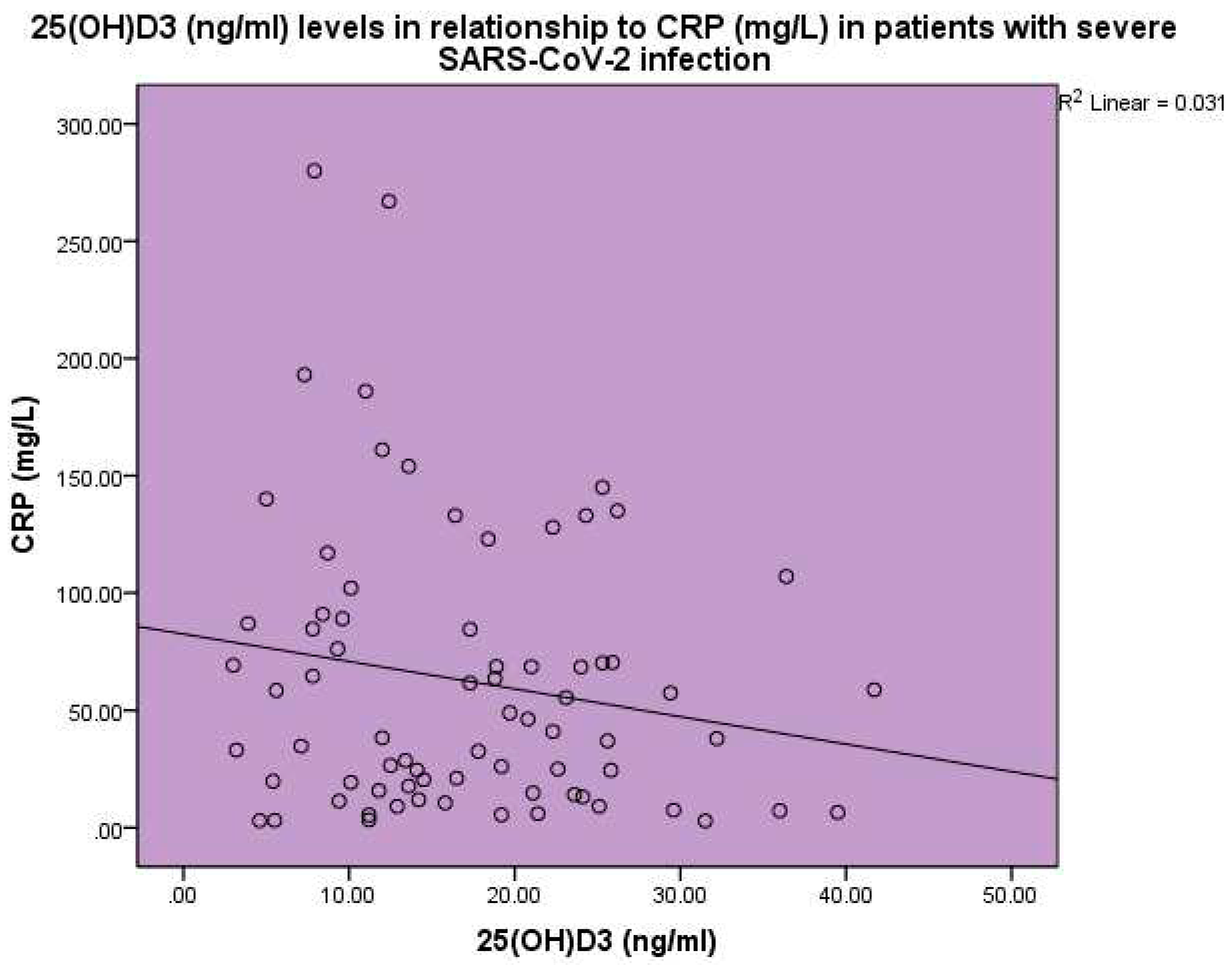
Increased CRP and ESR levels were observed in the SARS-CoV-2 patients. The concentration of 25(OH)D
3 was found to be inversely related to CRP, p<0.001, linear regression analysis, standardized coefficient of variation beta -0.176 (
Figure 3).
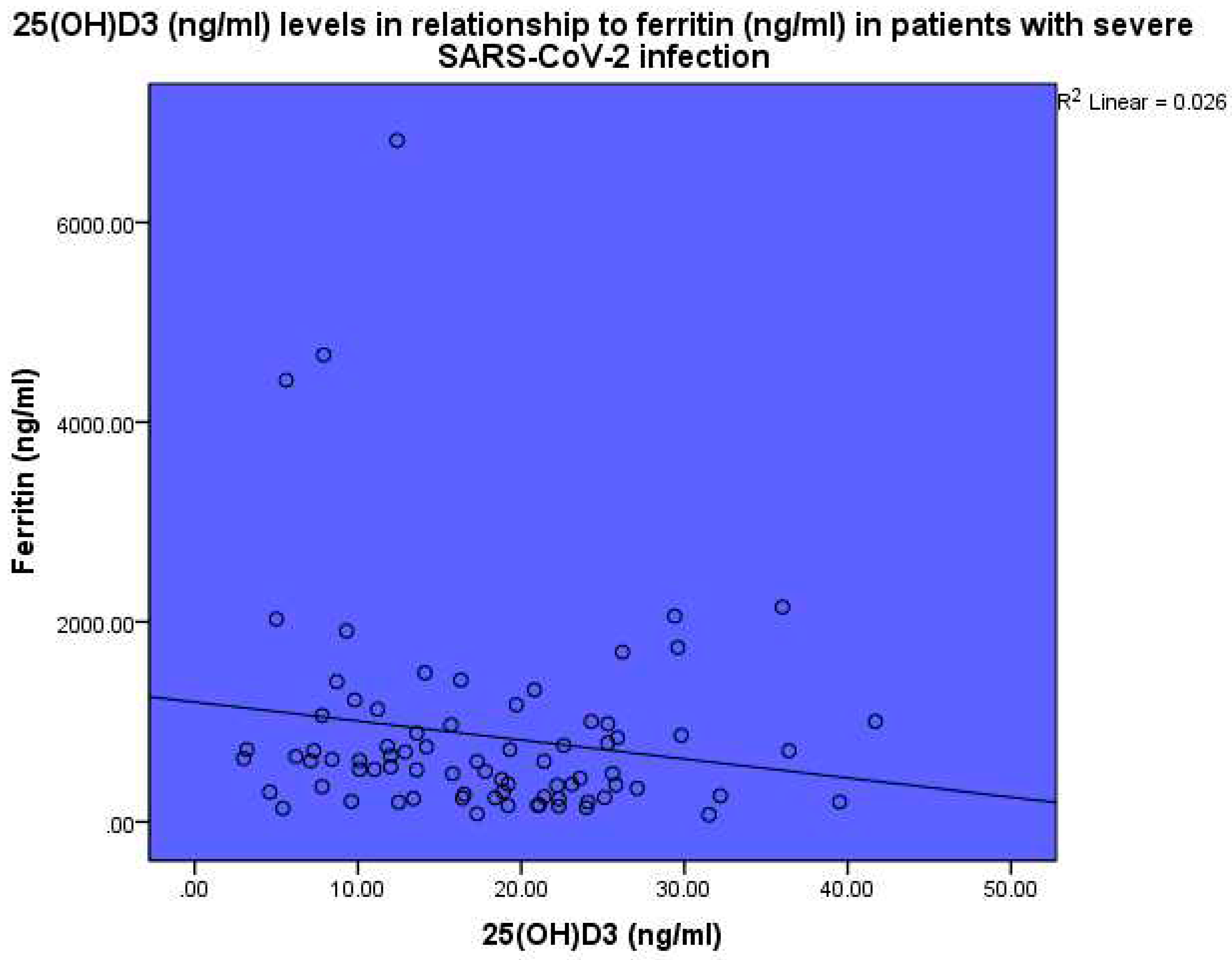
The levels of 25(OH)D
3 were also found to be inversely related to ferritin, p<0.001, linear regression analysis, standardized coefficient of variation beta -0.160 (
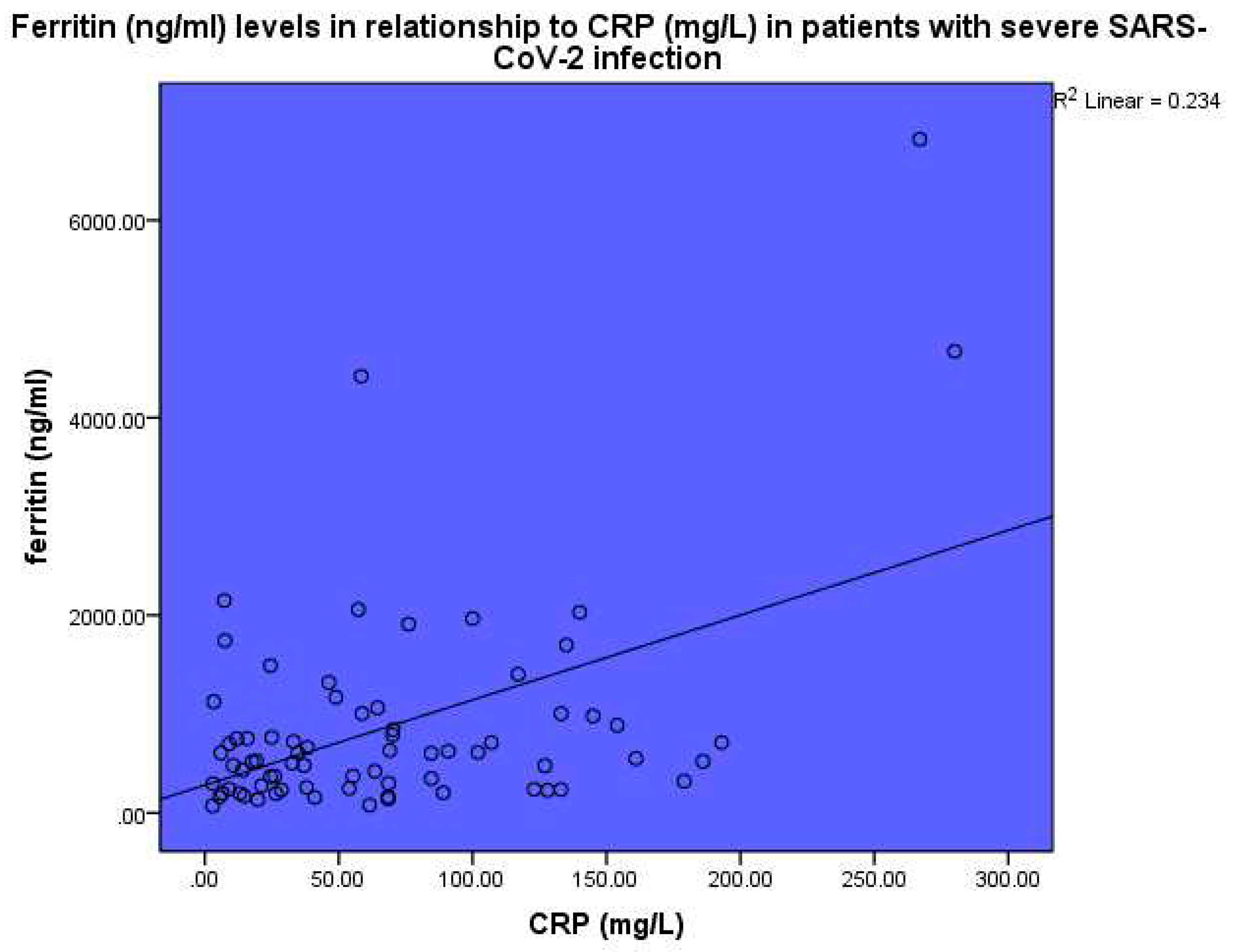
Figure 4). Ferritin (ng/ml) levels were found to be related to CRP, p<0.001, linear regression analysis, standardized coefficient of variation beta 0.484 (
Figure 5).
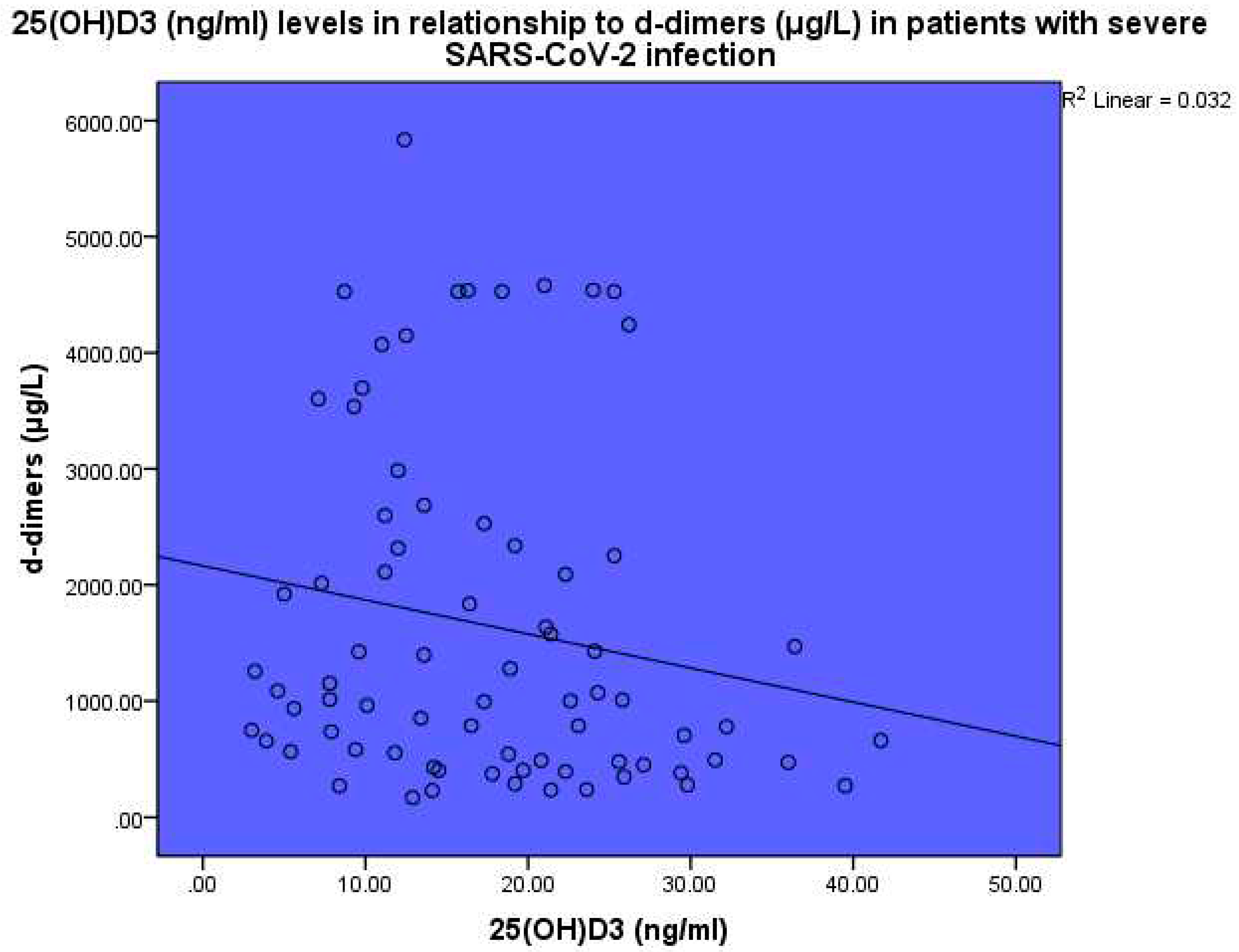
Thrombotic markers were found to be increased in the blood of the sick patients as compared to the expected normal levels. Levels of 25(OH)D
3 were found to be inversely related to d-dimer levels, p<0.001, linear regression analysis, standardized coefficient of variation beta -0.178 (
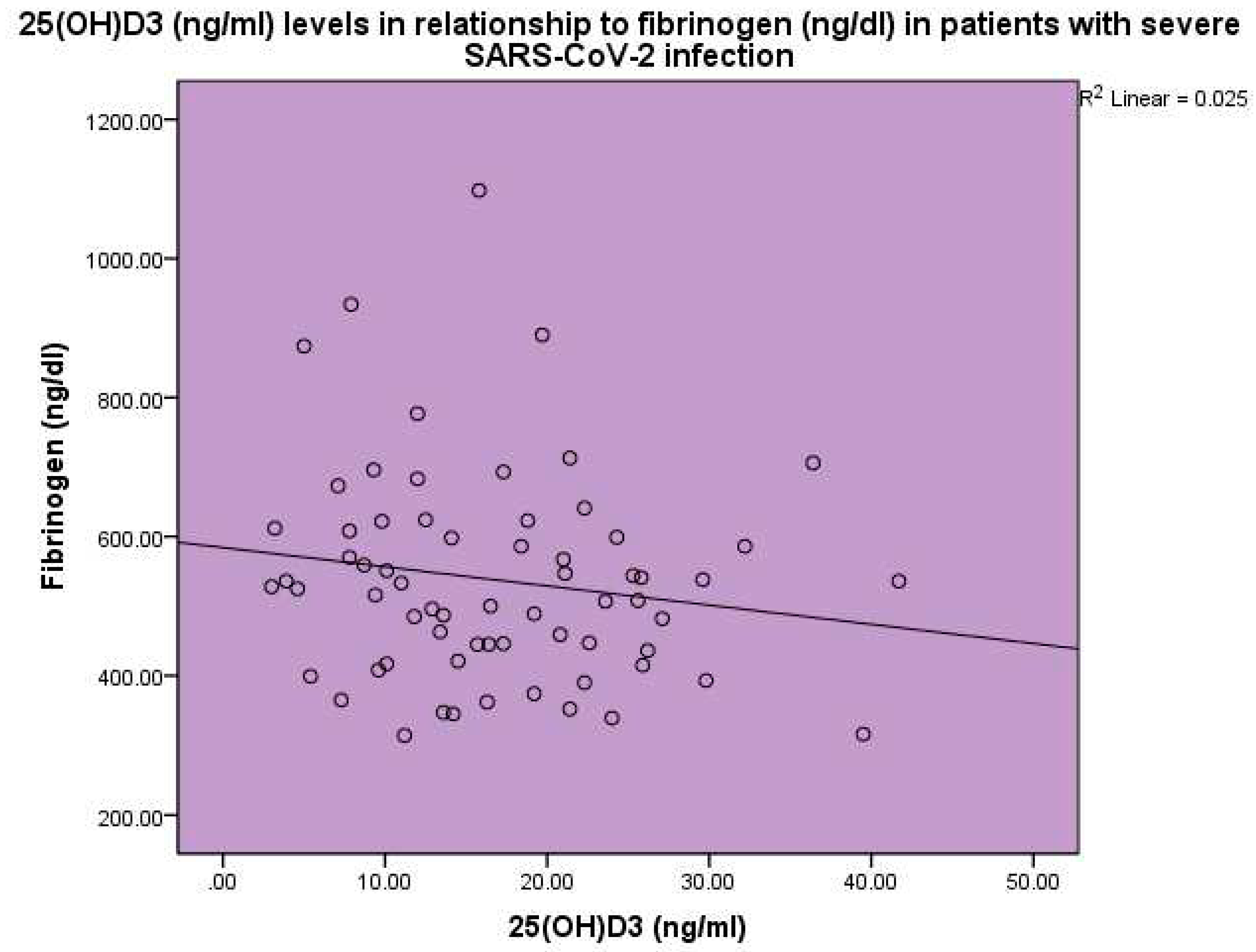
Figure 6). Levels of 25(OH)D
3 were found to be inversely related to fibrinogen levels, p<0.001, linear regression analysis, standardized coefficient of variation beta -0.158 (
Figure 7). Thyroid hormone levels were within normal range in the Covid 19 group of patients. In particular, TSH, FT
4 and FT
3 levels were within normal range.
3. Discussion
The SARS-CoV-2 infection may lead to a severe acute disease which necessitates hospitalization and may lead to intubation and death [
36,
37]. Various agents have been employed to combat the SARS-CoV-2 infection, amongst those vaccines and antiviral medications. As, technology was quick to employ the proper vaccines to combat the COVID-19 pandemic [
38,
39], antiviral medications were subsequently discovered [
40,
41,
42] and the use of various agents which might aid in fighting the disease gained the attention of the scientific community. Dexamethasone was found to improve the course of the disease and outcome. Vitamin D when insufficient may lead to a worse outcome, whereas, vitamin D administration may improve outcome [
13,
14,
15]. In the present study vitamin D insufficiency was observed in SARS-CoV-2 patients and it was found to be related to disease outcome.
Vitamin D deficiency has been observed in patients with severe SARS-C0V-2 infection [
4,
43,
44,
45,
46,
47,
48,
49,
50]. Bassatne et al. [
44] in a meta-analysis of 31 peer-reviewed observational studies found a positive trend between levels of 25(OH)D
3 <20 ng/ml and an increased risk of mortality, ICU admission, invasive ventilation and non-invasive ventilation. Chiodini et al. [
4] performed a meta-analysis of 54 studies on SARS-CoV-2 infection and vitamin D and they found that severe deficiency, deficiency and insufficiency of vitamin D was related to admission to the intensive care unit, mortality, COVID-19 infection and hospitalization. They concluded that patients with vitamin D deficiency present with an increased risk for the development of acute respiratory distress syndrome requiring admission to the intensive care unit, higher mortality and an increased susceptibility to SARS-CoV-2 infection and hospitalization. In our study we observed low vitamin D levels in patients with severe SARS-CoV-2 infection and a relationship between vitamin D deficiency and insufficiency with the outcome, i.e., fatal outcome as opposed to survival. Vitamin D is an immunomodulatory hormone which acts in a pleiotropic way by both enhancing innate immunity and the response of the organism against various infections and by suppressing the hyperinflammatory response to the viral insult [
51]. Vitamin D has been shown to contribute to the response to various pathogens [
11,
52], especially those attacking the respiratory system [
53]. Thus, vitamin D may enhance the innate immune response against the virus and may dampen the hyperinflammatory reaction against the COVID-19 virus [
51]. Vitamin D enhances the innate immune response against the SARS-CoV-2 virus by activating toll-like receptor 2 and by peptide synthesis, which act to combat infectious invasions [
54]. Vitamin D attenuates the release of proinflammatory cytokines by CD4+ lymphocytes thus inhibiting the development of a cytokine storm [
55]. Additionally, vitamin D has been shown to increase the bioavailability and expression of ACE2 [
56], which acts as a receptor for the virus and may contribute to the trapping and inactivation of the virus [
57].
Vitamin D supplementation has been proposed to play a significant role in the protection of the human organism against the COVID-19 infection. The effect of vitamin D supplementation on COVID-19 prognosis in high-risk older patients was tested in the COVIT-TRIAL, a randomized controlled trial [
58]. In the COVIT-TRIAL [
59], a multicenter, randomized, controlled, open-label study, performed in France, which involved collaboration of several medical centers patients were randomly allocated to either a single oral high-dose (400,000 IU) or standard-dose (50,000 IU) cholecalciferol administered under medical supervision within 72 hours after the diagnosis of COVID-19. The primary outcome was 14 day mortality from any cause. A group of 254 patients with a median age of 88 years met eligibility critera and formed the intention to treat group. Overall, 8 (6%) of 127 patients allocated to high-dose cholecalciferol, and 14 (11%) of 127 patients allocated to standard-dose cholecalciferol died within 14 days, p = 0.049. However, the protective effect of the single oral high-dose administration was not sustained at 28 days, not leading though to more frequent adverse effects as compared to the standard vitamin D dose. Bilezikian et al. [
60] suggested that vitamin D administration should be individualized, according to the needs of the patients and the existing comorbidities, such as kidney and liver disease, which may affect vitamin D metabolism.
Vitamin D levels were found to be inversely related to CRP. This finding has been previously observed [
61] in patients with autoimmunity and vitamin D deficiency [
62] and in patients hospitalized in the acute care unit [
61]. Thus, vitamin D may be a reverse acute phase reactant or a reverse index of the acute phase response [
63]. Ferritin levels were found to be increased as opposed to the expected normal range in patients with severe SARS-CoV-2 disease in this study and a negative association was observed between vitamin D and ferritin levels. Ferritin has been found to be increased in patients with COVID-19 disease and its levels were found to be related to disease severity and disease outcome. In a meta-analysis involving 52 studies the authors [
34] observed higher ferritin levels in patients with severe COVID-19 infection as compared to patients with non-severe infection. COVID-19 has been included in the hyperferritinemic syndromes [
64]. Hyperferritinemia in critically ill COVID-19 patients may be an index of severity as well as a pathogenic mediator [
65]. Ferritin in the context of severe COVID-19 disease may act as a possible enhancer of the cytokine storm and may be involved in a vicious pathogenic loop [
35,
66,
67].
In a retrospective cohort study, the association between serum levels of ferritin, soluble CD163, and IL-18 and their impact on the prognosis of COVID-19 was investigated [
67]
. Data of 70 hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were analyzed. Soluble CD163, ferritin, and IL-18 were measured and the correlation of these parameters with the respiratory deterioration and overall 30-day survival was assessed. A group of 60 patients survived 30 days from hospitalization. There were substantial differences between the subjects who were alive following 30 days compared to those who expired. Higher levels of IL-18 and ferritin were observed in the group with the fatal outcome as compared to those who survived. Soluble CD163, ferritin, and IL-18 were found to correlate with the severity of COVID-19 infection. It was suggested that measuring ferritin and IL-18 during the disease progression may help target them at the right time and refine the decision-making regarding the necessity for hospitalization [
67]
. As found in our study a relationship has been observed between CRP and ferritin as both are increased during an infection.
Viral infections are related with a tendency to develop thrombosis [
68]. The SARS-CoV-2 infection is related with the development of both arterial and venous thrombosis [
69]. In an autopsy study of lungs obtained from patients who died from COVID-19 histologic analysis showed widespread thrombosis with microangiopathy [
26]. Alveolar capillary microthrombi were prevalent in patients who died from COVID-19. In patients with SARS-CoV-2 infection viral effects, the increase in the vasoconstrictor angiotensin II, the decrease in the vasodilator angiotensin and the release of cytokines in the context of sepsis may lead to coagulopathy. Coagulopathy may be observed in as many as 50% of patients with severe COVID-19 disease.
Studies have shown an increase in d-dimer and fibrinogen levels in patients with COVID-19 disease during the course of the illness [
70]. A 3 to 4 fold rise in d-dimer levels may be associated with a poor prognosis [
70]. In a meta-analysis of 29 studies [
71] it was observed that in patients with SARS-CoV-2 infection increased d-dimer levels on admission were related to an increased risk of disease severity and mortality. In our study increased levels of d-dimer and fibrinogen levels were observed in severe SARS-CoV-2 infection as compared with healthy controls. D-dimer and fibrinogen levels were inversely related to the 25(OH)D
3 levels. In a small study short-term high dose vitamin D supplementation induced a significant decrease in fibrinogen concentrations, without however an effect on CRP, d-dimer or ferritin, leading to the conclusion that vitamin D might have an antithrombotic effect [
72].
In conclusion, lower vitamin D levels were observed in patients with severe SARS-CoV-2 infection as compared to control subjects. Vitamin D deficiency and insufficiency was related to disease outcome. Vitamin D levels were inversely related to CRP, ferritin, d-dimer and fibrinogen levels.
4. Materials and Methods
In a cohort of 88 patients, 47 male, 41 female, hospitalized in the COVID-19 Department over a period of 12 months CRP levels, ESR levels, hemoglobin levels, white blood cell count, ferritin levels, fibrinogen levels, prothrombin levels, d-dimer levels and 25(OH)D
3 levels were measured (
Table 1). The levels of 25(OH)D
3 were estimated upon admission to the COVID-19 unit. The levels of 25(OH)D
3 were also measured in a cohort of 88 control subjects matched for age and sex. Patients on treatment with calcium and vitamin D supplements were excluded from the study.
25(OH)D
3 levels were measured by a 1-step delayed chemiluminenescent microparticle immunoassay (Abbott Park IL) [
73]. It is a fully automated immunoassay offered by Abbott on the ARCHITECT platform with a within run CV of 3.0-5.4% and a between run CV of 4.7-6.3%.
CRP was analyzed by particle enhanced immunonephelometry according to the manufacturer’s instructions (CardioPhase hsCRP; Siemens Healthcare Diagnostics, Camberley, UK). Polystyrene particles coated with monoclonal antibodies specific to human CRP were aggregated when mixed with samples containing CRP, using equipment provided by Elite Medical, Marietta, GA, USA. These aggregates scattered a beam of light passed through the sample. The intensity of the scattered light was proportional to the concentration of CRP in the sample. The result was evaluated as compared with a standard of known concentration. The sensitivity of the assay was 0.175 mg/L with a coefficient of variation of 7.6% at 0.41 mg/L. No cross-reactivity is known for the assay. The intra-assay coefficient of variation ranged from 2.7% at 14 mg/L to 4.6% at 5.95 mg/L. The inter-assay coefficient of variation ranged from 2.0% at 14 mg/L to 4.0% at 5.95 mg/L.
TSH levels were measured in serum by the ARCHITECT TSH immunoassay (Abbott Park IL) which is a chemiluminescent microparticle immunoassay with an analytical sensitivity of <0.0025 μIU/ml, a precision of <10% and an interassay coefficient of variation of <20%. The ARCHITECT TSH assay is a two-step immunoassy which utilizes chemiluminescent microparticel immunoassay technology with flexible assay protocols, that are referred to as Chemiflex. In the first step, sample, anti-β TSH antibody coated paramagnetic microparticles and TSH Assay Diluent were combined. TSH present in the sample was bound to the anti-TSH antibody coated microparticles. After washing, anti-α TSH acridinium labeled conjugate was added in the second step. Pre-Trigger and Trigger Solutions were then added to the reaction mixture; the resulting chemiluminescent reaction was measured as relative light units. A direct relationship exists between the amount of TSH in the sample and the relative light units detected by the ARCHITECT i optical system.
Free T3 (FT3) levels were measured by the ARCHITECT FT3 assay which is a chemiluminescent microparticle immunoassay (Abbott Park IL), with an analytical sensitivity of <1.0 pg/ml and an analytical specificity of <0.001%. The ARCHITECT Free T3 assay is a two-step immunoassay for the determination of free T3 in human serum and plasma using Chemiluminescent Microparticle Immunoassay (CMIA) technology with flexible assay protocols. In the first step, sample and anti-T3 coated paramagnetic microparticles were combined. Free T3 (unbound) in the sample binded to the anti-T3 coated microparticles. After washing, T3 acridinium labeled conjugate was added in the second step. Pre-Trigger and Trigger Solutions were then added to the reaction mixture; the resulting chemiluminescent reaction was measured as relative light units. An inverse relationship existed between the amount of FT3 in the sample and the relative light units detected by the ARCHITECT i optical system.
Free T4 (FT4) levels were measured by the ARCHITECT FT4 assay which is a chemiluminescent microparticle immunoassay (Abbott Park IL) with an analytical sensitivity of <0.4 ng/dl and a precision of <10%. The ARCHITECT Free T4 assay is a two-step immunoassay to determine the presence of free thyroxine (Free T4) in human serum and plasma using Chemiluminescent Microparticle Immunoassay (CMIA) technology with flexible assay protocols. In the first step, sample and anti-T4 coated paramagnetic microparticles were combined. FT4 (unbound) present in the sample binded to the anti-T4 coated microparticles. After washing, T4 acridinium labeled conjugate was added in the second step. Pre-Trigger and Trigger Solutions were then added to the reaction mixture; the resulting chemiluminescent reaction was measured as relative light units. An inverse relationship exists between the amount of Free T4 in the sample and the relative light units detected by the ARCHITECT i optical system.
Ferritin levels were analyzed by the Atellica IM Fer assay (Siemens Healthineers), which is a 2-site sandwich immunoassay using direct chemiluminometric technology, which uses constant amounts of 2 anti-ferritin antibodies with an analytical sensitivity of <0.5 ng/ml and a limit of detection of <1.0 ng/ml. The Atellica IM Fer Assay utilizes 2 antiferritin antibodies, the first antibody is a goat polyclonal anti-ferritin antibody labeled with acridium ester and the second antibody is a mouse monoclonal anti-ferritin antibody, which is covalently coupled to paramagnetic particles. The measurement of ferritin was based on the direct relationship which exists between the amount of ferritin present in the sample and the amount of relative light units detected by the system.
D-dimer levels were studied by the Innovance D-Dimer immunoturbidimetric assay (Siemes Healthlineers) with a sensitivity of <0.5 mg/L and a precision, CV% between 5.9-8.4%. The age-adjusted sensitivity of the Innovance D-Dimer immunoturbidometric assay was 98.9% (CI 95%) and the age-adjusted specificity was 77.4% (CI 95%).
Fibrinogen levels were determined by the Multifibren U assay (Siemens Healthineers) which uses a modification of the Clauss method, with a precision, within series CV% of 2.9-7.2%. The Clauss fibrinogen assay is a quantitative, clot-based, functional assay. The assay measures the ability of fibrinogen to form fibrin clot after being exposed to a high concentration of purified thrombin. Multifibren U is an in vitro diagnostic reagent for the quantitative determination of fibrinogen in human sodium citrated plasma by means of automated and manual coagulometric methods. Fibrinogen determination by Multifibren U is standardized against the reference methods by RatnoffMenzie and Kjeldahl [
74]. Multifibren U utilizes a modification of the Clauss method. Citrated plasma was brought to coagulation by a large excess of thrombin. The coagulation time was dependent largely on the fibrinogen content of the specimen.
For the evaluation of the results of the present study 25(OH)D3 as far as disease outcome (survival versus non-survival) was concerned, the levels of 25(OH)D3 were classified as deficiency (0-10 ng/ml), insufficiency (10-20 ng/ml) and sufficiency >20 ng/ml.
The study was approved by the ethical committee of Asclepeion Hospital (approval number 15335, 24/11/2020). Informed consent was obtained from the patients involved in the study.
Statistical evaluation of the results was performed using the SPSS statistical package (IBM SPSS Statistics v27). Data were presented as mean ± SD. Student’s t-test was used to compare the patient group with the control group. Regression analysis was performed to analyse the relationship between 25(OH)D3 levels, CRP, ferritin, d-dimer and fibrinogen levels. Statistical significance was set at a p value of <0.05.
5. Conclusions
In conclusion, low vitamin D levels were observed in patients with severe SARS-CoV-2 infection which were related to the disease outcome and inversely related to ferritin, d-dimer and fibrinogen levels.
Author contributions: Conceptualization, L.A., I.K.A., S.N., A.K.; methodology, L.A, I.K.A, S.N., A.K..; software, O.M.; validation, I.K.A., O.M., P.A. and Y.S.; formal analysis L.A., I.K.A., P.A.; investigation, L.A, S.N., A.K., E.S., C.S..; resources O.M., E.S., C.S..; data curation, L.A., I.K.A., E.S., C.S.; writing—original draft preparation, L.A., I.K.A, Y.S.; writing—review and editing, P.A., Y.S.; visualization, I.K.A., S.N., A.K.; supervision, Y.S.; project administration, I.K.A., P.A., Y.S.; funding acquisition, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Asclepeion Hospital, Voula, Athens, Greece, approval number 15335, 24/11/2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farrag MA, Amer HM, Bhat R, et al. SARS-CoV-2: An Overview of Virus Genetics, Transmission, and Immunopathogenesis. Int J Environ Res Public Health. Jun 10 2021;18(12). [CrossRef]
- Shivalkar S, Pingali MS, Verma A, et al. Outbreak of COVID-19: A Detailed Overview and Its Consequences. Adv Exp Med Biol. 2021;1353:23-45. [CrossRef]
- Bhatti JS, Bhatti GK, Khullar N, Reddy AP, Reddy PH. Therapeutic Strategies in the Development of Anti-viral Drugs and Vaccines Against SARS-CoV-2 Infection. Mol Neurobiol. Nov 2020;57(11):4856-4877. [CrossRef]
- Chiodini I, Gatti D, Soranna D, et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front Public Health. 2021;9:736665. [CrossRef]
- Feentved Ødum SL, Kongsbak-Wismann M. Vitamin D and SARS-CoV-2. Basic Clin Pharmacol Toxicol. Jul 2023;133(1):6-15. [CrossRef]
- D’Ecclesiis O, Gavioli C, Martinoli C, et al. Vitamin D and SARS-CoV2 infection, severity and mortality: A systematic review and meta-analysis. PLoS One. 2022;17(7):e0268396. [CrossRef]
- Boulkrane MS, Ilina V, Melchakov R, et al. COVID-19 Disease and Vitamin D: A Mini-Review. Front Pharmacol. 2020;11:604579. [CrossRef]
- Charoenngam N, Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. Jul 15 2020;12(7). [CrossRef]
- Charoenngam N, Shirvani A, Holick MF. Vitamin D for skeletal and non-skeletal health: What we should know. J Clin Orthop Trauma. Nov-Dec 2019;10(6):1082-1093. 1082. [CrossRef]
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Mycobacterium tuberculosis, autoimmunity, and vitamin D. Clin Rev Allergy Immunol. Apr 2010;38(2-3):169-77. [CrossRef]
- Kim EW, Teles RMB, Haile S, Liu PT, Modlin RL. Vitamin D status contributes to the antimicrobial activity of macrophages against Mycobacterium leprae. PLoS Negl Trop Dis. Jul 2018;12(7):e0006608. [CrossRef]
- Grant WB, Lahore H, McDonnell SL, et al. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. Apr 2 2020;12(4). [CrossRef]
- Torres M, Casado G, Vigón L, et al. Changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D. Biomed Pharmacother. Jun 2022;150:112965. [CrossRef]
- Rawat D, Roy A, Maitra S, Shankar V, Khanna P, Baidya DK. “Vitamin D supplementation and COVID-19 treatment: A systematic review and meta-analysis”. Diabetes Metab Syndr. Jul-Aug 2021;15(4):102189. [CrossRef]
- Bae M, Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules. Nov 16 2020;25(22). [CrossRef]
- Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. Jama. Oct 6 2020;324(13):1307-1316. [CrossRef]
- Andreakos E, Papadaki M, Serhan CN. Dexamethasone, pro-resolving lipid mediators and resolution of inflammation in COVID-19. Allergy. 2021:626-628. vol. 3.
- Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. Feb 2021;76(2):428-455. [CrossRef]
- McPadden J, Warner F, Young HP, et al. Clinical characteristics and outcomes for 7,995 patients with SARS-CoV-2 infection. PLoS One. 2021;16(3):e0243291. [CrossRef]
- Pran L, Baijoo S, Slim H. Viral infection-induced thrombosis, novel coronavirus. J Vasc Surg. Aug 2020;72(2):764-765. [CrossRef]
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. Jun 2020;46(6):1089-1098. [CrossRef]
- Tiwari NR, Phatak S, Sharma VR, Agarwal SK. COVID-19 and thrombotic microangiopathies. Thromb Res. Jun 2021;202:191-198. [CrossRef]
- Bobescu E, Marceanu LG, Covaciu A, Vladau LA. Thrombosis, an important piece in the COVID-19 puzzle: From pathophysiology to therapy. Anatol J Cardiol. Sep 2021;25(9):601-608. [CrossRef]
- Porta-Etessam J, Yus M, González García N, Valcarcel A, Barrado-Cuchillo J, Pérez-Somarriba J. Brain inflammatory thrombogenic vasculopathy related with SARS-CoV-2 infection. Neurologia (Engl Ed). Nov-Dec 2020;35(9):701-703. Vasculopatía trombogénica inflamatoria del cerebro relacionada con la infección del SARS-CoV-2. [CrossRef]
- Nappi F, Nappi P, Gambardella I, Avtaar Singh SS. Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review. Metabolites. Sep 22 2022;12(10). [CrossRef]
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. Jul 9 2020;383(2):120-128. [CrossRef]
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. Jul 2020;95(7):834-847. [CrossRef]
- Qeadan F, Tingey B, Gu LY, Packard AH, Erdei E, Saeed AI. Prognostic Values of Serum Ferritin and D-Dimer Trajectory in Patients with COVID-19. Viruses. Mar 5 2021;13(3). [CrossRef]
- Wu WX, He DR. Low Vitamin D Levels Are Associated With the Development of Deep Venous Thromboembolic Events in Patients With Ischemic Stroke. Clin Appl Thromb Hemost. Dec 2018;24(9_suppl):69s-75s. [CrossRef]
- Mohammad S, Mishra A, Ashraf MZ. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules. Oct 24 2019;9(11). [CrossRef]
- Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. Aug 15 2002;33(4):457-63. [CrossRef]
- McCullough K, Bolisetty S. Iron Homeostasis and Ferritin in Sepsis-Associated Kidney Injury. Nephron. 2020;144(12):616-620. [CrossRef]
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. Nov 1 2017;29(9):401-409. [CrossRef]
- Cheng L, Li H, Li L, et al. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Lab Anal. Oct 2020;34(10):e23618. [CrossRef]
- Mahroum N, Alghory A, Kiyak Z, et al. Ferritin - from iron, through inflammation and autoimmunity, to COVID-19. J Autoimmun. Jan 2022;126:102778. [CrossRef]
- Tsai PH, Lai WY, Lin YY, et al. Clinical manifestation and disease progression in COVID-19 infection. J Chin Med Assoc. Jan 1 2021;84(1):3-8. [CrossRef]
- Yang R, Gui X, Gao S, Ke H, Xiong Y. Clinical progression and changes of chest CT findings among asymptomatic and pre-symptomatic patients with SARS-CoV-2 infection in Wuhan, China. Expert Rev Respir Med. Mar 2021;15(3):411-417. [CrossRef]
- Golob JL, Lugogo N, Lauring AS, Lok AS. SARS-CoV-2 vaccines: a triumph of science and collaboration. JCI Insight. May 10 2021;6(9). 10 May. [CrossRef]
- Patel R, Kaki M, Potluri VS, Kahar P, Khanna D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum Vaccin Immunother. Dec 31 2022;18(1):2002083. [CrossRef]
- Tao K, Tzou PL, Nouhin J, Bonilla H, Jagannathan P, Shafer RW. SARS-CoV-2 Antiviral Therapy. Clin Microbiol Rev. Dec 15 2021;34(4):e0010921. 0010. [CrossRef]
- Imai M, Ito M, Kiso M, et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N Engl J Med. 2023:89-91. vol. 1.
- Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. N Engl J Med. 2022:1475-1477. vol. 15.
- Mohan M, Cherian JJ, Sharma A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. Sep 2020;16(9):e1008874. [CrossRef]
- Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism. Jun 2021;119:154753. [CrossRef]
- Hsieh MC, Hsiao PJ, Liao MT, et al. The Role of Vitamin D in SARS-CoV-2 Infection and Acute Kidney Injury. Int J Mol Sci. Jul 1 2022;23(13). [CrossRef]
- Azzam AY, Ghozy S, Azab MA. Vitamin D and its’ role in Parkinson’s disease patients with SARS-CoV-2 infection. A review article. Interdiscip Neurosurg. Mar 2022;27:101441. [CrossRef]
- Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients. Sep 10 2020;12(9). [CrossRef]
- AlSafar H, Grant WB, Hijazi R, et al. COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents. Nutrients. May 19 2021;13(5). [CrossRef]
- Decyk A, Kobylińska M, Antosik K, Kurowska K. Vitamin D in SARS-CoV-2 infection. Rocz Panstw Zakl Hig. 2022;73(1):5-12. [CrossRef]
- Ferrari D, Locatelli M, Briguglio M, Lombardi G. Is there a link between vitamin D status, SARS-CoV-2 infection risk and COVID-19 severity? Cell Biochem Funct. Jan 2021;39(1):35-47. [CrossRef]
- Peng MY, Liu WC, Zheng JQ, et al. Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D. Int J Mol Sci. May 16 2021;22(10). 16 May. [CrossRef]
- Dennison CL, de Oliveira LB, Fraga LAO, et al. Mycobacterium leprae-helminth co-infections and vitamin D deficiency as potential risk factors for leprosy: A case-control study in south-eastern Brazil. Int J Infect Dis. Apr 2021;105:261-266. [CrossRef]
- Papagni R, Pellegrino C, Di Gennaro F, et al. Impact of Vitamin D in Prophylaxis and Treatment in Tuberculosis Patients. Int J Mol Sci. Mar 31 2022;23(7). [CrossRef]
- Liu WC, Zheng CM, Lu CL, et al. Vitamin D and immune function in chronic kidney disease. Clin Chim Acta. Oct 23 2015;450:135-44. [CrossRef]
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. Jul 2017;39(5):529-539. [CrossRef]
- Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. Nov 2017;16(5):7432-7438. [CrossRef]
- Xu Y, Baylink DJ, Chen CS, et al. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J Transl Med. Aug 26 2020;18(1):322. [CrossRef]
- Annweiler C, Beaudenon M, Gautier J, et al. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. Dec 28 2020;21(1):1031. [CrossRef]
- Annweiler C, Beaudenon M, Gautier J, et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med. May 2022;19(5):e1003999. 20 May. [CrossRef]
- Bilezikian JP, Formenti AM, Adler RA, et al. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev Endocr Metab Disord. Dec 2021;22(4):1201-1218. 1201. [CrossRef]
- Kostoglou-Athanassiou I, Pantazi E, Kontogiannis S, Kousouris D, Mavropoulos I, Athanassiou P. Vitamin D in acutely ill patients. J Int Med Res. Oct 2018;46(10):4246-4257. [CrossRef]
- Kostoglou-Athanassiou I, Athanassiou P, Lyraki A, Raftakis I, Antoniadis C. Vitamin D and rheumatoid arthritis. Ther Adv Endocrinol Metab. Dec 2012;3(6):181-7. [CrossRef]
- Antonelli MJ, Kushner I, Epstein M. The constellation of vitamin D, the acute-phase response, and inflammation. Cleve Clin J Med. Feb 1 2023;90(2):85-89. [CrossRef]
- Perricone C, Bartoloni E, Bursi R, et al. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res. Aug 2020;68(4):213-224. [CrossRef]
- Ruscitti P, Berardicurti O, Di Benedetto P, et al. Severe COVID-19, Another Piece in the Puzzle of the Hyperferritinemic Syndrome. An Immunomodulatory Perspective to Alleviate the Storm. Front Immunol. 2020;11:1130. [CrossRef]
- Cui Y, Zhang YC, Kang YL, Ren YQ, Miao HJ, Wang F. High-Volume Hemofiltration in Critically Ill Patients With Secondary Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome: A Prospective Study in the PICU. Pediatr Crit Care Med. Oct 2016;17(10):e437-e443. [CrossRef]
- . Volfovitch Y, Tsur AM, Gurevitch M, et al. The intercorrelations between blood levels of ferritin, sCD163, and IL-18 in COVID-19 patients and their association to prognosis. Immunol Res. Dec 2022;70(6):817-828. [CrossRef]
- Perkins MV, Joseph SB, Dittmer DP, Mackman N. Cardiovascular Disease and Thrombosis in HIV Infection. Arterioscler Thromb Vasc Biol. Feb 2023;43(2):175-191. [CrossRef]
- Ali MAM, Spinler SA. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc Med. Apr 2021;31(3):143-160. [CrossRef]
- Rostami M, Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. Nov 2020;13(11):1265-1275. [CrossRef]
- Nugroho J, Wardhana A, Maghfirah I, et al. Relationship of D-dimer with severity and mortality in SARS-CoV-2 patients : A meta-analysis. Int J Lab Hematol. Feb 2021;43(1):110-115. [CrossRef]
- Rastogi A, Bhansali A, Khare N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J. Feb 2022;98(1156):87-90. [CrossRef]
- Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. Mar 2012;58(3):531-42. [CrossRef]
- Stang LJ, Mitchell LG. Fibrinogen. Methods Mol Biol. 2013;992:181-92. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).