Submitted:
12 September 2023
Posted:
15 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Unveiling Adiponectin/AdipoRs Physiological Roles
2.1. Unlocking the Mysteries of APN/AdipoRs: A Journey Through Discovery, Structure, and Circulation Forms
2.2. Tissue Distribution, Mechanism, Physiological and Pathophysiological Relevance of APN/AdipoRs Pathway
3. Unlocking the Potential of APN/AdipoRs as Metabolic Regulators in Retinal Diseases
4. Current Understanding of the Pathophysiological Role of APN/AdipoRs in Neovascular AMD
5. Exercise, APN/AdipoRs signaling, and neovascular AMD
6. Adiponectin's antioxidative properties in ocular tissues
7. Unlocking Clarity: Adiponectin's Transformative Quest from Fat Reserves to Optical Resilience
8. Conclusion
8.1. Future directions for research and clinical applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: adiponectin. Nat Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016, 8, 93–100. [CrossRef]
- Tao, C.; Sifuentes, A.; Holland, W. L. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic β cells and adipocytes. Best practice & research. Clinical endocrinology & metabolism, 2014, 28, 43–58. [Google Scholar] [CrossRef]
- Lee, B.; Shao, J. Adiponectin and energy homeostasis. Rev Endocr Metab Disord. 2014, 15, 149–156 https://. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine. 2013, 64, 1–10 https://. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Krishna, A. Adiponectin/AdipoRs signaling as a key player in testicular aging and associated metabolic disorders. Vitam Horm. 2021; 115, 611-634. [CrossRef]
- Singh, A.; Choubey, M.; Bora, P.; and Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reproductive sciences, 2018, 25, 1462–1473. [Google Scholar] [CrossRef]
- Choubey, M.; Bora, P. Emerging Role of Adiponectin/AdipoRs Signaling in Choroidal Neovascularization, Age-Related Macular Degeneration, and Diabetic Retinopathy. Biomolecules. 2023, 13, 982. [Google Scholar] [CrossRef]
- Fu, Z.; Gong, Y.; Löfqvist, C. ; Hellström,A. ; Smith, L.E. Review: adiponectin in retinopathy. Biochim Biophys Acta. 2016, 1862, 1392–1400 https://. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J Mol Med. 2013, 91, 311–321 https://. [Google Scholar] [CrossRef]
- Kuo, J.Z. ; Guo X, Klein R, Klein BE, Genter P, Roll K, Hai Y, Goodarzi MO, Rotter JI, Chen YD, Ipp E. Adiponectin, Insulin Sensitivity and Diabetic Retinopathy in Latinos With Type 2 Diabetes. J Clin Endocrinol Metab. 2015, 100, 3348–55 https://. [Google Scholar] [CrossRef]
- Rahmouni, K.; Haynes, W.G. Endothelial effects of leptin: Implications in health and diseases. Curr Diab Rep. 2005, 5, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I; Sonmez, A.; Acikel, C.; et al. Adiponectin may play a part in the pathogenesis of diabetic retinopathy. Eur J Endocrinol. 2004, 151, 135–140 https://. [Google Scholar] [CrossRef]
- Fan, X. , Wu, Q. , Li, Y., Hao, Y., Ning, N., Kang, Z., Cui, Y., Liu, R., & Han, L. Association between adiponectin concentrations and diabetic retinopathy in patients with type 2 diabetes: a meta-analysis. Chinese medical journal. 2014, 127, 765–771. [Google Scholar]
- Fu, Z.; Löfqvist, C.A.; Liegl, R.; et al. Photoreceptor glucose metabolism determines normal retinal vascular growth. EMBO Mol Med. 2018, 10, 76–90 https://. [Google Scholar] [CrossRef]
- Iwabu, M.; Okada-Iwabu, M.; Yamauchi, T. Adiponectin/adiponectin receptor in disease and aging. npj Aging Mech Dis. 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.S.; Kaliappan, S.; Lyzogubov, V. V, et al. Expression of adiponectin in choroidal tissue and inhibition of laser-induced choroidal neovascularization by adiponectin. FEBS Lett. 2007, 581, 1977–1982 https://. [Google Scholar] [CrossRef]
- Lyzogubov, V.V.; Tytarenko, R.G.; Bora, N.S.; Bora, P.S. Inhibitory role of adiponectin peptide I on rat choroidal neovascularization. Biochim Biophys Acta. 2012, 1823, 1264–1272 https://. [Google Scholar] [CrossRef]
- Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression. Circ Res. 2009, 104, 1058–1065 https://. [CrossRef]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem, 1995, 270, 26746–26749 https://. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996, 271, 10697–10703 https://. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996, 221, 286–289 https://. [Google Scholar] [CrossRef]
- Nakano, Y. ; Tobe, T,; Choi-Miura, N. H.; Mazda, T.; Tomita, M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996, 120, 803–812 https://. [Google Scholar] [CrossRef]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003, 423, 762–769. [CrossRef]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007, 13, 332–339. [CrossRef]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J Mol Cell Biol. 2016, 8, 101–109 https://. [Google Scholar] [CrossRef]
- Tang, Y.T.; Hu, T.; Arterburn, M.; et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005, 61, 372–380 https://. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and Adiponectin Receptors, Endocrine Reviews. 2005; 26. [Google Scholar] [CrossRef]
- Capeau, J. The story of adiponectin and its receptors AdipoR1 and R2: to follow. J Hepatol. 2007, 47, 736–738 https://. [Google Scholar] [CrossRef]
- Zhu, N.; Pankow, J.S.; Ballantyne, C.M.; et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J Clin Endocrinol Metab. 2010, 95, 5097–5104 https://. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999, 257, 79–83 https://. [Google Scholar] [CrossRef] [PubMed]
- Sieminska, L.; Marek, B.; Kos-Kudla, B.; et al. Serum adiponectin in women with polycystic ovarian syndrome and its relation to clinical, metabolic and endocrine parameters. J Endocrinol Invest. 2004, 27, 528–534 https://. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001, 7, 941–946 https://. [Google Scholar] [CrossRef]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes, obesity & metabolism. 2006, 8, 264–280. [Google Scholar] [CrossRef]
- Yoda-Murakami, M.; Taniguchi, M.; Takahashi, K.; Kawamata, S.; Saito, K.; Choi-Miura, N.H.; Tomita, M. Change in expression of GBP28/adiponectin in carbon tetrachloride-administrated mouse liver. Biochemical and biophysical research communications, 2001, 285, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Delaigle, A.M.; Senou, M.; Guiot, Y.; Many, M.C.; Brichard, S.M. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: In vivo and in vitro studies. Diabetologia, 2006, 49, 1311–1323. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. British journal of pharmacology, 2012, 165, 313–327. [Google Scholar] [CrossRef]
- Hopkins, T.A.; Ouchi, N.; Shibata, R.; Walsh, K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007, 74, 11–18 https://. [Google Scholar] [CrossRef]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Baltazar, F.; Martin, L.J.; Krishna, A. Role of adiponectin as a modulator of testicular function during aging in mice. Biochim Biophys Acta Mol Basis Dis. 2019, 1865, 413–427. [Google Scholar] [CrossRef]
- Li, H.Y.; Hong, X.; Cao, Q.Q.; So, K.F. Adiponectin, exercise and eye diseases. International review of neurobiology, 2019, 147, 281–294. [Google Scholar] [CrossRef]
- Luo, N.; Liu, J.; Chung, B.H.; Yang, Q.; Klein, R.L.; Garvey, W.T.; Fu, Y. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes. 2010, 59, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; Suzuki, R. Satoh, H. ; Tsuchida, A.; Moroi, M.; Sugi, K.; Noda, T.; Ebinuma, H.; Ueta, Y.; Kondo, T.; Araki, E.; Ezaki, O.; Nagai, R.; Tobe, K.; Terauchi, Y.; Ueki, K.; Minokoshi, Y.; Kadowaki, T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell metabolism. 2007, 6, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; Jelicks, L.A.; Mehler, M.F.; Hui, D.Y.; Deshaies, Y.; Shulman, G.I.; Schwartz, G.J.; Scherer, P.E. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. The Journal of clinical investigation. 2007, 117, 2621–2637. [Google Scholar] [CrossRef] [PubMed]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Baltazar, F.; and Krishna, A. Direct actions of adiponectin on changes in reproductive, metabolic, and anti-oxidative enzymes status in the testis of adult mice. Gen Comp Endocrinol, 2019, 279, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Choubey, M.; Ranjan, A.; Bora, P.S.; and Krishna, A. Protective role of adiponectin against testicular impairment in high-fat diet/streptozotocin-induced type 2 diabetic mice. 2020, Biochimie, 168, 41–52. [CrossRef]
- Lin, T.; Qiu, Y.; Liu, Y.; Mohan, R.; Li, Q.; Lei, B. Expression of adiponectin and its receptors in type 1 diabetes mellitus in human and mouse retinas. Mol Vis. 2013, 19, 1769–78. [Google Scholar]
- Matsunami, T.; Sato, Y.; Ariga, S.; Sato, T.; Kashimura, H.; Hasegawa, Y.; Yukawa, M. Regulation of oxidative stress and inflammation by hepatic adiponectin receptor 2 in an animal model of nonalcoholic steatohepatitis. International journal of clinical and experimental pathology. 2010, 3, 472–481. [Google Scholar] [PubMed]
- Tsuchida, A.; Yamauchi, T.; Ito, Y.; Hada, Y.; Maki, T.; Takekawa, S.; Kamon, J.; Kobayashi, M.; Suzuki, R.; Hara, K.; Kubota, N.; Terauchi, Y.; Froguel, P.; Nakae, J.; Kasuga, M.; Accili, D.; Tobe, K.; Ueki, K.; Nagai, R.; Kadowaki, T. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. The Journal of biological chemistry. 2004, 279, 30817–30822. [Google Scholar] [CrossRef]
- Nigro, E. , Scudiero, O., Monaco, M. L., Palmieri, A., Mazzarella, G., Costagliola, C., Bianco, A., & Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. BioMed research international, 2014, 658913. [CrossRef]
- Gavrila, A.; Chan, J. L.; Yiannakouris, N.; Kontogianni, M.; Miller, L.C.; Orlova, C.; Mantzoros, C.S. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. The Journal of clinical endocrinology and metabolism, 2003, 88, 4823–4831. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Villuendas, G.; Botella-Carretero, J.I.; Alvarez-Blasco, F.; Sanchón, R.; Luque-Ramírez, M.; San Millán, J.L. Adiponectin and resistin in PCOS: a clinical, biochemical and molecular genetic study. Human reproduction. 2006, 21, 2257–2265. [Google Scholar] [CrossRef]
- Genchi, V.A.; Rossi, E.; Lauriola, C.; D’Oria, R.; Palma, G.; Borrelli, A.; Caccioppoli, C.; Giorgino, F.; Cignarelli, A. Adipose Tissue Dysfunction and Obesity-Related Male Hypogonadism. Int. J. Mol. Sci. 2022, 23, 8194. [Google Scholar] [CrossRef]
- Degawa-Yamauchi, M.; Moss, K.A.; Bovenkerk, J.E.; Shankar, S.S.; Morrison, C.L.; Lelliott, C.J.; Vidal-Puig, A.; Jones, R.; Considine, R.V. Regulation of adiponectin expression in human adipocytes: effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obesity research. 2005, 13, 662–669. [Google Scholar] [CrossRef]
- Ouchi, N.; Kenneth, W. Adiponectin as an anti-inflammatory factor. Clinica chimica acta. 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocrine reviews. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Terauchi, Y.; Yamauchi, T.; Kubota, T.; Moroi, M.; Matsui, J.; Eto, K.; Yamashita, T.; Kamon, J.; Satoh, H.; Yano, W.; Froguel, P.; Nagai, R.; Kimura, S.; Kadowaki, T.; Noda, T. Disruption of adiponectin causes insulin resistance and neointimal formation. The Journal of biological chemistry. 2002, 277, 25863–25866. [Google Scholar] [CrossRef]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; Komuro, R.; Ouchi, N.; Kihara, S.; Tochino, Y.; Okutomi, K.; Horie, M.; Takeda, S.; Aoyama, T.; Funahashi, T.; Matsuzawa, Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature medicine. 2002, 8, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Barbe, A.; Bongrani, A.; Mellouk, N.; Estienne, A. , Kurowska, P. ; Grandhaye, J.; Elfassy, Y.; Levy, R.; Rak, A.; Froment, P.; Dupont, J. Mechanisms of Adiponectin Action in Fertility: An Overview from Gametogenesis to Gestation in Humans and Animal Models in Normal and Pathological Conditions. International journal of molecular sciences. 2019, 20, 1526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Scherer, P.E. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008, 51, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Kikani, C.K.; Riojas, R.A.; Langlais, P.; Wang, L.; Ramos, F.J.; Fang, Q.; Christ-Roberts, C.Y.; Hong, J.Y.; Kim, R.Y.; Liu, F.; Dong, L.Q. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nature cell biology. 2006, 8, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, C.W. Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease. International journal of molecular sciences. 2019, 20, 1782. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Q.; Song, N.; Yan, Z.; Lin, R.; Wu, S.; Jiang, L.; Hong, S.; Xie, J.; Zhou, H.; et al. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat Commun. 2020, 11, 5807. [Google Scholar] [CrossRef]
- Li, Z.; Woo, J.M.; Chung, S.W.; Kwon, M.Y.; Choi, J.S.; Oh, H.J.; Yoon, K.C. Therapeutic effect of topical adiponectin in a mouse model of desiccating stress-induced dry eye. Investigative ophthalmology & visual science. 2013, 54, 155–162. [Google Scholar] [CrossRef]
- Liu, H.; Prokosch, V. Energy Metabolism in the Inner Retina in Health and Glaucoma. International journal of molecular sciences. 2021, 22, 3689. [Google Scholar] [CrossRef]
- Mizukami, Y.; Li, J.; Zhang, X.; Zimmer, M.A.; Iliopoulos, O.; Chung, D.C. Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer research. 2004, 64, 1765–1772. [Google Scholar] [CrossRef]
- Nakahara, T.; Hoshino, M.; Hoshino, S.; Mori, A.; Sakamoto, K.; Ishii, K. Structural and functional changes in retinal vasculature induced by retinal ischemia-reperfusion in rats. Experimental eye research. 2015, 135, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Fisher, C.R.; Kowluru, R.A. Mitochondrial Defects Drive Degenerative Retinal Diseases. Trends in molecular medicine. 2020, 26, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, X.; Guo, X.; Habtetsion, T.G.; Wang, Y.; Xu, X.; Kandala, S.; Li, Q.; Li, H.; Zhang, C.; et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2014, 34, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. BioMed research international. 2014, 801269. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxidative medicine and cellular longevity. 2018, 3420187. [Google Scholar] [CrossRef]
- Wong, B.W.; Marsch, E.; Treps, L.; Baes, M.; Carmeliet, P. Endothelial cell metabolism in health and disease: impact of hypoxia. The EMBO journal. 2017, 36, 2187–2203. [Google Scholar] [CrossRef]
- Omae, T.; Nagaoka, T.; Yoshida, A. Relationship Between Retinal Blood Flow and Serum Adiponectin Concentrations in Patients With Type 2 Diabetes Mellitus. Investigative ophthalmology & visual science. 2015, 56, 4143–4149. [Google Scholar] [CrossRef]
- Li, R.; Du, J.; Yao, Y.; Yao, G.; Wang, X. Adiponectin inhibits high glucose-induced angiogenesis via inhibiting autophagy in RF/6A cells. J Cell Physiol. 2019, 234, 20566–20576. [Google Scholar] [CrossRef]
- Palanisamy, K.; Nareshkumar, R.N.; Sivagurunathan, S.; Raman, R.; Sulochana, K.N.; Chidambaram, S. Anti-angiogenic effect of adiponectin in human primary microvascular and macrovascular endothelial cells. Microvascular research. 2019, 122, 136–145. [Google Scholar] [CrossRef]
- Srinivasan, V.; Sulochana, K.N. Effect of adiponectin on expression of vascular endothelial growth factor and pigment epithelium-derived factor: An in vitro study. The Indian Journal of Pharmacology. 2015, 47, 117–120. [Google Scholar] [CrossRef]
- Mallardo, M.; Costagliola, C.; Nigro, E.; Daniele, A. AdipoRon negatively regulates proliferation and migration of ARPE-19 human retinal pigment epithelial cells. Peptides, 2021, 146, 170676. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Toda, E.; Homma, K.; Guzman, NA.; Nagai, N.; Ogawa, M.; Negishi, K.; Arita, M.; Tsubota, K.; Ozawa, Y. ADIPOR1 deficiency-induced suppression of retinal ELOVL2 and docosahexaenoic acid levels during photoreceptor degeneration and visual loss. Cell Death Dis. 2021, 12, 458. [Google Scholar] [CrossRef]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018 Jun;36(5):411-420. [CrossRef]
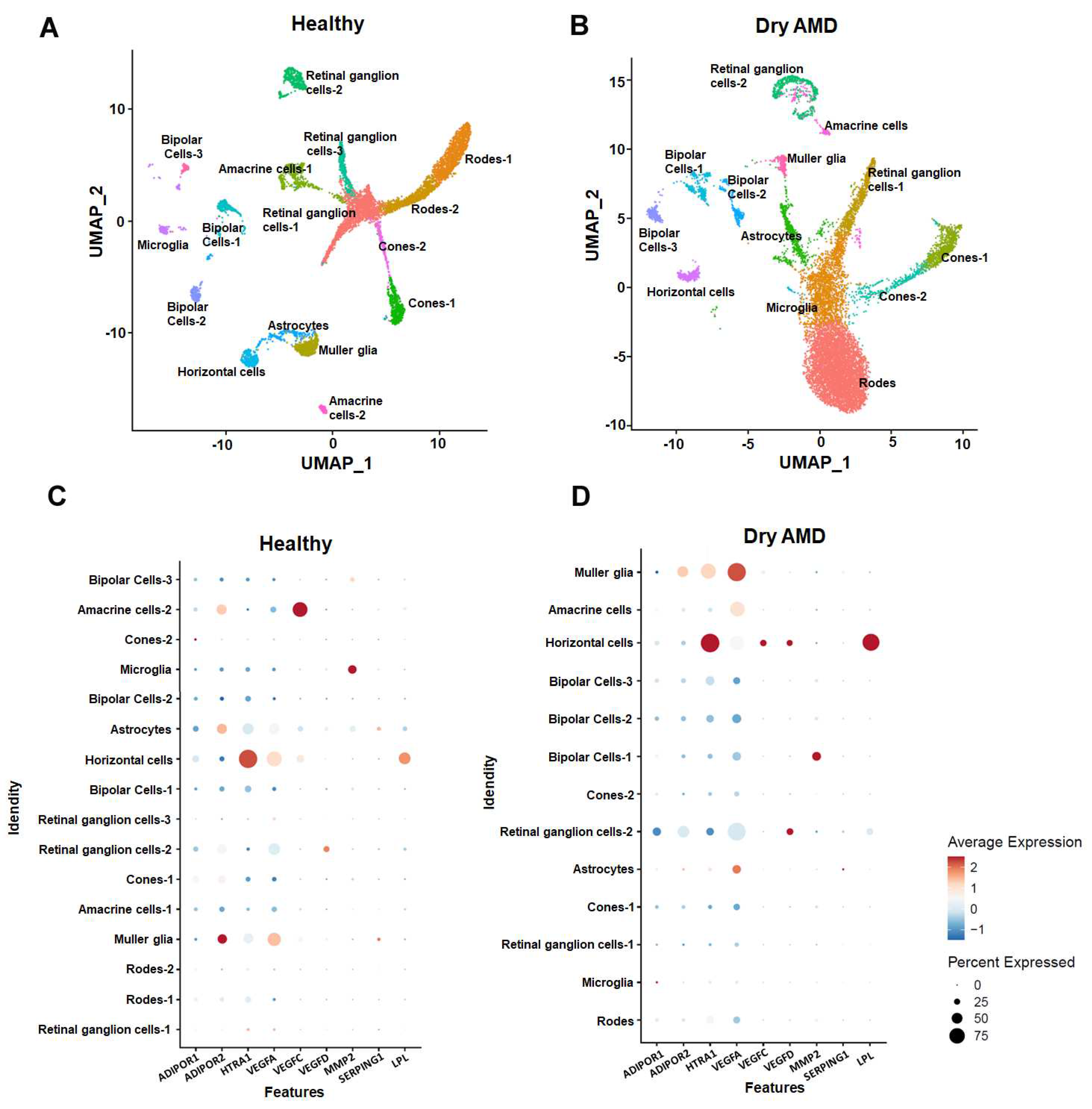
- Kuchroo M, DiStasio M, Song E, Calapkulu E, Zhang L, Ige M, Sheth AH, Majdoubi A, Menon M, Tong A, Godavarthi A, Xing Y, Gigante S, Steach H, Huang J, Huguet G, Narain J, You K, Mourgkos G, Dhodapkar RM, Hirn MJ, Rieck B, Wolf G, Krishnaswamy S, Hafler BP. Single-cell analysis reveals inflammatory interactions driving macular degeneration. Nat Commun. 2023 ;14(1):2589. /: https, 5 May. [CrossRef]
- Wu, K.H; Madigan, M.C.; Billson, F.A.; Penfold, P.L. Differential expression of GFAP in early v late AMD: a quantitative analysis. The British journal of ophthalmology, 2003, 87, 1159–1166. [Google Scholar] [CrossRef]
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol, 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Bushra, S.; Al-Sadeq, DW.; Bari, R.; Sahara, A.; Fadel, A.; Rizk, N. Adiponectin Ameliorates Hyperglycemia-Induced Retinal Endothelial Dysfunction, Highlighting Pathways, Regulators, and Networks. J Inflamm Res. 2022, 15, 3135–3166. [Google Scholar] [CrossRef]
- Sakaue TA, Fujishima Y, Fukushima Y, Tsugawa-Shimizu Y, Fukuda S, Kita S, Nishizawa H, Ranscht B, Nishida K, Maeda N, Shimomura I. Adiponectin accumulation in the retinal vascular endothelium and its possible role in preventing early diabetic microvascular damage. Sci Rep. 2022, 12, 4159. [CrossRef]
- Kaur, G.; Sharma, D.; Bisen, S.; Mukhopadhyay, C.S.; Gurdziel, K.; Singh, N.K. Vascular cell-adhesion molecule 1 (VCAM-1) regulates JunB-mediated IL-8/CXCL1 expression and pathological neovascularization. Communications biology. [CrossRef]
- Fu, Z.; Liegl, R.; Wang, Z.; Gong, Y.; Liu, C.H.; Sun, Y.; Cakir, B.; Burnim, S.B.; Meng, S.S.; Löfqvist, C.; et al. Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice. Investigative ophthalmology & visual science. 2017, 58, 3862–3870. [Google Scholar] [CrossRef]
- Logan, C.; Lyzogubov, V.; Bora, N.; Bora, P. Role of Adiponectin Peptide I (APNp1) in Age-Related Macular Degeneration. Biomolecules. 2022, 12, 1232. [Google Scholar] [CrossRef]
- Bora, P.S. New Discoveries in Retinal Cell Degeneration and Retinal Diseases. Biomolecules, 2023, 13, 1121. [Google Scholar] [CrossRef]
- Cui, B.; Guo, X.; Zhou, W.; Zhang, X.; He, K.; Bai, T.; Lin, D.; Wei-Zhang, S.; Zhao, Y.; Liu, S.; Zhou, H.; Wang, Q.; Yao, X.; Shi, Y.; Xie, R.; Dong, X.; Lei, Y.; Du, M.; Chang, Y.; Xu, H.; Yan, H. Exercise alleviates neovascular age-related macular degeneration by inhibiting AIM2 inflammasome in myeloid cells. Metabolism: clinical and experimental, 2023, 144, 155584. [Google Scholar] [CrossRef] [PubMed]
- 89. Kushwah, N;, Bora, K.; Maurya, M.; Pavlovich, M.C.; & Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants (Basel, Switzerland), 2023; 12, 1379. [CrossRef]
- Deng, H. , Ai, M., Cao, Y., Cai, L., Guo, X., Yang, X., Yi, G., & Fu, M. Potential Protective Function of Adiponectin in Diabetic Retinopathy. Ophthalmology and therapy, 2023; 12, 1519–1534. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




