Submitted:
13 September 2023
Posted:
15 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
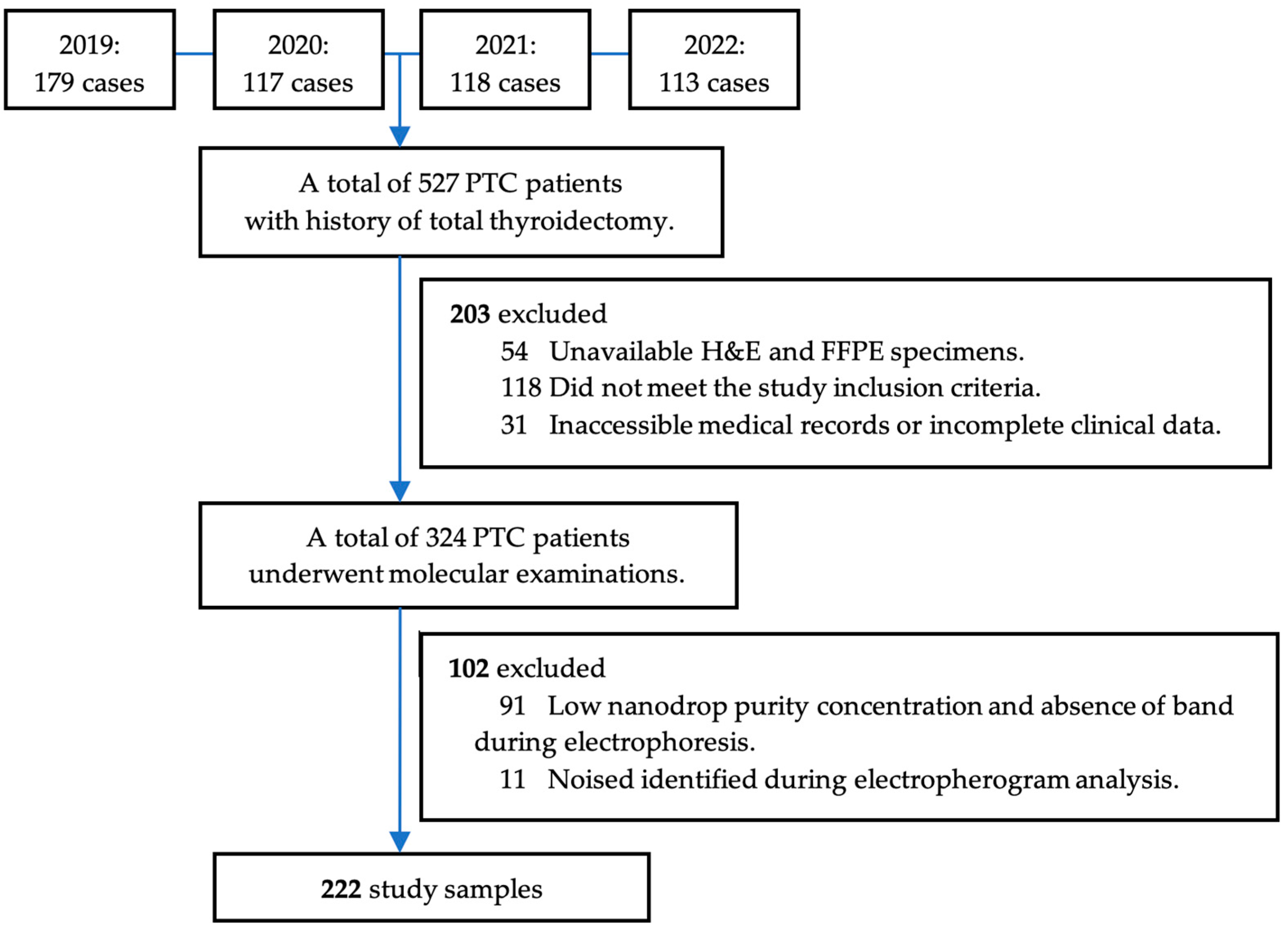
2.1. Study Design and Population
2.3. Mutational Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline characteristics
3.2. Bivariate analysis: correlation between clinico-histopathology characteristics with BRAFV600E and RAS mutational status
3.3. Multivariate analysis: establishing the BRAFV600E prediction model
3.4. Multivariate analysis: establishing the RAS mutation prediction model
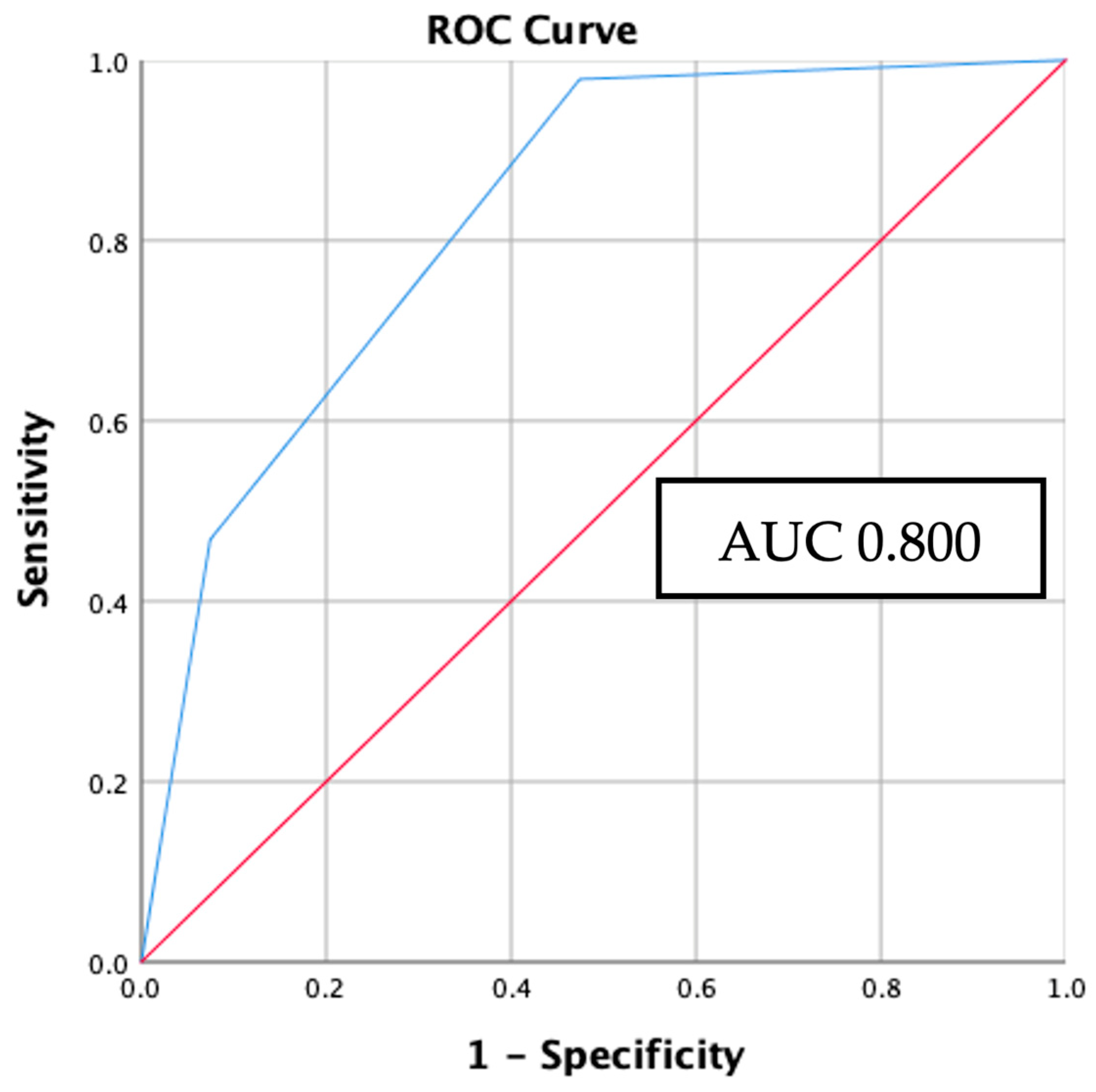
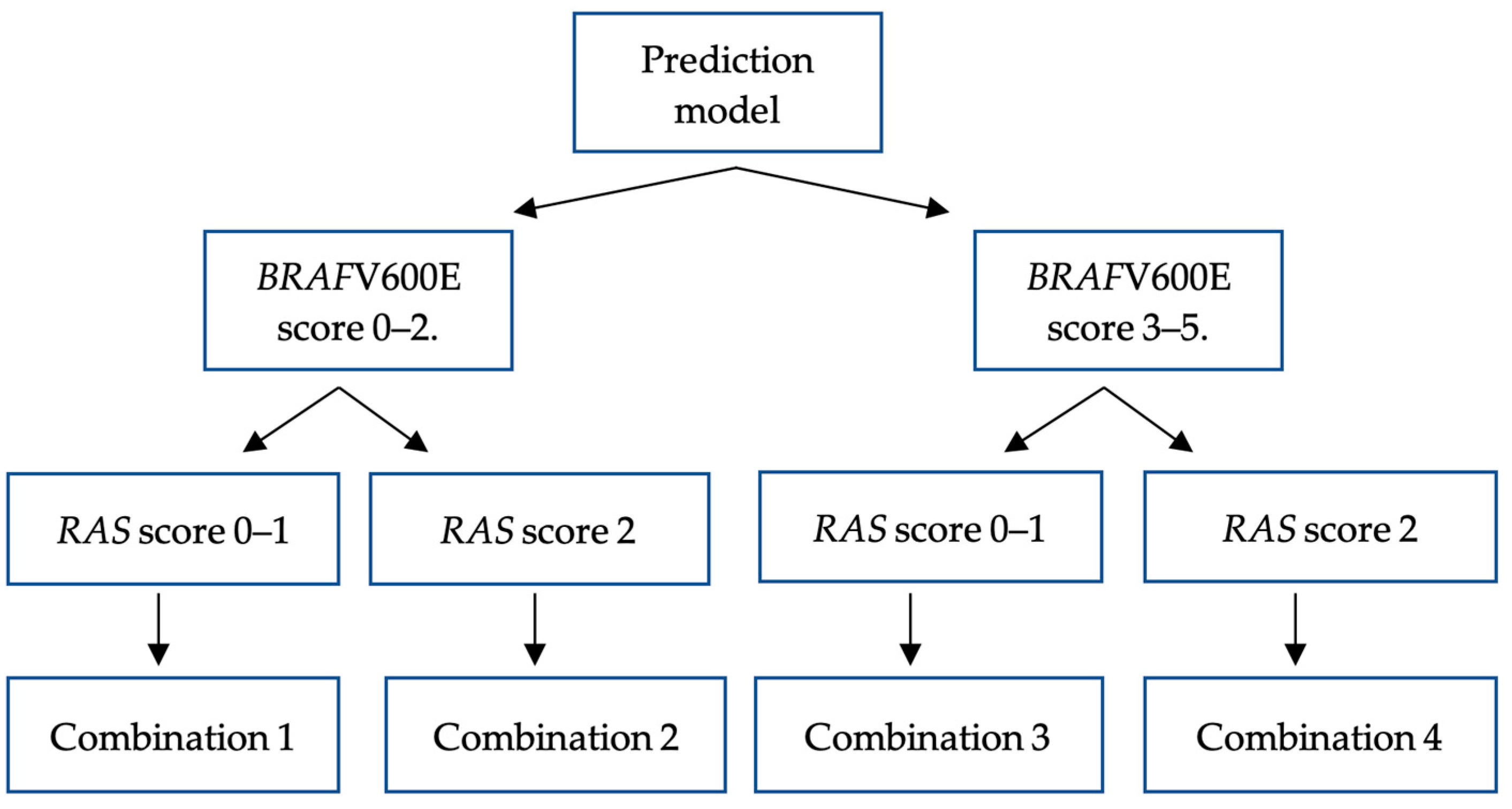
3.5. Internal validation: applicating BRAFV600E and RAS mutation prediction model to study samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Olson, E.; Wintheiser, G.; Wolfe, K.M.; Droessler, J.; Silberstein, P.T. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000-2013. Cureus 2019, 11, e4127–e4127. [Google Scholar] [CrossRef]
- Agrawal, N.; Akbani, R.; Aksoy, B.A.; Ally, A.; Arachchi, H.; Asa, S.L.; Auman, J.T.; Balasundaram, M.; Balu, S.; Baylin, S.B.; Behera, M.; Bernard, B.; Zmuda, E.; Zou, L. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.; Little, M.P.; Lubin, J.H.; Brenner, A. V; Wells, S.A.; Sigurdson, A.J.; Nikiforov, Y.E. The Increase in Thyroid Cancer Incidence during the Last Four Decades Is Accompanied by a High Frequency of BRAF Mutations and a Sharp Increase in RAS Mutations. J Clin Endocrinol Metab 2014, 99, E276–85. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF Mutation in Thyroid Cancer. Endocr Relat Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wei, B.; Shen, H.; Gao, Y.; Wang, L.; Liu, H. BRAF Mutation in Papillary Thyroid Carcinoma (PTC) and Its Association with Clinicopathological Features and Systemic Inflammation Response Index (SIRI). Am J Transl Res 2018, 10, 2726–2736. [Google Scholar]
- Brehar, A.C.; Brehar, F.M.; Bulgar, A.C.; Dumitrache, C. Genetic and Epigenetic Alterations in Differentiated Thyroid Carcinoma. J Med Life 2013, 6, 403–408. [Google Scholar]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS Mutations in Thyroid Benign Nodules and Non-Medullary Thyroid Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Soares, P.; Trovisco, V.; Rocha, A.S.; Lima, J.; Castro, P.; Preto, A.; Máximo, V.; Botelho, T.; Seruca, R.; Sobrinho-Simões, M. BRAF Mutations and RET/PTC Rearrangements Are Alternative Events in the Etiopathogenesis of PTC. Oncogene 2003, 22, 4578–4580. [Google Scholar] [CrossRef]
- Wang, P.; Han, L.; Yu, M.; Cao, Z.; Li, X.; Shao, Y.; Zhu, G. The Prognostic Value of PERK in Cancer and Its Relationship with Immune Cell Infiltration. Front Mol Biosci 2021, 8, 648752. [Google Scholar] [CrossRef]
- Wan, P.T. C.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; Marais, R.; Cancer Genome Project. Mechanism of Activation of the RAF-ERK Signaling Pathway by Oncogenic Mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Cao, Y.-M.; Zhang, T.-T.; Li, B.-Y.; Qu, N.; Zhu, Y.-X. Prognostic Evaluation Model for Papillary Thyroid Cancer: A Retrospective Study of 660 Cases. Gland Surg 2021, 10, 2170–2179. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; Robenshtok, E.; Fagin, J.A.; Puxeddu, E.; Fugazzola, L.; Czarniecka, A.; Jarzab, B.; O’Neill, C.J.; Sywak, M.S.; Lam, A.K.; Riesco-Eizaguirre, G.; Santisteban, P.; Nakayama, H.; Tufano, R.P.; Pai, S.I.; Zeiger, M.A.; Westra, W.H.; Clark, D.P.; Clifton-Bligh, R.; Sidransky, D.; Ladenson, P.W.; Sykorova, V. Association between BRAF V600E Mutation and Mortality in Patients with Papillary Thyroid Cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef]
- 13. WHO Classification of Tumours Editorial Board. Endocrine and neuroendocrine tumours, /: International Agency for Research on Cancer. https, 2023.
- Li, C.; Lee, K.C.; Schneider, E.B.; Zeiger, M.A. BRAF V600E Mutation and Its Association with Clinicopathological Features of Papillary Thyroid Cancer: A Meta-Analysis. Journal of Clinical Endocrinology and Metabolism 2012, 97, 4559–4570. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, A.J.; Zhu, Z.; Gandhi, M.; Steward, D.L.; Fidler, J.P.; Giordano, T.J.; Biddinger, P.W.; Nikiforov, Y.E. Correlation between Genetic Alterations and Microscopic Features, Clinical Manifestations, and Prognostic Characteristics of Thyroid Papillary Carcinomas. Am J Surg Pathol 2006, 30, 216–222. [Google Scholar] [CrossRef]
- Jung, C.K.; Bychkov, A.; Song, D.E.; Kim, J.H.; Zhu, Y.; Liu, Z.; Keelawat, S.; Lai, C.R.; Hirokawa, M.; Kameyama, K.; Kakudo, K. Molecular Correlates and Nuclear Features of Encapsulated Follicular-Patterned Thyroid Neoplasms. Endocrinology and Metabolism 2021, 36, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Ricarte-Filho, J.; Knauf, J.; Shaha, A.; Tuttle, M.; Fagin, J.A.; Ghossein, R.A. Molecular Genotyping of Papillary Thyroid Carcinoma Follicular Variant According to Its Histological Subtypes (Encapsulated vs Infiltrative) Reveals Distinct BRAF and RAS Mutation Patterns. Modern Pathology 2010, 23, 1191–1200. [Google Scholar] [CrossRef]
- Schulten, H.-J.; Salama, S.; Al-Ahmadi, A.; Al-Mansouri, Z.; Mirza, Z.; Al-Ghamdi, K.; Al-Hamour, O.A.; Huwait, E.; Gari, M.; Al-Qahtani, M.H.; Al-Maghrabi, J. Comprehensive Survey of HRAS, KRAS, and NRAS Mutations in Proliferative Thyroid Lesions from An Ethnically Diverse Population. Anticancer Res 2013, 33. [Google Scholar]
- Hara, H.; Fulton, N.; Yashiro, T.; Ito, K.; DeGroot, L.J.; Kaplan, E.L. N-Ras Mutation: An Independent Prognostic Factor for Aggressiveness of Papillary Thyroid Carcinoma. Surgery 1994, 116, 1010–1016. [Google Scholar]
- Odate, T.; Oishi, N.; Vuong, H.G.; Mochizuki, K.; Kondo, T. Genetic Differences in Follicular Thyroid Carcinoma between Asian and Western Countries: A Systematic Review. Gland Surg 2020, 9, 1813–1826. [Google Scholar] [CrossRef]
- Gomes, C.C.; Gayden, T.; Bajic, A.; Harraz, O.F.; Pratt, J.; Nikbakht, H.; Bareke, E.; Diniz, M.G.; Castro, W.H.; St-Onge, P.; Sinnett, D.; Han, H.; Rivera, B.; Mikael, L.G.; De Jay, N.; Kleinman, C.L.; Valera, E.T.; Bassenden, A. V; Berghuis, A.M.; Majewski, J.; Nelson, M.T.; Gomez, R.S.; Jabado, N. TRPV4 and KRAS and FGFR1 Gain-of-Function Mutations Drive Giant Cell Lesions of the Jaw. Nat Commun 2018, 9. [Google Scholar] [CrossRef]
- Harahap, A.S.; Subekti, I.; Panigoro, S.S.; Asmarinah; Lisnawati; Werdhani, R.A.; Agustina, H.; Khoirunnisa, D.; Mutmainnah, M.; Salinah; Siswoyo, A.D.; Ham, M.F. Profile of BRAFV600E, BRAFK601E, NRAS, HRAS, and KRAS Mutational Status, and Clinicopathological Characteristics of Papillary Thyroid Carcinoma in Indonesian National Referral Hospital. Appl Clin Genet 2023, 16, 99–110. [CrossRef]
- Yip, L.; Nikiforova, M.N.; Yoo, J.Y.; McCoy, K.L.; Stang, M.T.; Armstrong, M.J.; Nicholson, K.J.; Ohori, N.P.; Coyne, C.; Hodak, S.P.; Ferris, R.L.; LeBeau, S.O.; Nikiforov, Y.E.; Carty, S.E. Tumor Genotype Determines Phenotype and Disease-Related Outcomes in Thyroid Cancer: A Study of 1510 Patients. Ann Surg 2015, 262, 519–525; [Google Scholar] [CrossRef] [PubMed]
- Maik-Rachline, G.; Hacohen-Lev-Ran, A.; Seger, R. Nuclear ERK: Mechanism of Translocation, Substrates, and Role in Cancer. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Lee, A.W.; Mendoza, R.A.; Aman, S.; Hsu, R.; Liu, L. Thyroid Cancer Incidence Disparities among Ethnic Asian American Populations, 1990–2014. Ann Epidemiol 2022, 66, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.K.; Bychkov, A.; Song, D.E.; Kim, J.-H.; Zhu, Y.; Liu, Z.; Keelawat, S.; Lai, C.-R.; Hirokawa, M.; Kameyama, K.; Kakudo, K. Molecular Correlates and Nuclear Features of Encapsulated Follicular-Patterned Thyroid Neoplasms. Endocrinol Metab (Seoul) 2021, 36, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; Schuff, K.G.; Sherman, S.I.; Sosa, J.A.; Steward, D.L.; Tuttle, R.M.; Wartofsky, L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Pessôa-Pereira, D.; Medeiros, M.F. da S.; Lima, V.M. S.; Silva, J.C. da; Cerqueira, T.L. de O.; Silva, I.C. da; Fonseca, L.E.; Sampaio, L.J. L.; Lima, C.R. A. de; Ramos, H.E. Association between BRAF (V600E) Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma: A Brazilian Single-Centre Case Series. Arch Endocrinol Metab 2019, 63, 97–106. [Google Scholar] [CrossRef]
- Silver, J.A.; Bogatchenko, M.; Pusztaszeri, M.; Forest, V.-I.; Hier, M.P.; Yang, J.W.; Tamilia, M.; Payne, R.J. BRAF V600E Mutation Is Associated with Aggressive Features in Papillary Thyroid Carcinomas ≤ 1.5 Cm. Journal of Otolaryngology - Head & Neck Surgery 2021, 50. [Google Scholar] [CrossRef]

| Characteristics |
BRAFV600E N = 64 (%) |
Control N = 116 (%) |
p | OR | 95% CI | |
|---|---|---|---|---|---|---|
|
Clinical features Age (years) ≥ 55 < 55 |
17 (35.4) 47 (35.6) |
31 (64.4) 85 (64.4) |
0.981a |
0.992 1.000 |
0.497–1.978 Reference |
|
| Gender Man Woman |
18 (36.0) 46 (35.4) |
32 (64.0) 84 (64.6) |
0.938a |
1.027 1.000 |
0.520–2.028 Reference |
|
| Clinical stage Clinical stage IV Clinical stage III Clinical stage II Clinical stage I |
7 (58.3) 1 (20) 13 (43.3) 43 (32.3) |
5 (41.7) 4 (80) 17 (56.7) 90 (67.7) |
0.114b |
2.930 0.523 1.601 1.000 |
0.879–9.766 0.057–4.824 0.713–3.592 Reference |
|
| Stage group Late stage (III–IV) Early stage (I–II) |
8 (47.1) 56 (34.4) |
9 (52.9) 107 (65.6) |
0.298a |
1.698 1.000 |
0.621–4.643 Reference |
|
|
Histopathology features Tumor size (cm) ≥ 4 < 4 |
16 (34.8) 48 (35.8) |
30 (64.2) 86 (65.2) |
0.899a |
0.956 1.000 |
0.474–1.928 Reference |
|
| Nuclear score 3 2 |
56 (43.4) 8 (15.7) |
73 (56.6) 43 (84.3) |
< 0.001a |
4.123 1.000 |
1.796–9.466 Reference |
|
| Capsule Absent Present |
52 (44.1) 12 (19.4) |
66 (55.9) 50 (80.6) |
< 0.001a |
3.283 1.000 |
1.586–6.794 Reference |
|
| Histology variant Solid Oncocytic Classic Tall cell Follicular |
2 (33.3) 1 (10) 21 (37.5) 32 (60.4) 8 (14.5) |
4 (66.7) 9 (90) 35 (62.5) 21 (39.6) 47 (85.5) |
< 0.001b |
2.938 0.653 3.525 8.952 1.000 |
0.459–18.786 0.072–5.878 1.399–8.885 3.532–22.690 Reference |
|
| Histology group Aggressive Non-aggressive |
35 (50.7) 29 (26.1) |
34 (49.3) 82 (73.9) |
< 0.001a |
2.911 1.000 |
1.544–5.488 Reference |
|
| Multifocality Present Absent |
50 (35.7) 14 (35.0) |
90 (64.3) 26 (65.0) |
0.934a |
1.032 1.000 |
0.494–2.154 Reference |
|
| Lymphovascular invasion Present Absent |
31 (43.1) 33 (30.6) |
41 (56.9) 75 (69.4) |
0.086a |
1.718 1.000 |
0.924–3.197 Reference |
|
| Extrathyroidal extension Present Absent |
28 (49.1) 36 (29.3) |
29 (50.9) 87 (70.7) |
0.010a |
2.333 1.000 |
1.220–4.463 Reference |
|
| Nodes metastases Present Absent |
34 (47.2) 30 (27.8) |
38 (52.8) 78 (72.2) |
0.008a |
2.326 1.000 |
1.244–4.349 Reference |
|
| pERK1/2 expression High (>10%) Low (<10%) |
25 (51) 39 (29.8) |
24 (49) 92 (70.2) |
0.008a |
2.457 |
1.253–4.820 Reference |
|
| Characteristics |
RAS mutation N = 42 (%) |
Control N = 116 (%) |
p | OR | 95% CI |
|---|---|---|---|---|---|
|
Clinical factors Age (years) < 55 ≥ 55 |
31 (26.7) 11 (26.2) |
85 (73.3) 31 (73.8) |
0.947a |
1.028 1.000 |
0.461–2.291 Reference |
| Gender Woman Man |
32 (27.6) 10 (23.8) |
84 (72.4) 32 (76.2) |
0.635a |
1.219 1.000 |
0.538–2.764 Reference |
| Clinical stage Clinical stage I Clinical stage II Clinical stage III Clinical stage IV |
33 (26.8) 5 (22.7) 0 (0) 4 (44.4) |
90 (73.2) 17 (77.3) 4 (100) 5 (55.6) |
0.981b |
0.458 0.368 0.556 1.000 |
0.116–1.811 0.071–1.915 0.310–0.997 Reference |
| Stage group Early stage (I–II) Late stage (III–IV) |
38 (26.2) 4 (30.8) |
107 (73.8) 9 (69.2) |
0.721a |
0.799 1.000 |
0.232–2.746 Reference |
|
Histopathology factors Tumor size (cm) < 4 ≥ 4 |
32 (27.1) 10 (25.0) |
86 (72.9) 30 (75.0) |
0.696a |
1.116 1.000 |
0.490–2.541 Reference |
| Nuclear score 2 3 |
19 (30.6) 23 (24.0) |
43 (69.4) 73 (76.0) |
0.353a |
1.402 1.000 |
0.686–2.867 Reference |
| Capsule Present Absent |
22 (30.6) 20 (23.3) |
50 (69.4) 66 (76.7) |
0.302a |
1.452 1.000 |
0.715–2.948 Reference |
| Histology variant Follicular Solid Oncocytic Tall cell Classic |
32 (40.5) 1 (20) 0 (0) 0 (0) 9 (20.5) |
47 (59.5) 4 (80) 9 (100) 21 (100) 35 (79.5) |
< 0.001b |
2.648 0.972 1.257 1.257 1.000 |
1.121–6.253 0.960–9.799 1.082–1.460 1.082–1.460 Reference |
| Histology group Non-aggressive Aggressive |
41 (33.3) 1 (2.9) |
82 (66.7) 34 (97.1) |
< 0.001a |
17.000 1.000 |
2.247–128.615 Reference |
| Multifocality Present Absent |
28 (23.7) 14 (35.0) |
90 (76.3) 26 (65.0) |
0.163a |
0.578 1.000 |
0.266–1.255 Reference |
| Lymphovascular invasion Present Absent |
15 (25.4) 27 (26.5) |
41 (73.2) 75 (73.5) |
0.966a |
1.016 1.000 |
0.486–2.124 Reference |
| Extrathyroidal extension Absent Present |
35 (28.5) 7 (19.4) |
87 (71.3) 29 (80.6) |
0.270a |
1.667 1.000 |
0.668–4.157 Reference |
| Nodes metastasis Absent Present |
26 (25.0) 16 (29.6) |
78 (75.0) 38 (70.4) |
0.532a |
0.792 1.00 |
0.380–1.649 Reference |
| pERK1/2 expression High (>10%) Low (<10%) |
28 (53.8) 14 (13.2) |
24 (46.2) 92 (86.8) |
< 0.001a |
7.667 |
3.503-16.778 Reference |
| Variables | B coefficient | SE | Wald | p | adjOR | 95% CI | B/SE | Score |
|---|---|---|---|---|---|---|---|---|
| Nuclear score (3) | 1.213 | 0.480 | 6.375 | 0.012 | 3.364 | 1.312 – 8.626 | 2.527 | 1 |
| Capsule (absent) | 0.975 | 0.412 | 5.605 | 0.018 | 2.651 | 1.183 – 5.941 | 2.366 | 1 |
| Histology group (aggressive) | 0.858 | 0.375 | 5.218 | 0.022 | 2.358 | 1.130 – 4.921 | 2.288 | 1 |
| pERK1/2 (>10%) | 1.460 | 0.410 | 12.668 | < 0.001 | 4.308 | 1.927 – 9.627 | 3.560 | 2 |
| Total score | Probability | Sensitivity | Specificity |
|---|---|---|---|
| 0 | 5% | 100% | 0% |
| 1 | 12.33% | 100% | 12% |
| 2 | 25.25% | 95% | 39% |
| 3 | 43% | 63% | 65% |
| 4 | 62% | 30% | 94% |
| 5 | 82% | 14% | 100% |
| Variables | B coefficient | SE | Wald | p | adjOR | 95% CI | B/SE | Score |
|---|---|---|---|---|---|---|---|---|
| pERK1/2 (>10%) | 2.101 | 0.430 | 23.865 | < 0.001 | 8.171 | 3.518 – 18.981 | 4.886 | 1 |
| Variant (follicular) | 1.628 | 0.454 | 12.877 | < 0.001 | 5.092 | 2.092 – 12.387 | 3.585 | 1 |
| Total score | Probability | Sensitivity | Specificity |
|---|---|---|---|
| 0 | 5% | 100% | 0% |
| 1 | 27% | 98% | 48% |
| 2 | 70% | 45% | 91% |
| Models outcome |
BRAF- V600E n (%) |
p | adj OR |
95% CI |
RAS n (%) |
p | adj OR |
95% CI | Control n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Comb. 1 | 22 (34.4) | Ref | Ref | Ref | 14 (33.3) | Ref | 1.00 | Ref | 68 (58.6) |
| Comb. 2 | 2 (3.1) | 0.882 | 0.883 | 0.171–4.568 | 7 (16.7) | 0.010 | 4.857 | 1.470–16.049 | 7 (6) |
| Comb. 3 | 37 (57.8) | < 0.001 | 3.091 | 1.594–5.995 | 9 (21.4) | 0.725 | 1.181 | 0.467–2.989 | 37 (31.9) |
| Comb. 4 | 3 (4.7) | 0.295 | 2.318 | 0.481–11.168 | 12 (28.6) | < 0.001 | 14.571 | 4.095–51.855 | 4 (3.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





