1. Introduction
Idiosyncratic drug induced liver injury (DILI) is an upredictable reaction of exposed individual on a certain drug, with a variable latency and wide spectrum of clinical and patohistological presentations, ranging from mild asymptomatic liver injury to the acute liver failure requiring transplantation [
1]. According to the results of the Spanish DILI Registry from 2021, herbal and dietary products hold responsibility for approximately 3.4% of DILI cases. According to the US Drug-Induced Liver Injury Network (DILIN) and the Iceland study, these rates were significantly higher in previous decades when they reached 16% [
2,
3,
4].
Drug-induced autoimmune hepatitis (DIAIH) presents DILI phenotype that mimics idiopathic autoimmune hepatitis when considering the clinical, biochemical, serological and histological parameters [
1]. Herein we present a case of a 48-year-old male that was hospitalized due to severe hepatocellular liver injury two months after the self-treatment with the muscle-building dietary supplement.
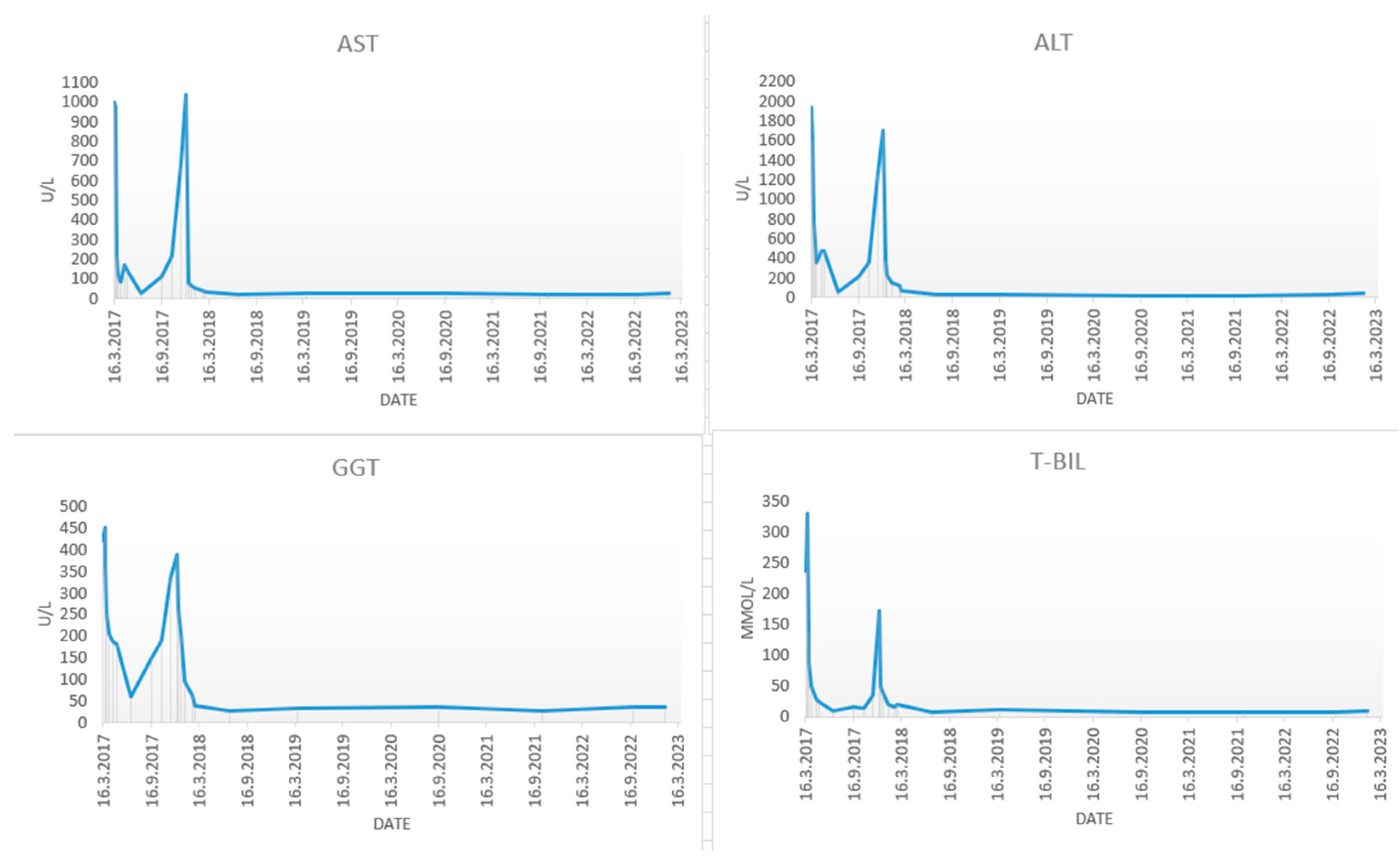
In March 2017, a 48-year-old male was admitted to the Department of gastroenterology and hepatology due to severe liver injury. He had no previous liver or other chronic diseases. Patient reported recently taking a dietary supplement for muscle mass growth based on arginine-alpha-ketoglutarate, L-citrulline, L-tyrosine, creatine malate and beet extract. Upon admission he presented with malaise and icterus. Laboratory parameters revealed increased bilirubin and aminotransferase levels, with normal alkaline phosphatase (
Figure 1).
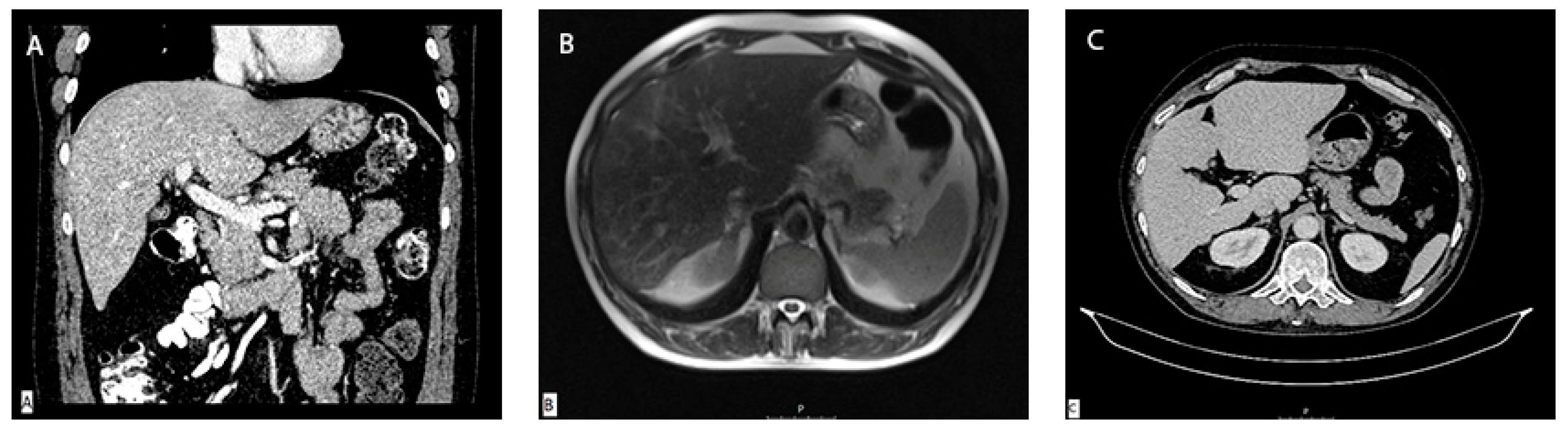
Diagnostic panel excluded acute viral hepatitis as well as metabolic diseases, and the patient denied alcohol consumption or treatment with any concomitant drugs or supplements. Immunology panel revealed positive antinuclear (ANA), anti-mitochondrial (AMA) and AMA-M2 antibodies with increased immunoglobulin G (IgG) levels. Liver sonography and abdominal MSCT scan were ordinary (
Figure 2A). He was treated with corticosteroid therapy and ursodeoxycholic acid (UDCA) followed by a significant decrease in laboratory parameters. Calculated Roussel Uclaf Causality Assessment Method (RUCAM) score was 5, indicating the possible causality with the aforementioned supplement.
He was perceived as a hepatocellular DILI and further treated as out-hospital patient with tapering doses of corticosteroid therapy. In June 2017., three months after the admission, corticosteroid therapy was withdrawn due to complete normalization of laboratory parameters. In September 2017., an increase in aminotransferase levels was detected and the corticosteroid therapy was initiated again. In October 2017, liver MR was performed and described a rough structure of the right liver lobe, more pronounced in the peripheral zones, where coarser thickened septa with initial retraction of the liver parenchyma were observed, suggesting the initial cirrhotic changes (
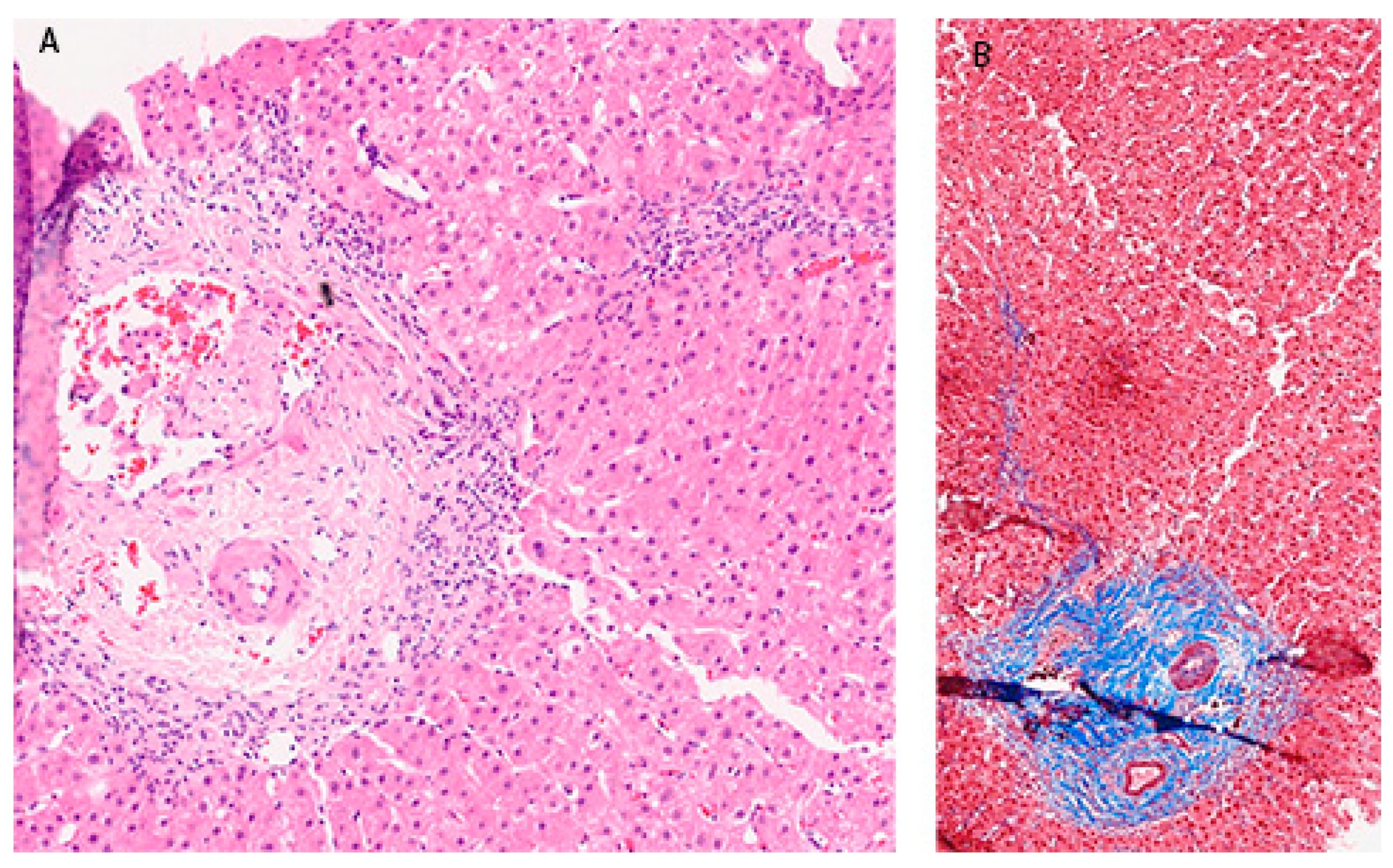
Figure 2B). In December 2017. he was admitted again due to significant increase in aminotransferase and bilirubin levels. Biopsy of the right liver lobe was performed, showing abundant T lymphocyte and plasma cell infiltration with numerous piecemeal necrosis, without cholestasis. The finding corresponded with chronic active hepatitis, morphologically dominantly autoimmune hepatitis of medium activity (modified hepatitis activity index (mHAI) according to Scheuer 8-9/18) with liver fibrosis (stage 2/6) (
Figure 3). After increasing the corticosteroid dose, a decrease in laboratory parameters was detected. He was further treated as ambulatory patient with tapering corticosteroid doses and UDCA. The patient remained under biochemical control under low dose corticosteroid therapy, with no further relapses.
In March 2019, two years after the initial admission, control abdominal MSCT scan was performed and showed atrophic right liver lobe with irregular contours and hypertrophic left lobe indicating presence of liver cirrhosis (
Figure 2C). Since the left liver lobe seemed spared of fibrotic changes, it was biopsied and revealed insignificant inflammatory infiltrate without piecemeal necrosis, cholestasis or advanced fibrosis.
In 2023, multiparametric ultrasound with elastography was performed and confirmed advanced liver fibrosis with laboratory parameters indicating controlled inflammatory activity (normal bilirubin, aminotransferase and IgG levels).
4. Discussion
DIAIH is one of the rare DILI phenotypes, hardly distinguishable from classic idiopathic AIH, usually coupled with the antibiotic therapy (minocycline and nitrofurantoin), but also with other classes of drugs presented by diclofenac, indomethacin, halothane, infliximab, methyldopa, hydralazine and statins [
1,
5]. When trying to distinguish between idiopathic AIH and DIAIH, clinicians use a wide armamentarium of methods, reaching from the comprehensive patient history to the genetic testing and liver biopsy.
Regarding the clinical presentation, DIAIH tends to show more severe clinical picture with jaundice, resulting in higher hospitalization requirement [
6]. Patients usually present with nonspecific symptoms including anorexia, nausea, abdominal discomfort and malaise, sometimes followed by manifestations of hypersensitivity reactions [
7]. Both disorders are accompanied with increase in aminotransferase and bilirubin levels, without remarkable cholestasis. According to the serology markers, 96% of patients with the DIAIH have positive autoantibodies characteristic for AIH (ANA, anti-smooth muscle antibody (ASMA), anti-liver-kidney microsomal antibody (anti-LKM)), and 90% of patients have elevated IgG levels. However, we must keep in mind an additional confusing factor of the high antibody prevalence among asymptomatic individuals in general population [
1]. Additionally, carriers of HLA alleles DRB1*03:01/*04:01 have higher risk of idiopathic AIH, while HLA DRB1*15:01 is known as the DILI- risk allel [
1].
As per the Spanish DILI Registry, established in 1994, there were 26 DIAIH cases up to 2018, and the diagnosis was based on convincing temporal relationship between the drug intake and the liver injury, no prior evidence of AIH, and the fulfilment of the simplified AIH criteria. When comparing features of patients with DIAIH with the complete DILI cohort, they revealed a higher predominance of female patients and higher percentage of hepatocellular injury, as well as longer treatment requirement in the DIAIH group [
2].
Regarding the histology patterns, DIAIH also presents with the lymphocyte, eosinophilic and plasma cell infiltrates in portal and periportal spaces and interface hepatitis. According to Febres-Aldana et al., necroinflammatory and regenerative changes, as well as portal and lobular densities of neutrophils and eosinophils do not differ between the groups (p ≥ 0.05), but patients with idiopathic AIH more often show signs of collagen deposition and fibrosis (p < 0.05) [
8]. Therefore, advanced stages of fibrosis support the diagnosis of AIH over DIAIH. Additionally, portal infiltrates in DIAIH are predominantly made of cytotoxic (CD8+) T cells, and of mature B cells (CD20+) in idiopathic AIH [
1]. Still, there is no specific histopathologic sign pivotal for diagnosing DIAIH over AIH. Suzuki et al. compared the patohistologic diagnosis among the experts in field, and found an unanimous agreement in only 46% of cases [
9].
The most important distinguishment is provided at the end of the road, after withdrawal of immunosuppression following the remission accomplishment. Namely, DIAIH does not relapse over a long-term follow-up, while patients with idiopathic AIH relapse in 63% of cases in the first year after therapy cessation [
8,
10,
11,
12]. However, it has been reported that 10–18% of patients may develop chronic DILI during long-term follow-up [
13]. According to the USA- DILIN, chronic DILI is diagnosed when the liver disease persists 6 months after the DILI onset [
3,
6]. In 2011, an international expert working group defined persistent DILI as abnormal liver biochemistry lasting more than 3 or 6 months for hepatocellular or cholestatic injury, respectively. They defined chronic DILI as liver injury lasting more than 12 months [
14]. Similarly, in 2019. EASL defined chronic DILI as biochemical or imaging evidence of liver disease persisting one year after the acute DILI onset [
1]. Several forms of chronic DILI are described in the literature and include autoimmune-like DILI, vanishing bile duct syndrome, drug-induced steatohepatitis, secondary sclerosing cholangitis, sinusoidal obstruction syndrome, development of fibrosis, and lastly even liver cirrhosis with portal hypertension [
5,
10]. To emphasize, severe liver fibrosis following DILI occurs rarely, and is described only in isolated case reports [
6].
Acute onset, absence of advanced liver fibrosis, and no relapse after corticosteroid withdrawal are the main features distinguishing DIAIH from the idiopathic AIH. However, the diagnostic path of the presented patient seems to wander between the two disorders and leaves us in doubt even years after the initial admission. Due to fulminant clinical onset with jaundice and according to RUCAM-determined possible causality with the dietary supplement, he was initially perceived as DILI, but the unexpected relapse after corticosteroid withdrawal and further development of liver cirrhosis eventually led to diagnosis of idiopathic AIH. Could it be that our patient developed chronic DIAIH rather than classical AIH?
Nevertheless, such a rapid development of liver fibrosis occurring in a 7-month interval seems too prompt. Did the patient already have existing liver fibrosis due to formerly unrecognized AIH and the drug acted as a trigger for severe necroinflammation and stimulus for development of more advanced fibrosis?
Regarding liver patohistology, we have acquired a knowledge of its insufficiency in making the definite distinction between the disorders in question. As Lewis JH charmingly pointed up- not all that glitters are necessarily the gold standard, and similarly, not all the cases in clinical medicine are strictly categorizable into a certain box, leaving some questions open for good [
15].
5. Conclusions
Regular patient monitoring and clinical open-mindedness with adjustment of therapeutic approaches according to the disease course are more important than strict labelling of the disease.
Author Contributions
Conceptualization, D.B. and A.T.; methodology, D.B. and A.T.; formal analysis, D.B., AT., K.V., M.R..; investigation, D.B., AT., K.V., M.R..; writing—original draft preparation, D.B., AT., K.V., M.R..; writing—review and editing, D.B., AT., K.V., M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of University of Split School of Medicine (protocol code 003-08/23-03/0015 and date of approval 4.7.2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Association for the Study of the Liver. Clinical Practice Guideline Panel: Chair; Panel members; EASL Governing Board representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol 2019, 70, 1222–1261. [Google Scholar]
- Stephens C, Robles-Diaz M, Medina-Caliz I, Garcia-Cortes M, Ortega-Alonso A, et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J Hepatol 2021, 75, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008, 135, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Björnsson ES, Andrade RJ. Long-term sequelae of drug-induced liver injury. J Hepatol 2022, 76, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Alonso A, Andrade RJ. Chronic liver injury induced by drugs and toxins. J Dig Dis 2018, 19, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Czaja, AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci 2011, 56, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Febres-Aldana CA, Alghamdi S, Krishnamurthy K, Poppiti RJ. Liver Fibrosis Helps to Distinguish Autoimmune Hepatitis from DILI with Autoimmune Features: A Review of Twenty Cases. J Clin Transl Hepatol 2019, 7, 21–26. [Google Scholar]
- Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology 2011, 2, 931–9. [Google Scholar]
- Björnsson ES, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, et al. Drug-induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol 2017, 15, 1635–1636. [Google Scholar] [CrossRef] [PubMed]
- Czaja, AJ. Difficult treatment decisions in autoimmune hepatitis. World J Gastroenterol 2010, 16, 934–947. [Google Scholar] [CrossRef] [PubMed]
- deLemos AS, Foureau DM, Jacobs C, Ahrens W, Russo MW, et al. Drug-induced liver injury with autoimmune features. Semin Liver Dis 2014, 34, 194–204. [Google Scholar] [CrossRef] [PubMed]
- He T, Ren L, Gong M, Guo Y, Wang L, et al. The progression of chronicity and autoimmune hepatitis in recurrent drug-induced liver injury. Clin Res Hepatol Gastroenterol 2022, 46, 102009. [Google Scholar] [CrossRef] [PubMed]
- Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011, 89, 806–15. [Google Scholar] [CrossRef] [PubMed]
- Lewis, JH. Diagnosis: Liver biopsy differentiates DILI from autoimmune hepatitis. Nat Rev Gastroenterol Hepatol 2011, 8, 540–542. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).