1. Introduction
Enterococci are Gram (+), catalase (-), spore-type, facultative anaerobic lactic acid bacteria. They are found in the output flora of many mammals, birds, reptiles, amphibians, fish and insects, as well as in the output flora of humans [
1] . Despite the spread of more than fifty species in the genus of Enterococci, the pollutant Enterococcus faecium (E. faecium) and Enterococcus faecalis (E. faecalis) are responsible for the disease in humans [
2]. E. faecalis is the most pathogenic species, but E. faecium is more and more resistant to antibacterials in general. For example; They play a role in hospital country of origin such as bacteremia, neonatal sepsis, endocarditis, storage mediated urinary tract systems, burn and surgical wound services, and more rarely meningitis [
3]. Fecal carriers of the main reservoir of VRE strains are considered. Spread and transmission of VRE in hospital settings is through direct contact with colonized or infected viruses or indirect contact through the hands of healthcare workers; it may happen that gloves, thermometers (especially rectal thermometers), bed guards, and contaminated treatment devices can be placed [
4]. Some vancomycin resistance gene settings (van A, van B, van D, van E, van G, van L, van M, and van N) can be adjusted [
5]. VRE resistance variants can be used with the van A or van B genes in clinical samples from North America, Europe, Asia and Africa [
6]. The World Health Organization (WHO) has a rich comprehensive list of pathogens in the center of urgent need for new antibiotics that have recently become widespread. Vancomycin-resistant E. faecium (VRE fm) has been listed in the high quality category [
7].
Our study is to determine the enterococci species that cause VRE epidemic in the intensive care units of our hospital, and the genomic structures of VRE fms, which are frequently detected in our hospital, and their resistance to antibacterials.
2. Materials and Methods
Our study started with the detection of the increase of VRE fm isolates in our hospital after VRE fm growth in the blood culture of the patient who was hospitalized in the anesthesia intensive care unit with the diagnosis of respiratory failure on 02.12.2021. In accordance with the decisions of the infection control committee of our hospital, rectal swab samples were taken from all intensive care units and VRE follow-up was performed. The samples sent to our laboratory by taking Stuart transport medium were inoculated on blood agar and enterococcosel agar chromogenic VRE medium and incubated at 35 °C for 24-48 hours. Growing colonies were identified using the Vitek 2 Compact (BioMerieux, France) automated identification device after conventional methods. Minimal inhibition concentration (MIC) values were determined for antibiotic susceptibility using both the Vitek 2 device and the E-test (AB Biodisk, Sweden) method. Antibiotic susceptibility results were evaluated in accordance with the European Committee of Antimicrobial Susceptibility Testing (EUCAST) criteria [
8]. Samples were stored at -80°C until the working day. Multiple swab samples were continued to be collected from each patient until no new VRE was detected for three consecutive weeks. However, only one sample of each patient with the same enterococcal growth was included in the study.
AP-PCR and PFGE methods were used to determine cross-contamination in VREfm isolates under investigation due to the fact that it was detected most frequently among VRE isolates. The AP-PCR method was performed as previously described by Menekse et al. [
9]. DNA isolation was performed in the QIAsymphony SP automated nucleic acid isolation device using the QIAamp DNA mini kit. Subsequently, amplification was performed with the M13 primer (5’-GAG GGT GGC GGT TCT-3’). Amplicons were electrophoresed on a 2% agarose gel at 100 V for 1 hour and then at 50 V for 470 minutes and imaged with the Kodak Gel Logic 200 Imaging System (Eastman Kodak Company, Rochester, NY, USA). For the PFGE method, the protocol optimized by Turabelidze et al. [
10]. was applied. 30 units of Sma-I enzyme were used for restriction enzyme digestion. Band profiles for both methods were analyzed using the GelCompar II software system (version 6.5; Applied Maths, Sint-Martens-Latem, Belgium). Dice correlation coefficient was used to calculate similarity for band analysis and UPGMA (“Unweighthed Pairvise Grouping Mathematical Avenaging” method of grouping unweighted pairs with mathematical mean) was used for cluster analysis. Considering the similarity coefficients of the isolates, the strains showing more than 90% similarity with each other were considered in the same clone.
Demographic, clinical and microbiological information of the patients were obtained prospectively from the electronic medical record system and infection prevention databases.
3. Results
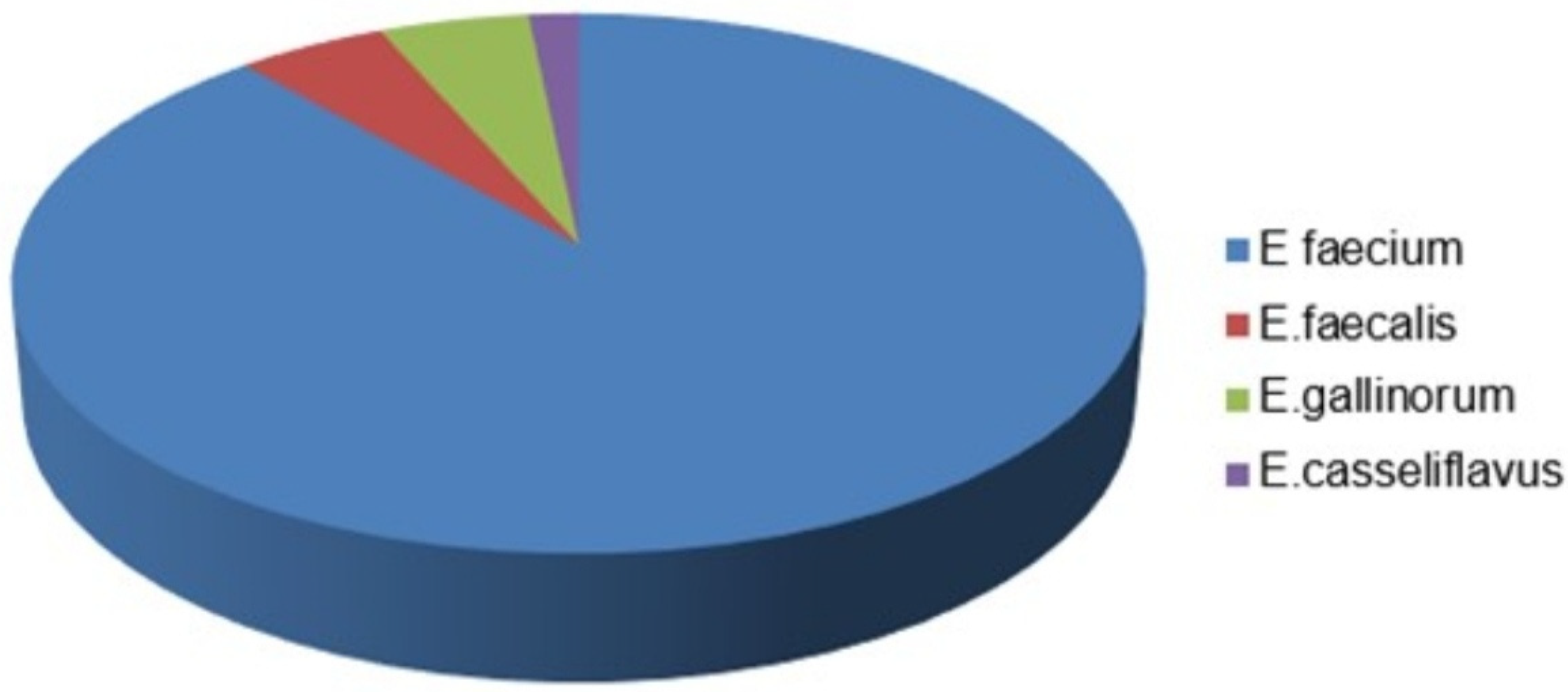
2601 rectal swab samples were taken from the adult and pediatric intensive care units of our hospital for VRE screening. VRE was detected in 178 (6.84%) of the samples. Of the patients, 1450 (55.74%) were male and 1151 (44.25%) were female, with a mean age of 61. When the distribution was made according to the samples requested from the intensive care units, the intensive care anesthesia intensive care unit (72/849) was the most requested and at the same time the highest VRE was seen. Intensive care units, where VRE is most frequently detected after anesthetized intensive care; general intensive care (6/76), chest intensive care (16/204), internal medicine intensive care (38/518), neurology intensive care (18/258) and pediatric intensive care (28/496). VRE was not detected in any of the samples sent from the neonatal intensive care unit (0/200). Of the 178 samples with VRE, only one of the VRE isolates in which more than one of the same agents was detected from the same patient was included in the study, and thus the study was continued with 61 isolates. Of 61 isolates with VRE, 54 (89%) were E. faecium, 3 (4.91%) E.faecalis, 3 (4.91%) Enterococcus gallinorum (E.gallinorum), 1 (2%) Enterococcus casseliflavus (E.casseliflavus) (
Figure 1).
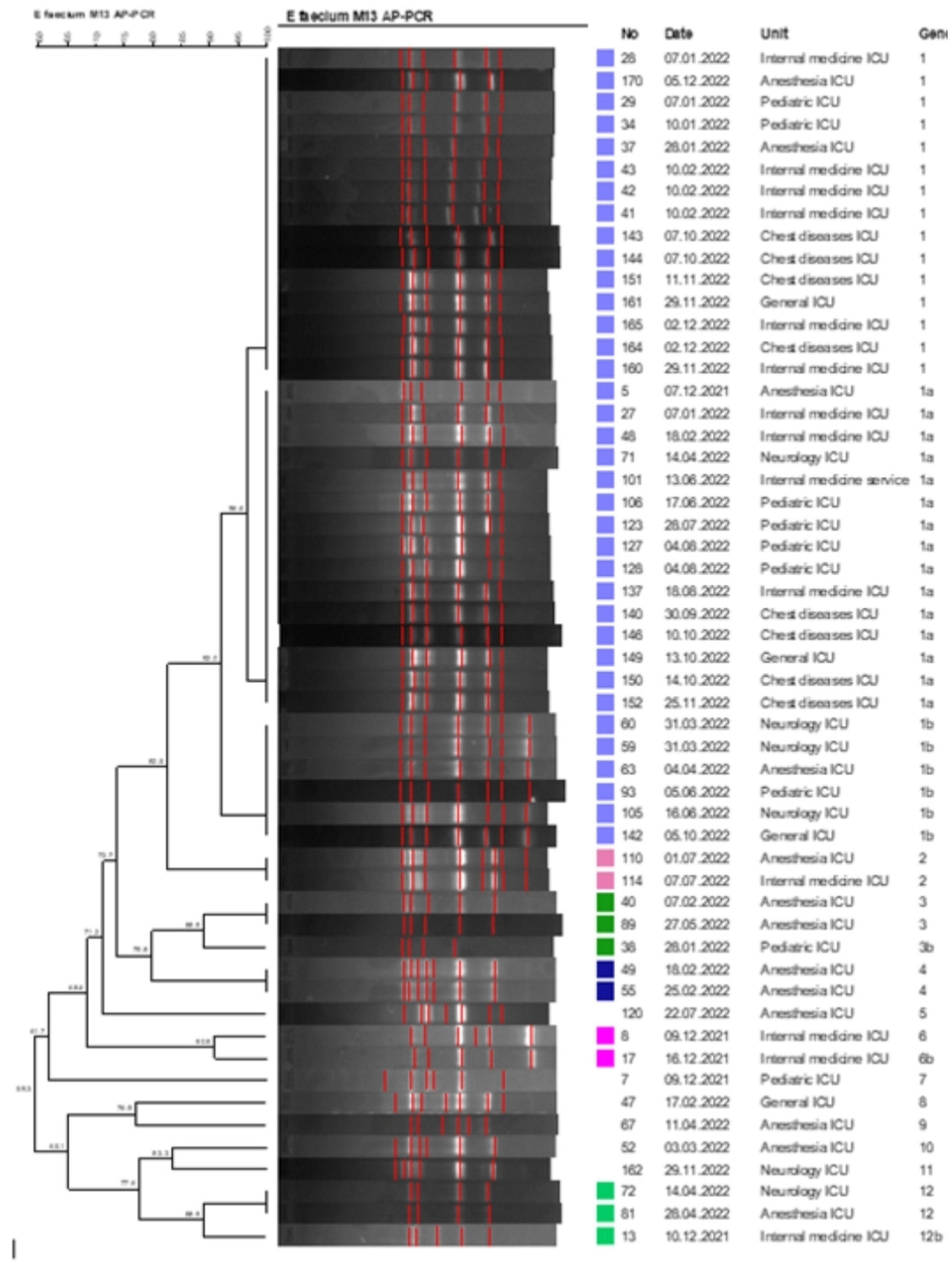
In our study, the clonal relationship of 54 VREfm isolates, which were detected most frequently, was studied by AP-PCR and PFGE molecular methods. It was determined that all of the VREfm isolates had the van A gene and consisted of a total of 12 different genotypes. Clustered isolates were collected in 6 different clusters (tolerance 1.0, optimization 1.0, cutoff 90%). 48 of 54 VREfm isolates were in any cluster, and the aggregation rate was 88.8%. The largest cluster was determined as genotype 1 cluster with 36 isolates. It was determined that the isolates withinthe genotype 1 cluster may be associated with a possible epidemic (
Figure 2).
VRE fm examples; 100% to ampicillin, 100% to levofloxacin, 100% to ciprofloxacin, 100% to high-level gentamicin, 100% to trimethoprim-sulfamethoxazole, 100% to teicoplanin, 7.40% to tigecycline and 1.85% to linazolide. VRE fm was detected in 10 (16.39%) pre-screening (8 urine, 2 blood samples) and 5 (8.19%) post-screening (4 urine, 1 blood samples) clinical samples of 54 patients with VRE fm isolated in rectal swab samples.
4. Discussion
Vancomycin is a class of antibiotics in the glycopeptide group and is used as a last resort drug in the treatment of life-threatening infections caused by multi-drug resistant Gram-positive cocci [
11]. It is reported that patients hospitalized in oncology, transplantation units, intensive care units, undergoing cardiothoracic surgery, intra-abdominal surgery, hospitalized for a long time, treated with multiple antibiotics, and patients with indwelling urinary or central venous catheters are at high risk of VRE colonization and/or infection [
12]. For European Union Countries, it has been reported that vancomycin resistance of invasive E. faecium strains increased from 11.6% in 2016 to 16.8% in 2020. While the VRE in Turkey was 14.6% in 2016, it was found to be 15.4% in 2020. In recent years, it has been reported to be 15.8% in our country [
13,
14]. In studies conducted in our country for the detection of VRE in rectal swab samples, Bulut et al. [
15]. reported the rate of VRE as 4.3%. Alçi et al. [
12]. found the rate of VRE in rectal swab samples to be 8.1%. In the study of Kınıklı et al. [
16]. 159 rectal swab samples taken from patients hospitalized in the intensive care unit confirmed with automated antimicrobial susceptibility and E-test methods, while VRE was detected in 21 (13%) samples, but they did not detect VRE in 138 (87%) samples. Kirişçi Ö. et al. [
17]. in their study the rates of VRE in rectal swab samples; They found that it was 5.5% in 2013, 4.1% in 2014, 4.8% in 2015, 9.7% in 2016 and 11.6% in 2019. The average VRE rate between 2013 and 2019 was 6%. Kaçar F. et al. [
18]. in their study in which all nosocomial infections due to enterococci were examined, reported that the rate of VRE was found to be 0.24%, lower than the data of Turkey. Enterococci are inherently resistant to many antibiotics, such as cephalosporins, fusidic acid, low-level aminoglycosides, sulfanamides, macrolides, which are widely used in the treatment of other Gram-positive cocci (such as staphylococci and streptococci). Unlike E. faecalis, E. gallinarum, E. casseliflavus, it is naturally resistant to clindamycin and quinopristin-dalfopristine. In addition, E. gallinarum and E. casseliflavus are intrinsically resistant to vancomycin [
19].
In our study, 178 (6.84%) of the 2,601 rectal swab samples sent from the intensive care units were found to have VRE. Only one of the recurrent isolates of the same agent was included in the study and the study was continued with 61 isolates. Of the isolates, 54 (85.52%) were E. faecium, 3 (4.91%) E. faecalis, 3 (4.91%) E. gallinarum, 1 (1.63%) E. casseliflavus. While the intensive care unit was the anesthesia intensive care unit, where the highest number of samples were sent and the highest number of VRE positivity was detected (8.48%), no VRE was detected in any rectal swab sample in the neonatal intensive care unit. 54 isolates detected in VRE fm; It was found that it was resistant to ampicillin, levofloxacin, ciprofloxacin, high-level gentamicin, trimethoprim-sulfamethoxazole, 100% to teicoplanin, 7.40% to tigecycline, and 1.85% to linazolide.
In VRE fm molecular typing studies conducted in our country, Arslan U et al. [
20]. found the van A gene in all isolates, and E.faecium isolates carrying the van A gene were studied with MLST and PFGE methods in terms of clonal association; Four pulsotypes (AD) and one sporadic isolate were determined by the PFGE method. Eight of 29 strains were identified as A1 type, 9 as A2 type, 6 as A3 type, 2 as A4 type and 4 as A5 by MLST method. Gözalan A et al. [
21]. studies showed that all VRE-positive isolates carried the van A gene and 41 (75%) of them were positive for virulence genes, 16 different types were identified, seven of which were clusters of two to 14 strains each. The aggregation rates of rectal swab, blood and urine isolates were 72.7%, 61.5% and 87.5%, respectively. They stated that the genetic similarity observed between VRE fm isolates suggested that there was cross-contamination in the hospital. In the study of Güldemir D. et al. [
22]. E. faecium isolates formed one main group and four clusters, and 10 E. faecalis strains formed one major group and three clusters. It has been shown that 26 E. faecium isolates are in the same group with 97% similarity. The clustering rate was 77% (20/26) and it was determined that the clusters contained between 2-14 isolates. In their study performed on patients hospitalized in the intensive care unit, Sakin F et al. [
23]. detected the van A gene in all of the samples in the molecular analysis of 23 VRE fm isolates isolated from rectal swab samples using the PFGE method. In the study of Aşkın N and Otlu B, only the van A gene was detected in the strains, and according to the PFGE results, 31 of 47 strains were clonally associated with a clustering rate of 66%.[
24].
In a study conducted in France in VRE fm molecular typing studies abroad, VRE outbreaks occurring in 195 hospitals between 2001 and 2008 were investigated; According to the PFGE analysis results, 161 different patterns were detected and it was reported that a predominant clone was prevalent in hospitals. 504 VRE reports were recorded from 195 hospitals, corresponding to 2475 cases of infection (n=243) or colonization (n=2232) and 74 clustered 1case divisions. Usually, a few dominant clones and a few minor clones spanned in a single hospital, they detected 13 different sequence types, all belonging to the clonal complex CC17, in a subset of 46 representatives of PFGE clones [
25]. Liu et al. [
26]. using PFGE molecular diagnostic method, 27 E. faecium isolates containing the valve gene were identified as VRE fm in 89 E. faecium isolates. Major clonal VRE fm strains persisting between 2013 and 2015, CT1/ST78/PFGE cluster A containing transposon type Ⅰ were detected, and other CT4/ST363/PFGE cluster of VRE fm strains were found to contain transposon type Ⅰ. They found that three patients received different clonal E. faecium strains during the hospitalization and one patient was infected with the VRE fm strain. In a recent study by Purohit G et al. in India, E. faecium was detected in 64.8% of various clinical specimens from which enterococci were isolated (n=250), of which 25.2% were found to be resistant to vancomycin and 87.3% were reported to carry the van A gene. They also found that 70.2% of patients with VRE infection (n=47) developed on the basis of colonization [
27].
5. Conclusion
As we saw in our study, VRE continues to be a problem for hospital intensive care units. Although it takes time to develop new antibiotics and regulatory approval for clinical use in the treatment of VRE, resistance to existing antibiotics is increasing day by day. Molecular analyzes can help control enterococcal infections by identifying the source and transmission networks of an epidemic and guiding infection control interventions.
Institutional Review Board Statement
Approval for our study was obtained from the Ethics Committee of Fırat University Faculty of Medicine (Decision No: 2023/08-04, Date: 08.06.2023).
Conflicts of Interest
The authors declared no conflict of interest regarding this article.
References
- Comerlato CB, Ritter AC, Miyamoto KN, Brandelli A. Proteomic study of Enterococcus durans LAB18S growing on prebiotic oligosaccharides. Food Microbiol. 2020; 89:103430. [CrossRef]
- Zaheer R, Cook SR, Barbieri R, et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci Rep 10, 3937 2020. [CrossRef]
- Ramos S, Silva V, Dapkevicius MdLE, Igrejas G, Poeta P. Enterococci, from Harmless Bacteria to a Pathogen. Microorganisms. 2020; 8(8):1118. [CrossRef]
- Orsi GB, Ciorba V. Vancomycin resistant enterococci healthcare associated infections. Ann Ig. 2013; Nov-Dec;25(6):485-92. [CrossRef]
- Rios R, Reyes J, Carvajal LP, Rincon S, Panesso D, Echeverri AM, et al. Genomic epidemiology of vancomycin-resistant Enterococcus faecium (VREfm) in Latin America: revisiting the global VRE population structure. Sci Rep 2020;10:5636. [CrossRef]
- Miller WR, Murray BE, Rice LB, Arias CA. Resistance in Vancomycin-Resistant Enterococci. Infect Dis Clin North Am. 2020 Dec;34(4):751-771. [CrossRef]
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL. et al. WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018 Mar;18(3):318-327. [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0.2022.Available:https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf.
- Menekşe Ş, Tanrıverdi ES, Oğuş H, Altınay E, Kaya Ç, Çağlayan E, et al. Stenotrophomonas maltophilia outbreak with a commercial blood gas injector as the culprit and interventions for source and prevention: A possible passage between patient and ECMO water heater device. Am J Infect Control. 2023 May;51(5):533-538. [CrossRef]
- Turabelidze D, Kotetishvili M, Kreger A, Morris JG Jr, Sulakvelidze A. Improved pulsed-field gel electrophoresis for typing vancomycin-resistant enterococci. J Clin Microbiol. 2000 Nov;38(11):4242-5. [CrossRef]
- Marcone GL, Binda E, Berini F, Marinelli F. Old and new glycopeptide antibiotics: From product to gene and back in the post-genomic era. Biotechnol Adv. 2018 Mar-Apr;36(2):534-554. [CrossRef]
- Alçi G, Güneşer D, Güner A, Karahasan A. Hastanede yatan hastalardan alınan rektal sürüntü örneklerinde vankomisine dirençli enterokok taranması: Stratejik değerlendirme. ANKEM Derg. 2021;35(3):70-6. /: https. [CrossRef]
- World Health Organization Regional Office for Europe. Antimicrobial Resistance Map. Avaliable from: https://worldhealthorg.shinyapps.io/WHO-AMR-Dashboard/?_ga=2.172166563.1827800992.16686544401324205868.1668654440 Accessed: 13 January 2023. 13 January.
- World Health Organization Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022 – 2020 data. Copenhagen: WHO Regional Office for Europe; 2022. Avaliable from: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data Accessed: 13 January 2023. 13 January.
- Bulut A, Şengül H, Kaşıkcı ÖM. Vankomisine dirençli enterokok sürveyans çalışması: bir devlet hastanesi örneği. JAREN. 2018;4(1):21-7. [CrossRef]
- Kınıklı S, Cesur S, Hatipoğlu ÇA, Arslan K, Karakök T, Demircan ŞA, et al. Vankomisine dirençli enterokokların saptanmasında iki farklı kromojenik besiyerinin karşılaştırılması. Turk J Clin Lab 2019; 10: 319-323. [CrossRef]
- Kirişçi Ö ve Çalışkan A Rektal tarama örnekleri ile klinik örneklerde üreyen vankomisine dirençli enterokokların irdelenmesi:yedi yıllık sürveyans, retrospektif kesitsel bir çalışma. ANKEM Derg. 2020;34(3):105-11. [CrossRef]
- Kacar F, Eroğlu E, Tarakçı A, Çölkesen FD, Özdemir Armağan Ş, Can S. Vankomisine Dirençli Enterekok Enfeksiyonlarının İrdelenmesi. J Turk Soc Intens Care 2022;20:44-50. [CrossRef]
- Türk Mikrobiyoloji Cemiyeti, EUCAST Dirençli Olması Beklenen Fenotipler. Avaliable from: https://www.tmconline.org/userfiles/file/Diren%C3%A7li-Olmas%C4%B1-Beklenen-Fenotipler-2022.pdf Accessed: 11 January 2023.
- Arslan U, Demir E, Oryaşin E, Türk Dağı H, Tuncer I, Fındık D, Bozdoğan B. Kan kültürlerinden izole edilen vankomisine dirençli Enterococcus faecium suşlarının MLST tipleri [MLST types of vancomycin-resistant Enterococcus faecium strains isolated from blood cultures]. Mikrobiyol Bul. 2013 Jul;47(3):432-41. Turkish. [CrossRef]
- Gozalan A, Coskun-Ari FF, Ozdem B, Unaldi O, Celikbilek N, Kirca F, et al. Molecular characterization of vancomycin-resistant Enterococcus faecium strains isolated from carriage and clinical samples in a tertiary hospital, Turkey. J Med Microbiol. 2015 Jul;64(7):759-766. [CrossRef]
- Güldemir D, Karagöz A, Dal T, Tekin A, Özekinci T, Durmaz R. Hastane kaynaklı enterokok izolatlarının pulsed-field jel elektroforezis yöntemiyle moleküler tiplendirilmesi. Turk Hij Den Biyol Derg, 2015; 72(1): 1-10. [CrossRef]
- Sakin, F., Aslantaş, Ö., & Bağcı, F. 2019; Molecular characterization of vancomycin resistant Enterococcus faecium isolates from patients admitted to intensive care unit in Hatay state hospital. Turkish Journal of Intensive Care. [CrossRef]
- Asgin N, Otlu B. Antibiotic Resistance and Molecular Epidemiology of Vancomycin-Resistant Enterococci in a Tertiary Care Hospital in Turkey. Infect Drug Resist. 2020 Jan 21;13:191-198. [CrossRef]
- Bourdon N, Fines-Guyon M, Thiolet JM, Maugat S, Coignard B, Leclercq R, Cattoir V. Changing trends in vancomycin-resistant enterococci in French hospitals, 2001-08. J Antimicrob Chemother. 2011 Apr;66(4):713-21. [CrossRef]
- Liu S, Li Y, He Z, Wang Y, Wang J, Jin D. A molecular study regarding the spread of vanA vancomycin-resistant Enterococcus faecium in a tertiary hospital in China. J Glob Antimicrob Resist. 2022 Dec;31:270-278.
- Purohit G, Gaind R, Dawar R, Verma PK, Aggarwal KC, Sardana R, Deb M. Characterization of Vancomycin Resistant Enterococci in Hospitalized Patients and Role of Gut Colonization. J Clin Diagn Res. 2017 Sep;11(9):DC01-DC05. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).