Submitted:
13 September 2023
Posted:
15 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Tarsocrural desmitis
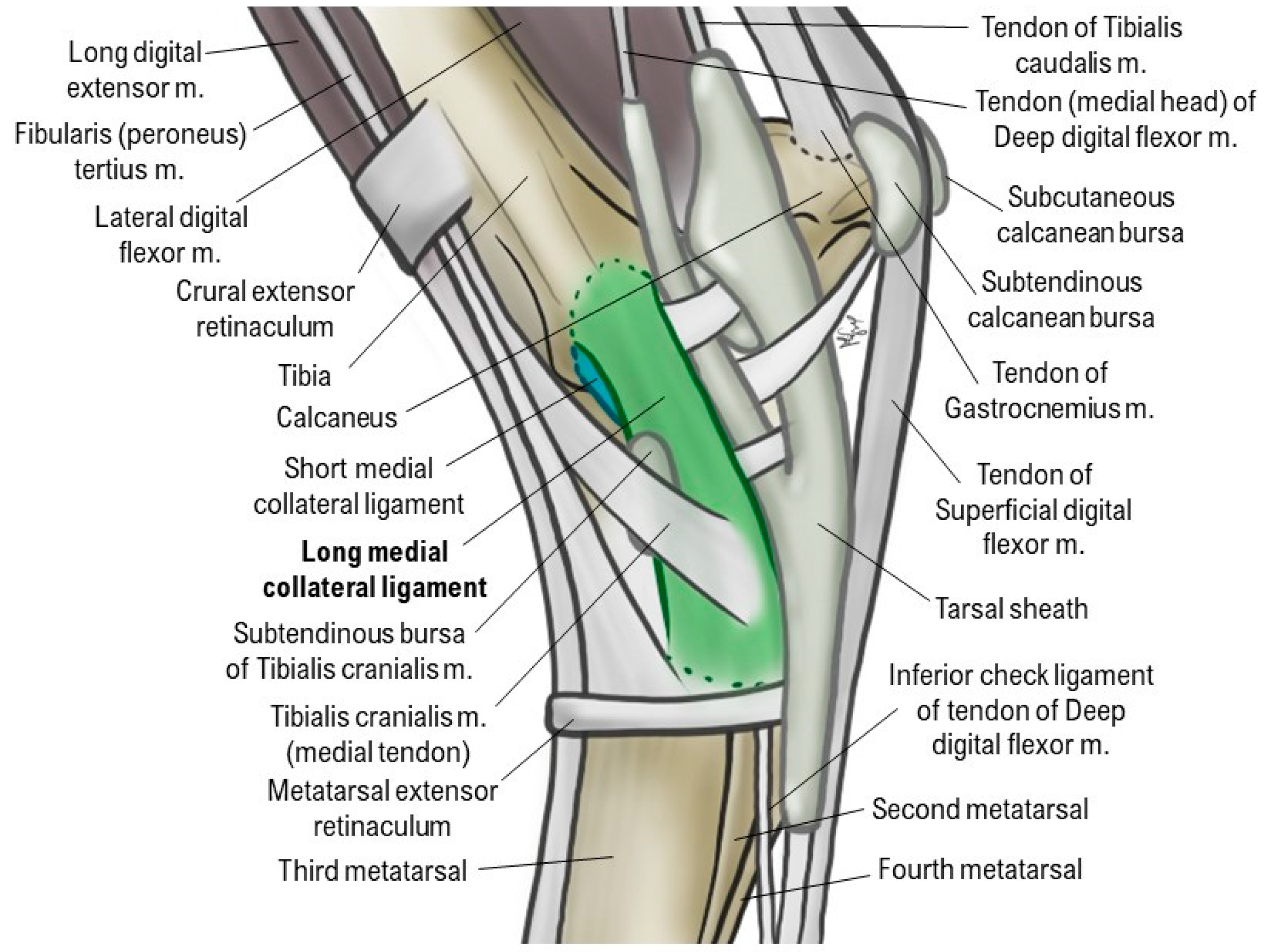
1.1.1. Anatomy and Biomechanics
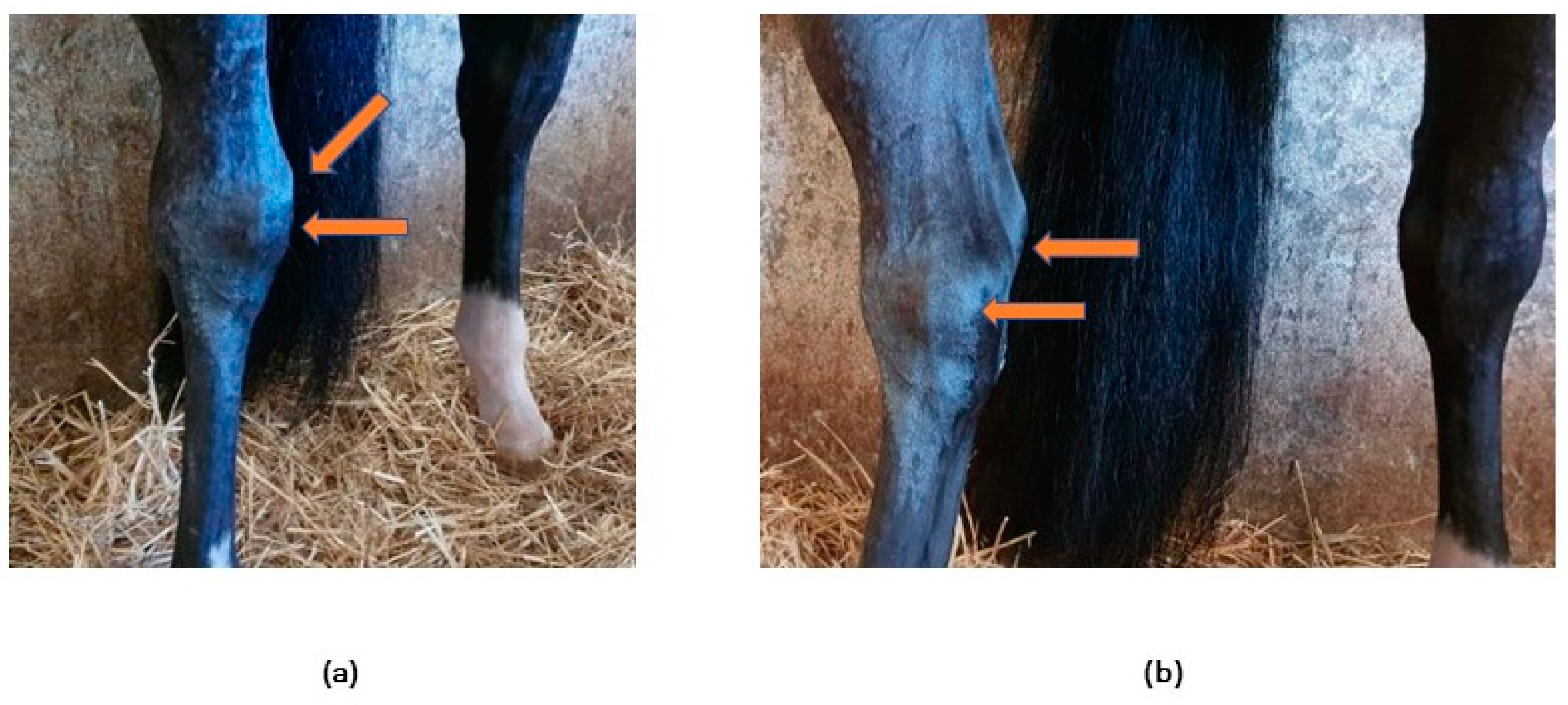
1.1.2. Clinical signs
1.1.3. Diagnostic complementary exams
1.1.4. Treatment: current regenerative therapies
1.2. Regenerative medicine in desmitis: Cell- based and Cell-free therapies
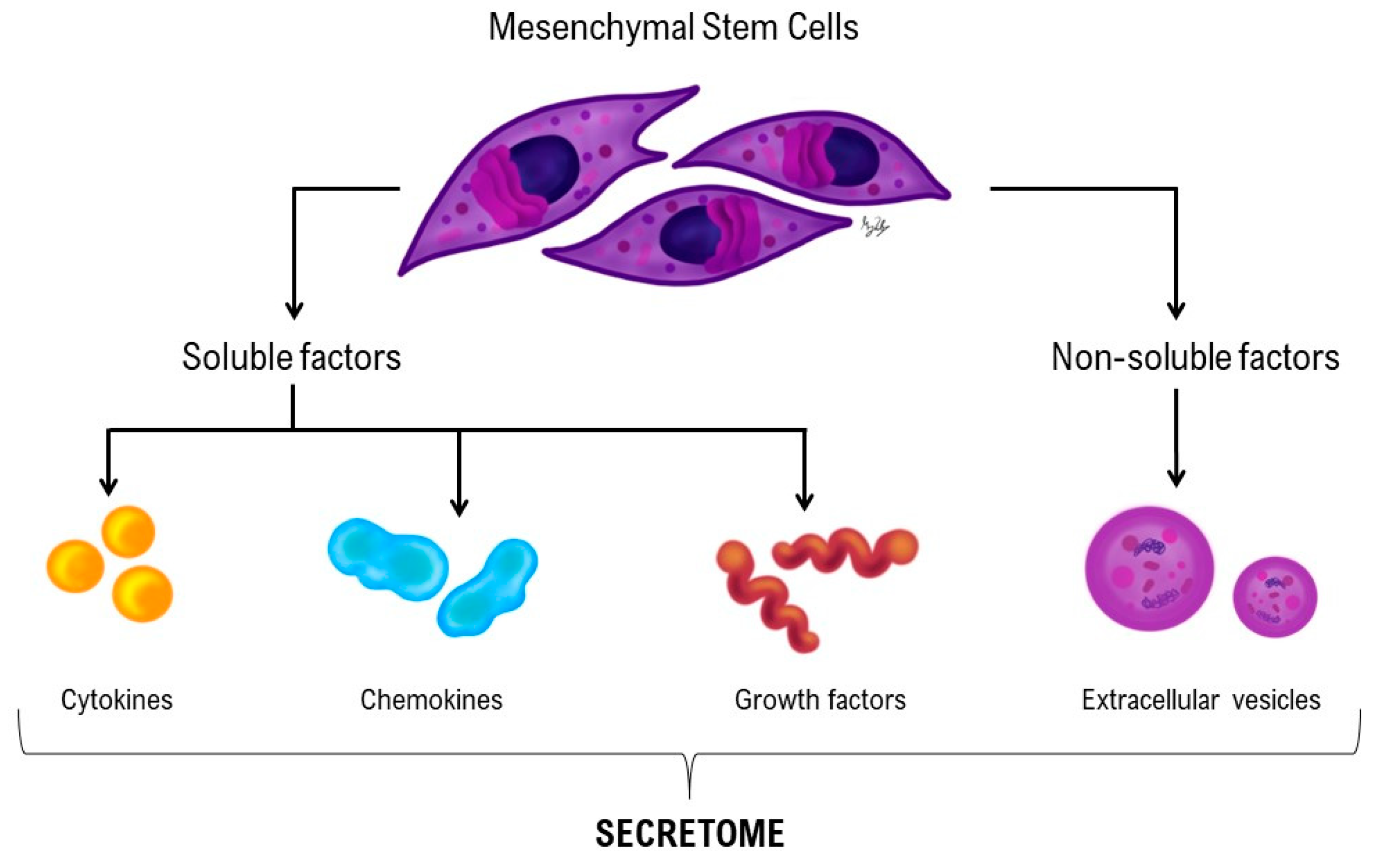
2. Materials and Methods
2.1. Ethics and Regulation
2.2. Patient identification
2.3. Patient clinical evaluation
2.4. Diagnostic Complementary exams
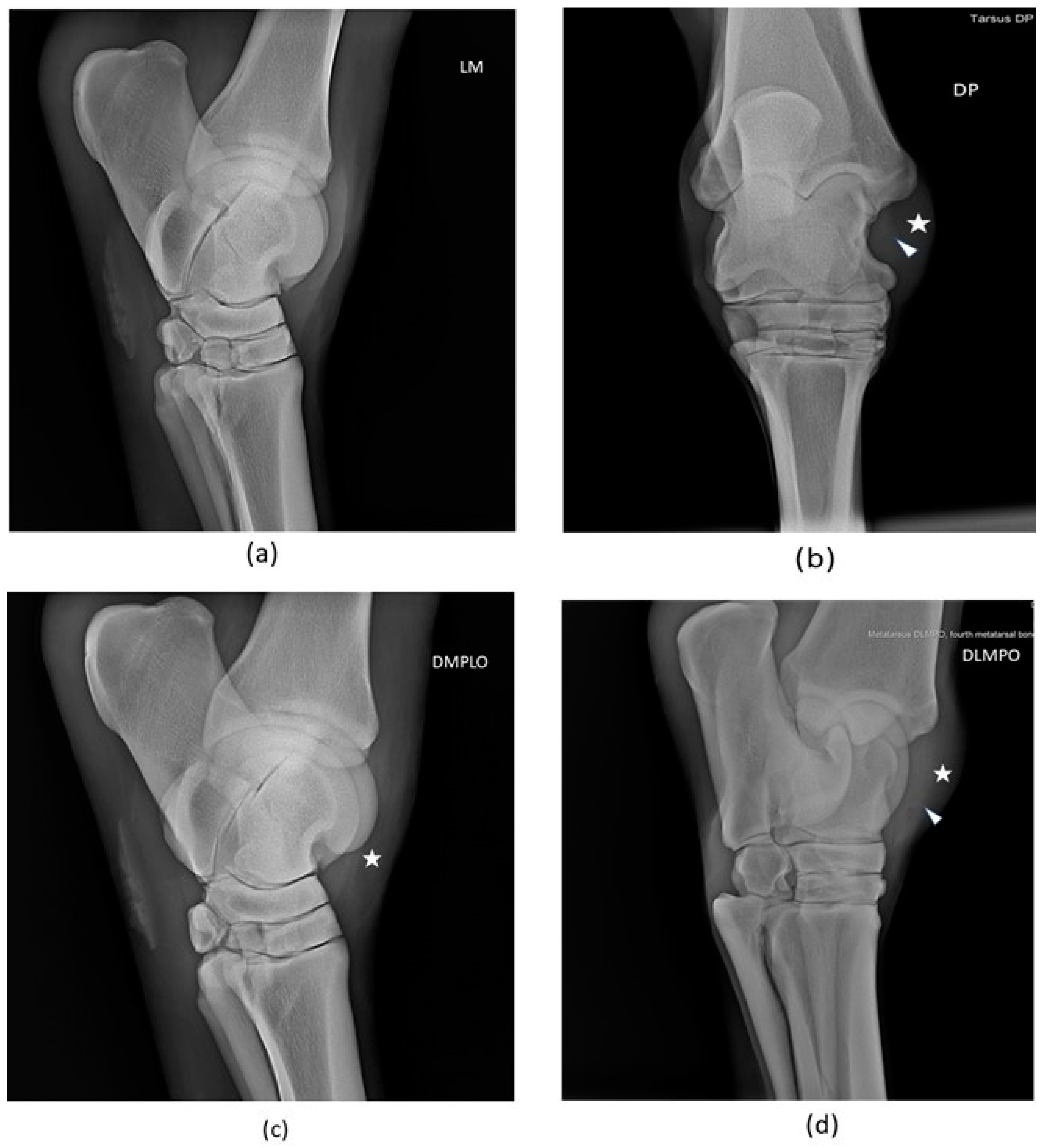
2.4.1. Radiological examination
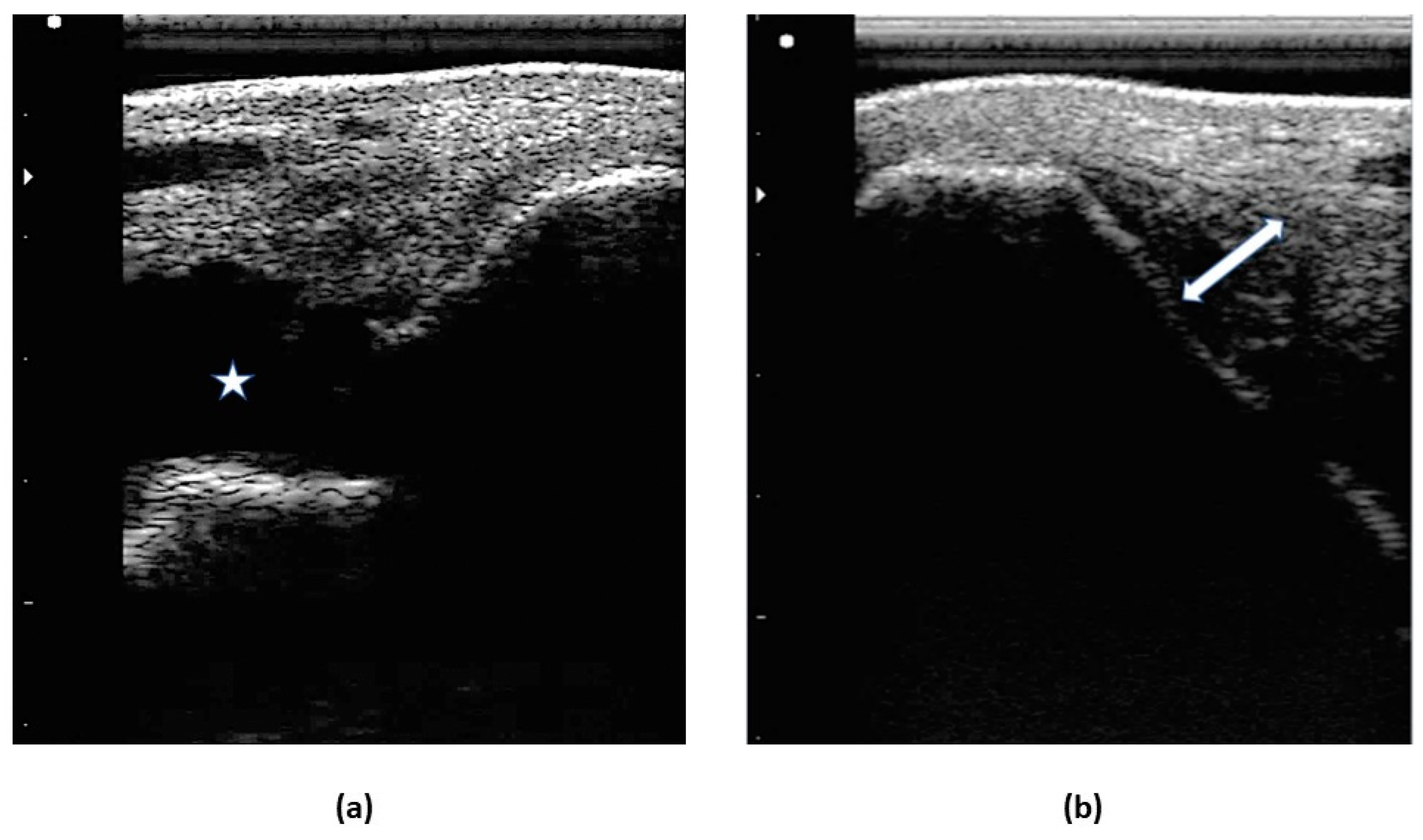
2.4.2. Ultrasound examination
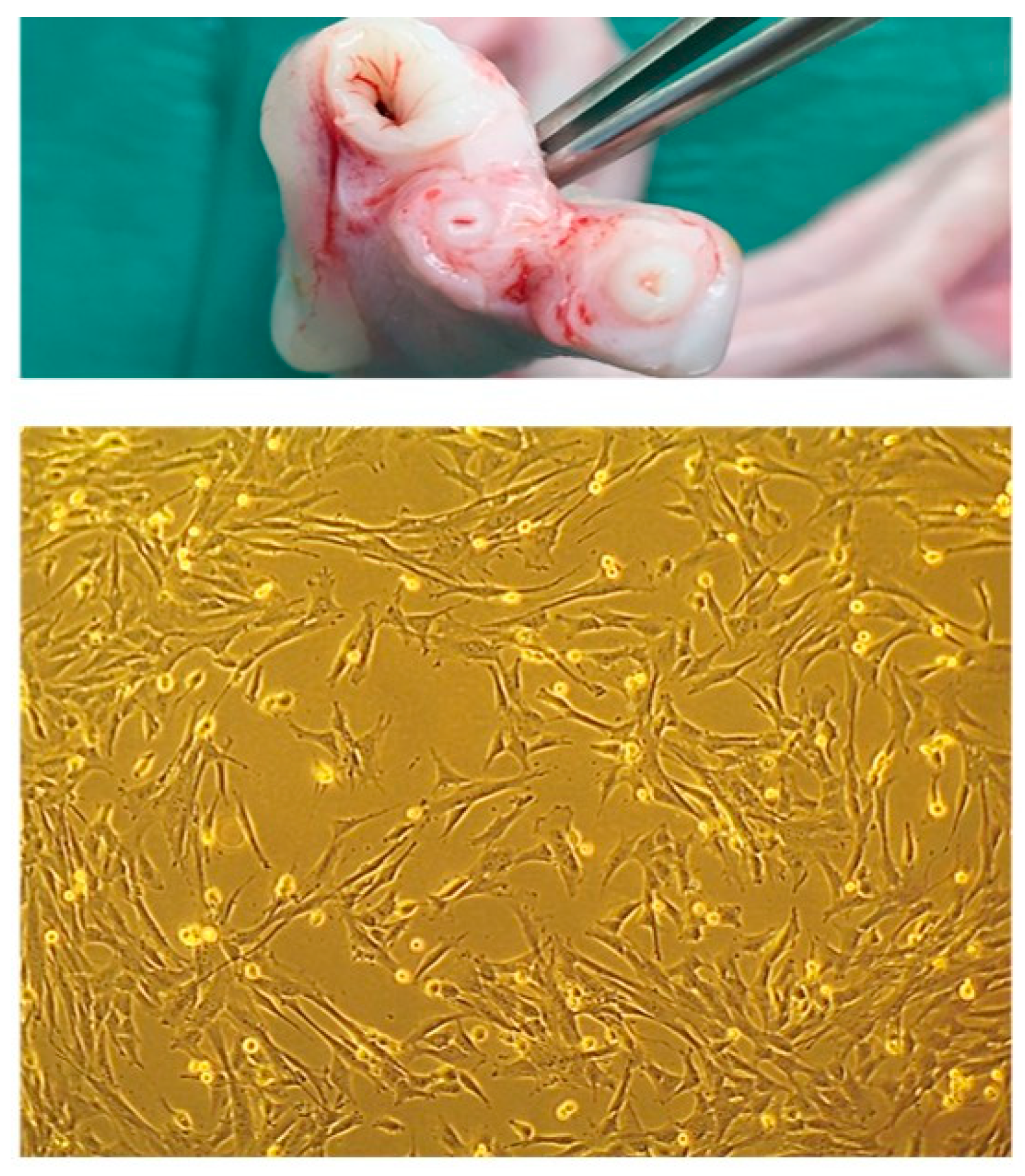
2.5. Donor selection and SM collection
2.6. eSM-MSCs isolation, culture and characterization
2.7. UC-MSC’s isolation, culture and characterization
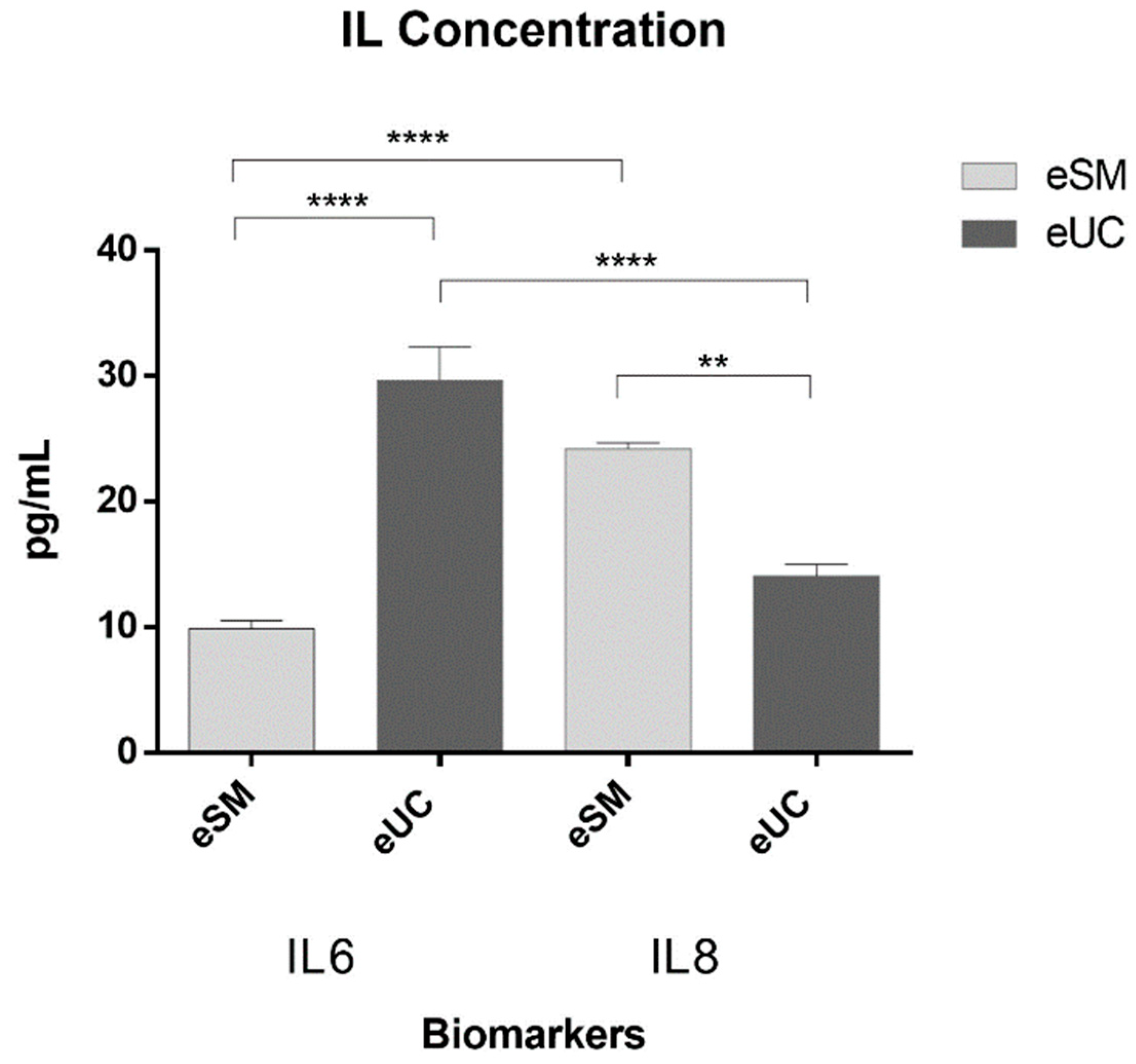
2.8. Secretome – Conditioned Medium preparation and analysis
2.9. eSM-MSCs + eUC-MSC CM solution preparation
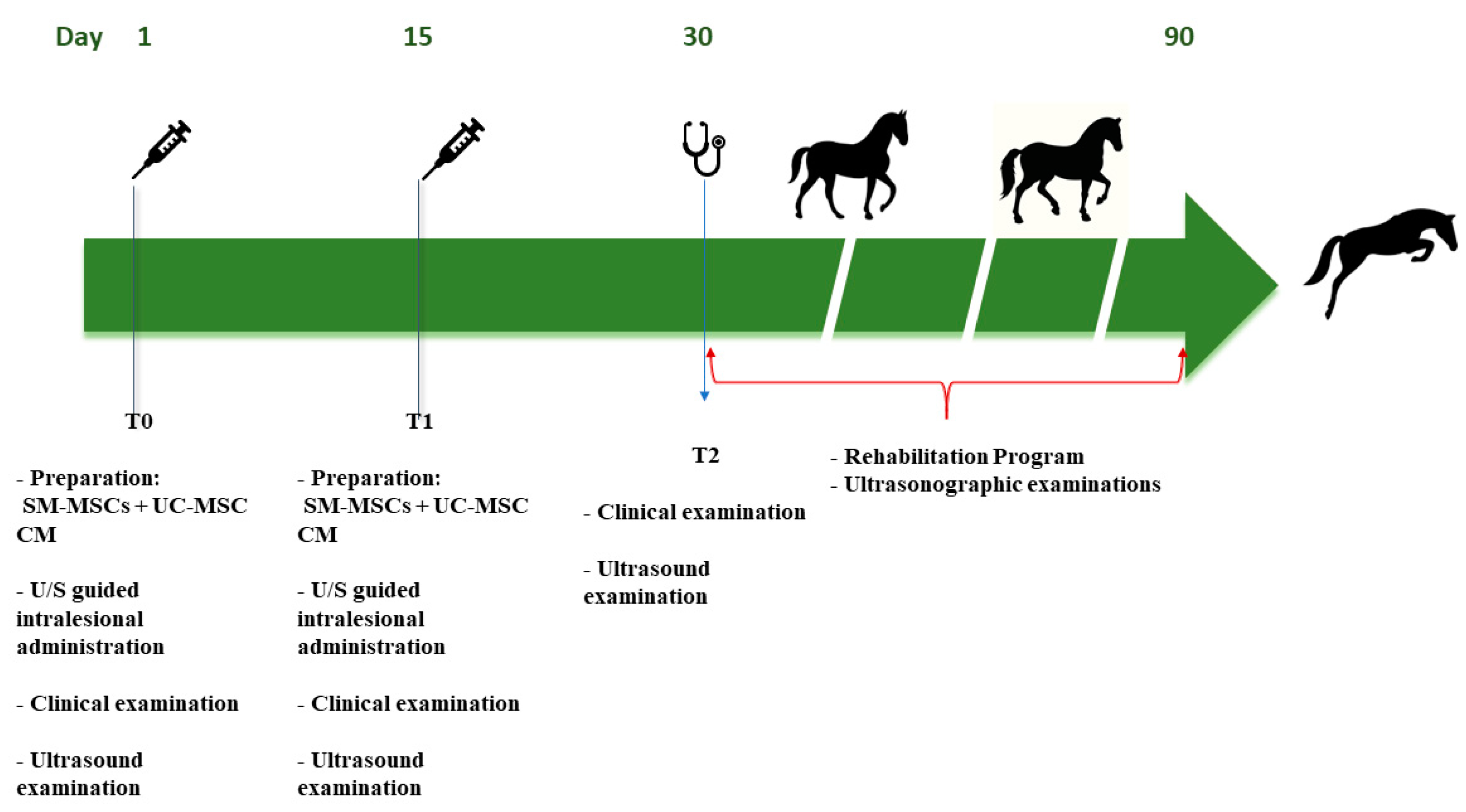
2.10. Treatment Protocol
2.10.1. Intralesional eSM-MSCs + eUC-MSCs CM administration
2.10.2. Post-treatment monitoring - clinical evaluations
3. Results
3.1. Clinical evaluation
3.1.1. Radiological examination
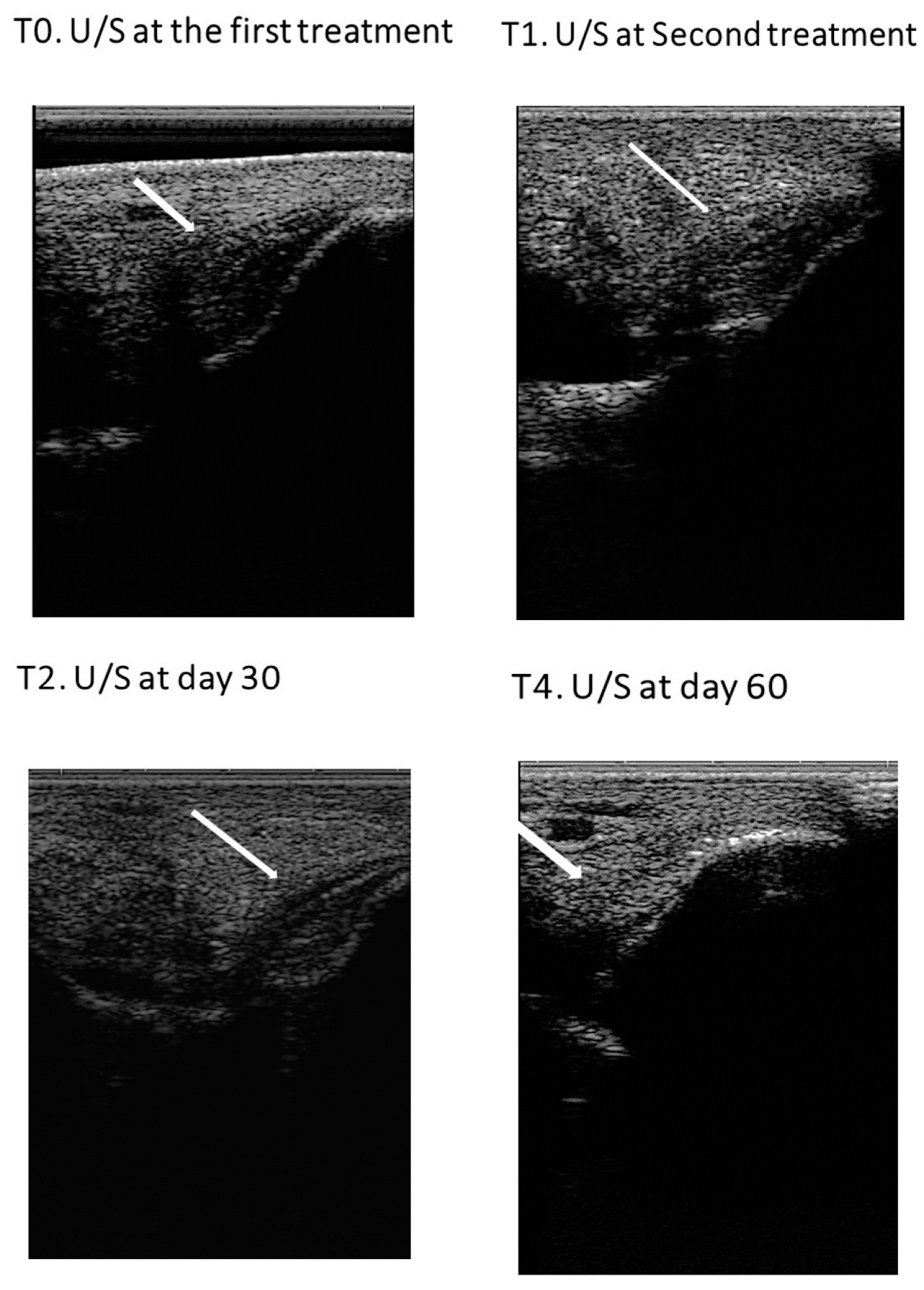
3.1.2. Ultrasound examination
3.2. MSCs isolation and characterization
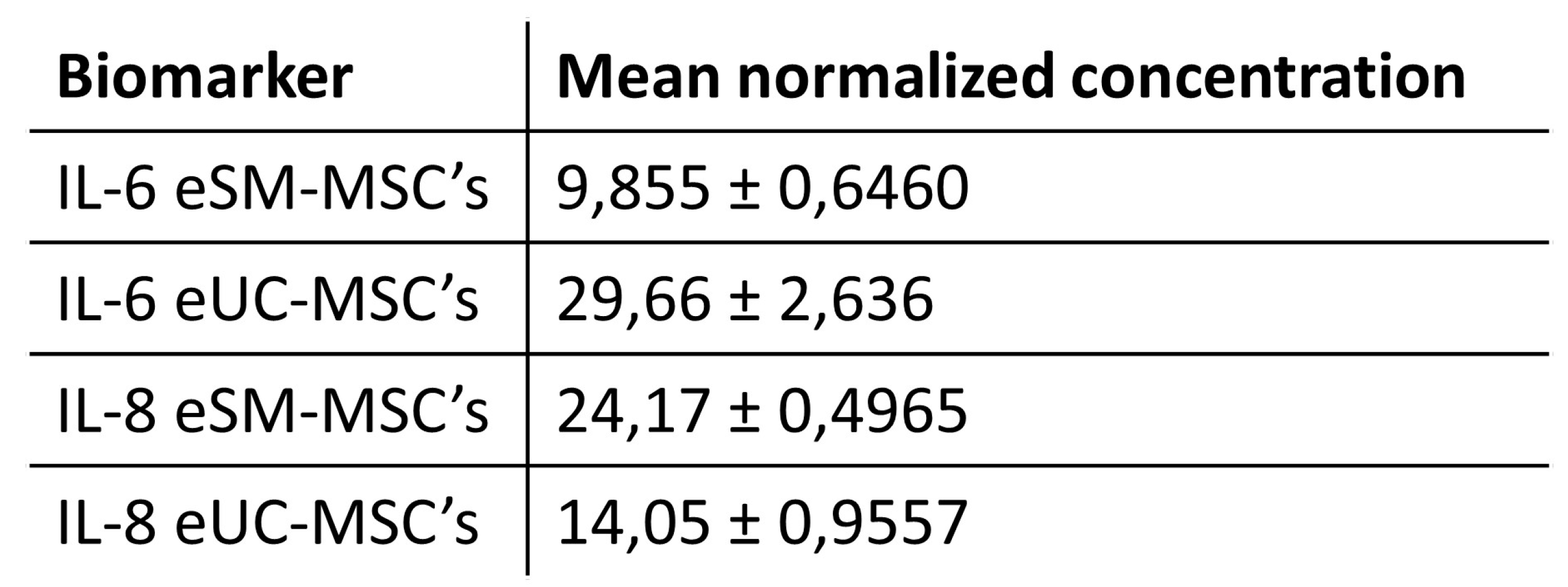
3.3. Secretome: conditioned medium analysis
3.4. Treatment Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D -Bidimensional |
| 3D - Tridimensional |
| AAEP - American Association of Equine Practitioners |
| ACS - Autologous conditioned serum |
| APS - Autologous protein solution |
| BM - MSC Bone Marrow Mesenchymal Stem Cells |
| CL - Collateral ligament |
| CM - Conditioned Medium |
| cm2 - Square centimetre |
| d - Days |
| DLPMO - Oblique Dorsolateral-plantaromedial |
| DMEM - Dulbecco’s Modified Eagle Medium |
| DMPLO - Oblique dorsomedial-plantarolateral |
| DMSO - Dimethylsulphoxide |
| DP - Dorso plantar |
| DPBS - Dulbecco′s Phosphate Buffered Saline |
| ECM - Extracellular matrix |
| eSM - MSCs Equine synovial membrane derived mesenchymal stem cells |
| eUC-MSCs Equine Umbilical cord-stroma derived mesenchymal stem cell |
| EV - Extracellular Vesicles |
| FBS - Fetal Bovine Serum |
| FGF-2 - Basic Fibroblast Growth Factor |
| G-CSF - Granulocyte Colony Stimulating Factor |
| GM-CSF - Granulocyte-macrophage Colony Stimulating Factor |
| IL - Interleukins |
| IL-1Ra - Interleukin one receptor antagonist |
| IRAP - Interleukin receptor antagonist protein |
| ISCT - International Society for Cellular Therapy |
| IV - Endovenous |
| KC/GRO - Human Growth-regulated oncogene/Keratinocyte Chemoattractant |
| Kg - Kilogram |
| Kv - Kilovolts |
| LLCL - Long lateral collateral ligament |
| LM - Lateromedial |
| LMCL - Long medial collateral ligament |
| mA - miliamperes |
| MCB - Master Cell Banks |
| MCP-1 - Monocyte Chemoattractant Protein-1 |
| mg - milligram |
| MHz - Megahertz |
| min - minutes |
| ml - millilitre |
| MMP-3 - Matrix metaloproteinase-3 |
| MSCs - Mesenchymal Stem Cells |
| OA - Osteoarthritis |
| ORBEA - Organismo Responsável pelo Bem-estar Animal |
| P - Passage |
| PBS - Phosphate-buffered saline |
| pg - picograms |
| PRP - Platelet-rich plasma |
| rpm - Rotations per minute |
| SEM - Standard error mean |
| SLCL - Short lateral collateral ligament |
| SMCL - Short medial collateral ligament |
| TGF-β - Transforming Growth factor-β |
| TNF-α - Tumor Necrosis Factor-α |
| U/S - Ultrasound |
| VEGF-R1 - Vascular endothelial growth factor |
References
- Ortved, K.F. Regenerative Medicine and Rehabilitation for Tendinous and Ligamentous Injuries in Sport Horses. Veter- Clin. North Am. Equine Pr. 2018, 34, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Duddy, H.R.; Schoonover, M.J.; Hague, B.A. Outcome following local injection of a liquid amnion allograft for treatment of equine tendonitis or desmitis – 100 cases. BMC Veter- Res. 2022, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stashak, T.S. Adams' lameness in horses.; Verlag M. & H. Schaper, 2008. [Google Scholar]
- Clegg, P. Differential diagnosis of a swollen hock in the horse. Pr. 2003, 25, 328–341. [Google Scholar] [CrossRef]
- Kümmerle, J.M.; Kummer, M.R. Arthroscopically Accessible Anatomy of the Tarsal Collateral Ligaments in the Horse. Veter- Surg. 2013, 42, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A. The horse in action: anatomy and biomechanics. Equine Heal. 2016, 2016, 15–17. [Google Scholar] [CrossRef]
- Schuurman, S.O.; Kersten, W.; Weijs, W.A. The equine hind limb is actively stabilized during standing. J. Anat. 2003, 202, 355–362. [Google Scholar] [CrossRef]
- Lohse, C.L.; Trout, D.R. Equine Limb Anatomy: Peroneus Tertius Muscle Relationships. Anat. Histol. Embryol. 1984, 13, 313–318. [Google Scholar] [CrossRef]
- Lamb, L.; Zubrod, C.; Hague, B.; Brakenhoff, J.; Major, M. Clinical outcome of collateral ligament injuries of the tarsus. Can. Veter- J. = La Rev. Veter- Can. 2012, 53, 518–24. [Google Scholar]
- Desmaizières, L.; Cauvin, E.R. Carpal collateral ligament desmopathy in three horses. Veter- Rec. 2005, 157, 197–201. [Google Scholar] [CrossRef]
- Blaik, M.A.; Hanson, R.R.; Kincaid, S.A.; Hathcock, J.T.; Hudson, J.A.; Baird, D.K. LOW-FIELD MAGNETIC RESONANCE IMAGING OF THE EQUINE TARSUS: NORMAL ANATOMY. Veter- Radiol. Ultrasound 2000, 41, 131–141. [Google Scholar] [CrossRef]
- Barker, W.; et al. Soft tissue injuries of the tarsocrural joint: a retrospective analysis of 30 cases evaluated arthro-scopically. Equine Veterinary Journal 2013, 45(4), 435–441. [Google Scholar] [CrossRef]
- Garrett, K.S. Ultrasonography of the hock. In Atlas of equine ultrasonography; 2022; pp. 173–188. [Google Scholar]
- Tokateloff, N.; Carmalt, J.; Manning, S. Trauma resulting in hemarthrosis and long medial collateral ligament desmitis of the tarsocrural joint in a horse. Can. Veter- J. = La Rev. Veter- Can. 2011, 52, 519–23. [Google Scholar]
- Dyson, S. Lameness associated with recurrent haemarthrosis in a horse. Equine Veter- J. 1986, 18, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Judy, C.E.; Galuppo, L.D. Evaluation of iatrogenic hemarthrosis of the metacarpophalangeal joint as a method of induction of temporary reversible lameness in horses. Am. J. Veter- Res. 2005, 66, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Ogata, K.; Miura, H.; Arizono, T.; Sugioka, Y. Spontaneous hemarthrosis of the knee in the elderly: Etiology and treatment. Arthrosc. J. Arthrosc. Relat. Surg. 1994, 10, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, C.E.; Eggleston, R.B.; Peroni, J.F.; Parks, A.H. Desmitis of the medial tarsal collateral ligament in 7 horses. Equine Veter- Educ. 2011, 24, 72–80. [Google Scholar] [CrossRef]
- Branch, M.V.; Murray, R.C.; Dyson, S.J.; Goodship, A.E. Magnetic Resonance Imaging of the Equine Tarsus. Clin. Tech. Equine Pr. 2007, 6, 96–102. [Google Scholar] [CrossRef]
- Vanderperren, K.; Raes, E.; Hoegaerts, M.; Saunders, J.H. Diagnostic imaging of the equine tarsal region using radiography and ultrasonography. Part 1: The soft tissues. Veter- J. 2009, 179, 179–187. [Google Scholar] [CrossRef]
- PARK, R.D.; NELSON, R.; HOOPES, P.J. Magnetic resonance imaging of the normal equine digit and met-acarpophalangeal joint. Veterinary radiology 1987, 28, 105–116. [Google Scholar] [CrossRef]
- Kraft, S.L.; Gavin, P. Physical Principles and Technical Considerations for Equine Computed Tomography and Magnetic Resonance Imaging. Veter- Clin. North Am. Equine Pr. 2001, 17, 115–130. [Google Scholar] [CrossRef]
- Fraschetto, C.; Dancot, M.; Vandersmissen, M.; Denoix, J.-M.; Coudry, V. Conservative management of equine tarsal collateral ligament injuries may allow return to normal performance. J. Am. Veter- Med Assoc. 2023, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dik, K.J. ULTRASONOGRAPHY OF THE EQUINE TARSUS. Veter- Radiol. Ultrasound 1993, 34, 36–43. [Google Scholar] [CrossRef]
- Mair, T.S.; Kinns, J.; Jones, R.D.; Bolas, N.M. Magnetic resonance imaging of the distal limb of the standing horse. Equine Veter- Educ. 2010, 17, 74–78. [Google Scholar] [CrossRef]
- Bramlage, L.R. Traumatic and developmental lesions of the tarsus. in Proc. Am. Assoc. Equine Pract. 2006.
- Whitcomb, M.B. Ultrasonography of the equine tarsus. In Proceedings of the 52nd Annual Convention of the American Association of Equine Practitioners, San Antonio, Texas, USA, 2006. 2006. American Association of Equine Practitioners (AAEP)., 2-6 December.
- Chandra, V.; Mankuzhy, P.; G. , T.S. Mesenchymal Stem Cells in Veterinary Regenerative Therapy: Basic Physiology and Clinical Applications. Curr. Stem Cell Res. Ther. 2022, 17, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Platonova, S.; Korovina, D.; Viktorova, E.; Savchenkova, I. Equine Tendinopathy Therapy Using Mesenchymal Stem Cells. KnE Life Sci. 2021, 533–541. [Google Scholar] [CrossRef]
- Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative Medicine for Equine Musculoskeletal Diseases. Animals 2021, 11, 234. [Google Scholar] [CrossRef]
- Tuemmers, C.; Rebolledo, N.; Aguilera, R. Effect of the application of stem cells for tendon injuries in sporting horses. Arch. de Med. Veter- 2012, 44, 207–215. [Google Scholar] [CrossRef]
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341. [Google Scholar] [CrossRef]
- González-González, A.; et al. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative med-icine. World journal of stem cells 2020, 12, 1529. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Maguire, G. Stem cell therapy without the cells. Commun. Integr. Biol. 2013, 6, e26631. [Google Scholar] [CrossRef]
- Moll, G.; Hoogduijn, M.J.; Ankrum, J.A. Editorial: Safety, Efficacy and Mechanisms of Action of Mesenchymal Stem Cell Therapies. Front. Immunol. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Sun, D.Z.; Abelson, B.; Babbar, P.; Damaser, M.S. Harnessing the mesenchymal stem cell secretome for regenerative urology. Nat. Rev. Urol. 2019, 16, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Shin, S.; Do, M.; Oh, B.H.; Song, Y.; Bui, V.D.; Lee, E.S.; Jo, D.-G.; Cho, Y.W.; Kim, D.-H.; et al. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J. Control. Release 2020, 330, 15–30. [Google Scholar] [CrossRef]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochem. (Moscow) 2019, 84, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.-H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef]
- Harman, R.M.; Marx, C.; Van de Walle, G.R. Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Raik, S. Kumar, and S. Bhattacharyya, Insights into cell-free therapeutic approach: Role of stem cell “soup-ernatant”. Biotechnology and Applied Biochemistry 2018, 65, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 2021, 12, 1–30. [Google Scholar] [CrossRef]
- Broeckx, S.Y.; Seys, B.; Suls, M.; Vandenberghe, A.; Mariën, T.; Adriaensen, E.; Declercq, J.; Van Hecke, L.; Braun, G.; Hellmann, K.; et al. Equine Allogeneic Chondrogenic Induced Mesenchymal Stem Cells Are an Effective Treatment for Degenerative Joint Disease in Horses. Stem Cells Dev. 2019, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Committee, A.H.S. Guide to veterinary services for horse shows; American Association of Equine Practitioners: Lexington, 1999. [Google Scholar]
- Practitioners, A.A.o.E. Guide for veterinary service and judging of equestrian events. 1991. AAEP.
- Reis, I.L.; Lopes, B.; Sousa, P.; Sousa, A.C.; Branquinho, M.; Caseiro, A.R.; Pedrosa, S.S.; Rêma, A.; Oliveira, C.; Porto, B.; et al. Allogenic Synovia-Derived Mesenchymal Stem Cells for Treatment of Equine Tendinopathies and Desmopathies—Proof of Concept. Animals 2023, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.J.; et al. Position Statement: Minimal criteria for reporting veterinary and animal medicine research for mesenchymal stromal/stem cells in orthopaedic applications. Frontiers in Veterinary Science 2022, 199. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. J. Orthop. Res. 2015, 33, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Schils, S.; Turner, T. Review of early mobilization of muscle, tendon, and ligament after injury in equine reha-bilitation. In Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners, Bal-timore, Maryland, USA, 4-8 December 2010; American Association of Equine Practitioners (AAEP), 2010. [Google Scholar]
- Kaneps, A.J. Practical Rehabilitation and Physical Therapy for the General Equine Practitioner. Veter- Clin. North Am. Equine Pr. 2016, 32, 167–180. [Google Scholar] [CrossRef]
- Davidson, E.J. Controlled Exercise in Equine Rehabilitation. Veter- Clin. North Am. Equine Pr. 2016, 32, 159–165. [Google Scholar] [CrossRef]
- Davidson, E.J. Lameness Evaluation of the Athletic Horse. Veter- Clin. North Am. Equine Pr. 2018, 34, 181–191. [Google Scholar] [CrossRef]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Veter- J. 2011, 44, 25–32. [Google Scholar] [CrossRef]
- Bami, M.; Sarlikiotis, T.; Milonaki, M.; Vikentiou, M.; Konsta, E.; Kapsimali, V.; Pappa, V.; Koulalis, D.; O Johnson, E.; Soucacos, P.N. Superiority of synovial membrane mesenchymal stem cells in chondrogenesis, osteogenesis, myogenesis and tenogenesis in a rabbit model. Injury 2020, 51, 2855–2865. [Google Scholar] [CrossRef]
- De Bari, C.; et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis & Rheumatism 2001, 44, 1928–1942. [Google Scholar]
- Mocchi, M.; Dotti, S.; Del Bue, M.; Villa, R.; Bari, E.; Perteghella, S.; Torre, M.L.; Grolli, S. Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells 2020, 9, 1453. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Al Naem, M.; Bourebaba, L.; Kucharczyk, K.; Röcken, M.; Marycz, K. Therapeutic mesenchymal stromal stem cells: Isolation, characterization and role in equine regenerative medicine and metabolic disorders. Stem Cell Rev. Rep. 2019, 16, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Hosseini-Beheshti, E.; Grau, G.E.; Zreiqat, H.; Little, C.B. Stem Cell-Derived Extracellular Vesicles for Treating Joint Injury and Osteoarthritis. Nanomaterials 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2015, 2016, 1–11. [Google Scholar] [CrossRef]
- Pawitan, J.A. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. BioMed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed]
- Shireman, P.K.; Contreras-Shannon, V.; Ochoa, O.; Karia, B.P.; Michalek, J.E.; McManus, L.M. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J. Leukoc. Biol. 2007, 81, 775–785. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Chen, X.; Li, G.; Chan, K.-M.; Heng, B.C.; Yin, Z.; Ouyang, H.-W. Concise Review: Stem Cell Fate Guided By Bioactive Molecules for Tendon Regeneration. STEM CELLS Transl. Med. 2018, 7, 404–414. [Google Scholar] [CrossRef]
- Shiomi, A.; Usui, T.; Mimori, T. GM-CSF as a therapeutic target in autoimmune diseases. Inflamm. Regen. 2016, 36, 1–9. [Google Scholar] [CrossRef]
- Wright, C.R.; Ward, A.C.; Russell, A.P. Granulocyte Colony-Stimulating Factor and Its Potential Application for Skeletal Muscle Repair and Regeneration. Mediat. Inflamm. 2017, 2017, 7517350. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.; Marvin, J.C.; Vaughn, B.; Andarawis-Puri, N. Innate tissue properties drive improved tendon healing in MRL/MpJ and harness cues that enhance behavior of canonical healing cells. FASEB J. 2020, 34, 8341–8356. [Google Scholar] [CrossRef]
- Al-Sadi, O.; et al. Tenocytes, pro-inflammatory cytokines and leukocytes: a relationship? Muscles, ligaments and tendons journal 2011, 1, 68. [Google Scholar]
- Abumaree, M.; Al Jumah, M.; Pace, R.A.; Kalionis, B. Immunosuppressive Properties of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 8, 375–392. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and mus-culoskeletal therapies. Stem cell research & therapy 2016, 7, 1–14. [Google Scholar]
- Galun, E.; Rose-John, S. The regenerative activity of interleukin-6. Tissue-Protective Cytokines: Methods and Protocols 2013, 59–77. [Google Scholar]
- Hirota, H.; Kiyama, H.; Kishimoto, T.; Taga, T. Accelerated Nerve Regeneration in Mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J. Exp. Med. 1996, 183, 2627–2634. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Domeij-Arverud, E.; Leclerc, P.; Amoudrouz, P.; Nader, G.A. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surgery, Sports Traumatol. Arthrosc. 2012, 21, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, G.; Li, K.; Zheng, H.; Wang, G.; Yu, B.; Zhang, K. Interleukin-6 Promotes Proliferation but Inhibits Tenogenic Differentiation via the Janus Kinase/Signal Transducers and Activators of Transcription 3 (JAK/STAT3) Pathway in Tendon-Derived Stem Cells. Medical science monitor: international medical journal of experimental and clinical research 2018, 24, 1567–1573. [Google Scholar] [CrossRef]
- Lin, T.W.; Cardenas, L.; Glaser, D.L.; Soslowsky, L.J. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J. Biomech. 2006, 39, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.B.; Pingel, J.; Kjaer, M.; Langberg, H.; Hammerman, M.; Blomgran, P.; Dansac, A.; Aspenberg, P.; Patel, S.H.; D’lugos, A.C.; et al. Interleukin-6: a growth factor stimulating collagen synthesis in human tendon. J. Appl. Physiol. 2011, 110, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-O.; Sim, J.A.; Choi, J.U.; Lee, B.K.; Park, H.G. The effect of interleukin-8 in the early stage after anterior cruciate ligament reconstruction with remnant preservation. Knee Surg. Relat. Res. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- Ohls, R.K.; Maheshwari, A. Hematology, Immunology and Infectious Disease: Neonatology Questions and Controversies: Expert Consult-Online and Print; Elsevier Health Sciences, 2012. [Google Scholar]
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53–53. [Google Scholar] [CrossRef]
- Wang, H.-N.; Rong, X.; Yang, L.-M.; Hua, W.-Z.; Ni, G.-X. Advances in Stem Cell Therapies for Rotator Cuff Injuries. Front. Bioeng. Biotechnol. 2022, 10, 866195. [Google Scholar] [CrossRef]
- Scala, M.; Lenarduzzi, S.; Spagnolo, F.; Trapasso, M.; Ottonello, C.; Muraglia, A.; Barla, A.; Squillario, M.; Strada, P. Regenerative medicine for the treatment of Teno-desmic injuries of the equine. A series of 150 horses treated with platelet-derived growth factors. Vivo 2014, 28. [Google Scholar]
- Raes, E.V.; Vanderperren, K.; Pille, F.; Saunders, J.H. Ultrasonographic findings in 100 horses with tarsal region disorders. Veter- J. 2010, 186, 201–209. [Google Scholar] [CrossRef]
- Bell, C.; Torske, K.; Lobb, B. Collateral ligament reconstruction in two horses following traumatic avulsion fracture using a knotless suture anchor construct. Equine Veter- Educ. 2016, 30, 360–366. [Google Scholar] [CrossRef]
- Salz, R.O.; Elliott, C.R.B.; Zuffa, T.; Bennet, E.D.; Ahern, B.J. Treatment of racehorse superficial digital flexor tendonitis: A comparison of stem cell treatments to controlled exercise rehabilitation in 213 cases. Equine Veter- J. 2023. [Google Scholar] [CrossRef]
- Rose, P.L.; Moore, I. Imaging diagnosis--avulsion of the medial collateral ligament of the tarsus in a horse. Veterinary Radiology & Ultrasound. The Official Journal of the American College of Veterinary Radi-ology and the International Veterinary Radiology Association 2003, 44, 657–659. [Google Scholar]
- Shokry, M.; Mostafa, A.; Tohamy, A.; El-Sharkawi, M. Autologous mesenchymal stem cells for treatment of acute superficial digital flexor tendonitis in athletic horses: clinical study of 15 cases. Pferdeheilkunde Equine Med. 2020, 36, 43–48. [Google Scholar] [CrossRef]
- Lui, P.; et al. What are the validated animal models for tendinopathy? Scandinavian journal of medicine & science in sports 2011, 21, 3–17. [Google Scholar]
- Melotti, L.; Carolo, A.; Elshazly, N.; Boesso, F.; Da Dalt, L.; Gabai, G.; Perazzi, A.; Iacopetti, I.; Patruno, M. Case Report: Repeated Intralesional Injections of Autologous Mesenchymal Stem Cells Combined With Platelet-Rich Plasma for Superficial Digital Flexor Tendon Healing in a Show Jumping Horse. Front. Veter- Sci. 2022, 9, 843131. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Friedman, R.J. Animal Models of Articular Cartilage Defect. Animal models in orthopaedic research 2020, 309–325. [Google Scholar] [CrossRef]
- Lönker, N.S.; Fechner, K.; El Wahed, A.A. Horses as a Crucial Part of One Health. Veter- Sci. 2020, 7, 28. [Google Scholar] [CrossRef]
| Parameter | Score | Clinical implication |
|---|---|---|
| AAEP Grading | 0 | No Lameness |
| 1 | Lameness not consistent | |
| 2 | Lameness consistent under certain circumstances | |
| 3 | Lameness consistently observable on a straight line. | |
| 4 | Obvious lameness at walk: marked nodding or shortened stride | |
| 5 | Minimal weight bearing lameness in motion or at rest | |
| Flexion Test | 0 | No flexion response |
| 1 | Mild flexion response | |
| 2 | Moderate flexion response | |
| 3 | Severe flexion response | |
| Pain to pressure | 0 | No pain to pressure |
| 1 | Mild pain to pressure | |
| 2 | Moderate pain to pressure | |
| 3 | Severe pain to pressure |
| Week | Exercise |
|---|---|
| 0-2 | 2 days: stall confinement Handwalk: 10 min Day 15: new treatment |
| 3-4 | 2 days: stall confinement Handwalk: 10 min VET-CHECK + U/S |
| 5 | Handwalk: 15 min |
| 6 | Handwalk: 20 min VET-CHECK + U/S |
| 7 | Handwalk: 25 min |
| 8 | Handwalk: 30 min VET-CHECK + U/S |
| 9-10 | Handwalk: 30 min + 5 min trot |
| 11-12 | Handwalk: 30 min + 10 min trot VET-CHECK + U/S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





