Submitted:
13 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Recruitment of Participants and Peripheral Blood Sample Collection
Metabolite Analysis
RNA Extraction and cDNA Synthesis
Primer Designing and Quantitative Real-Time PCR (RT-qPCR)
Statistical Analysis
3. Results
Demographic and Clinical Characteristics
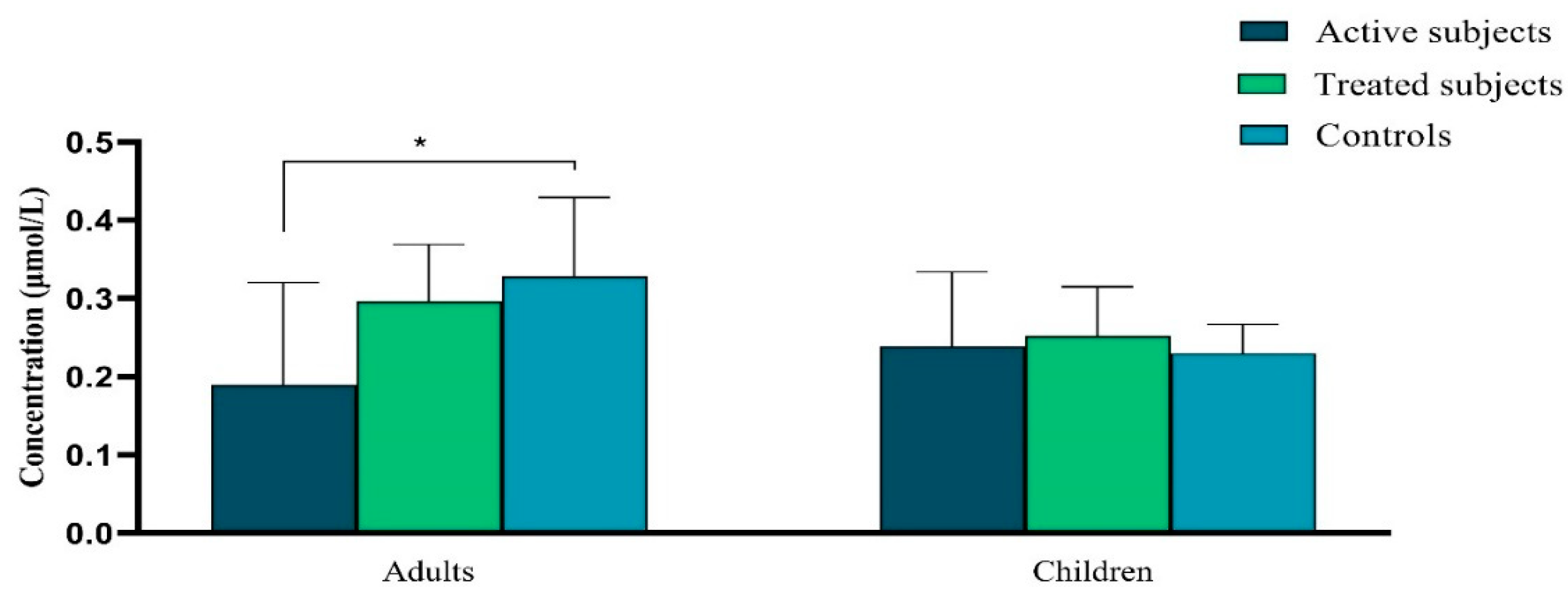
Vitamin A Levels
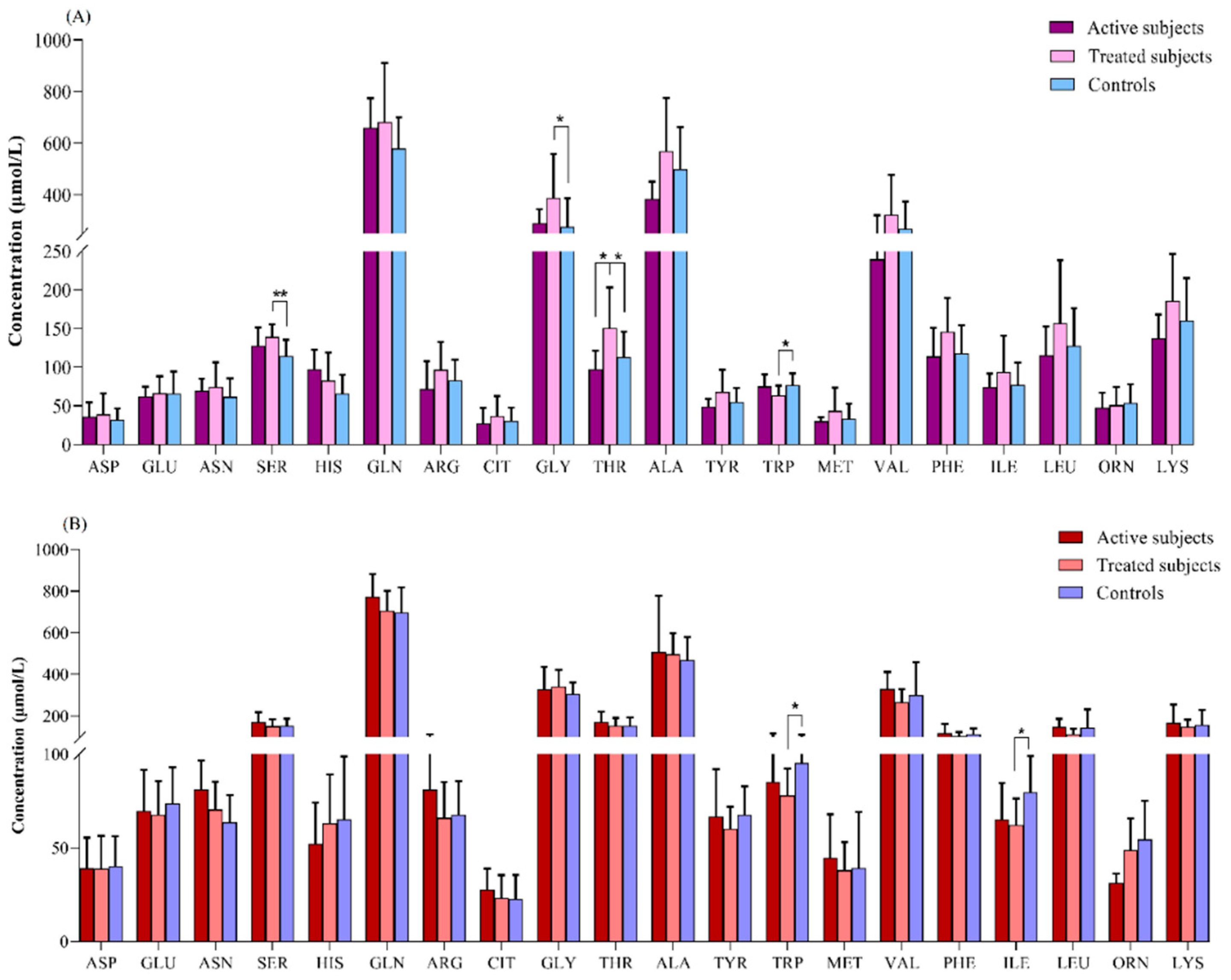
Amino Acids Profiles
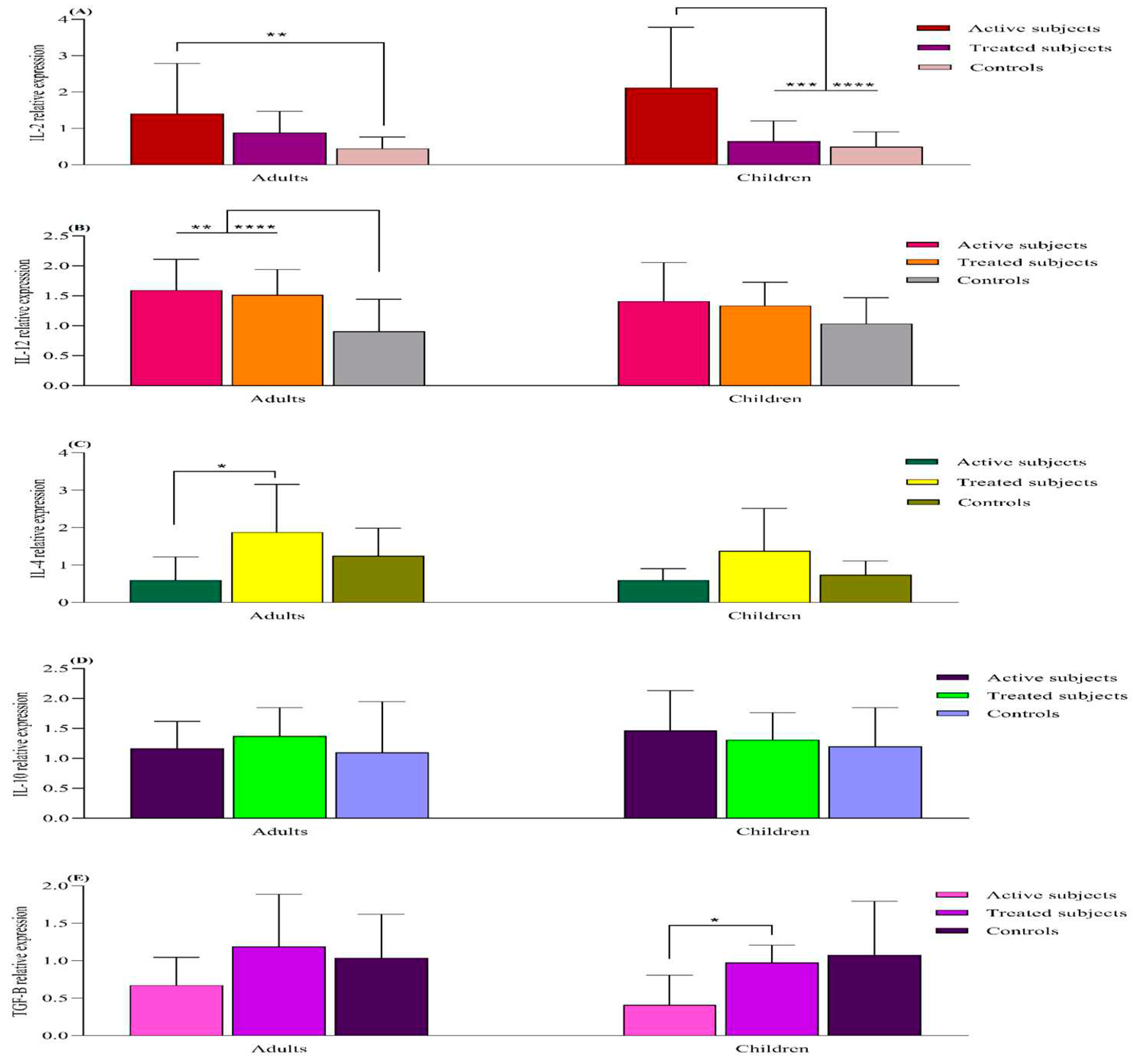
mRNA Expression Analysis
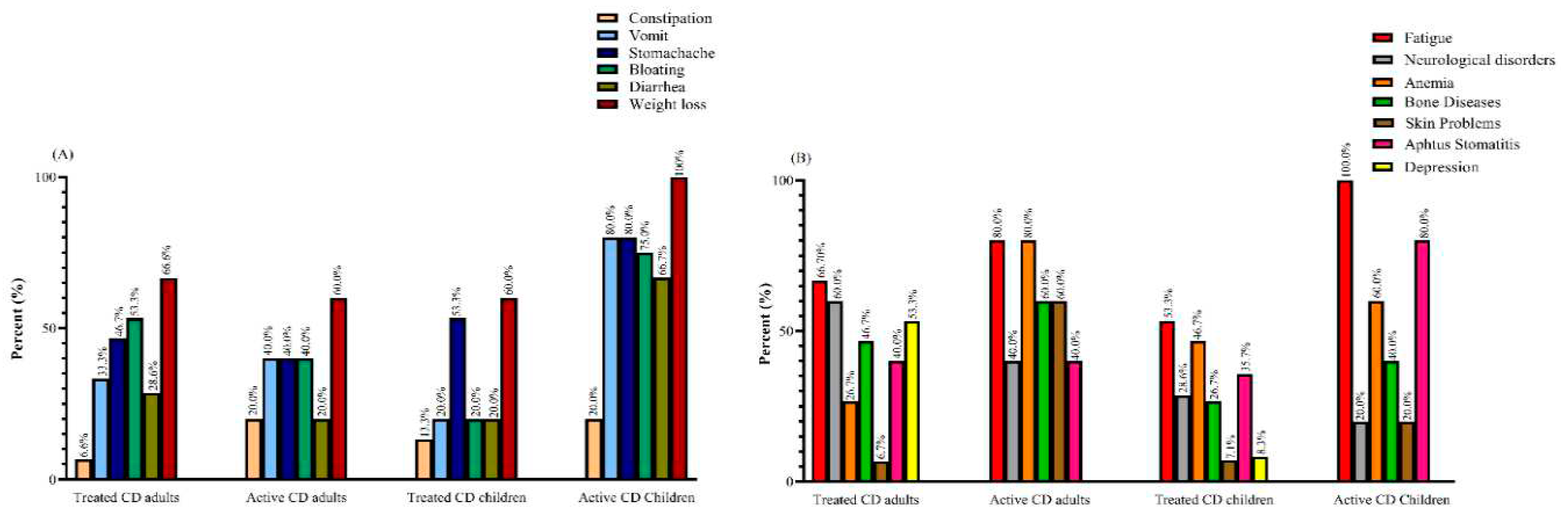
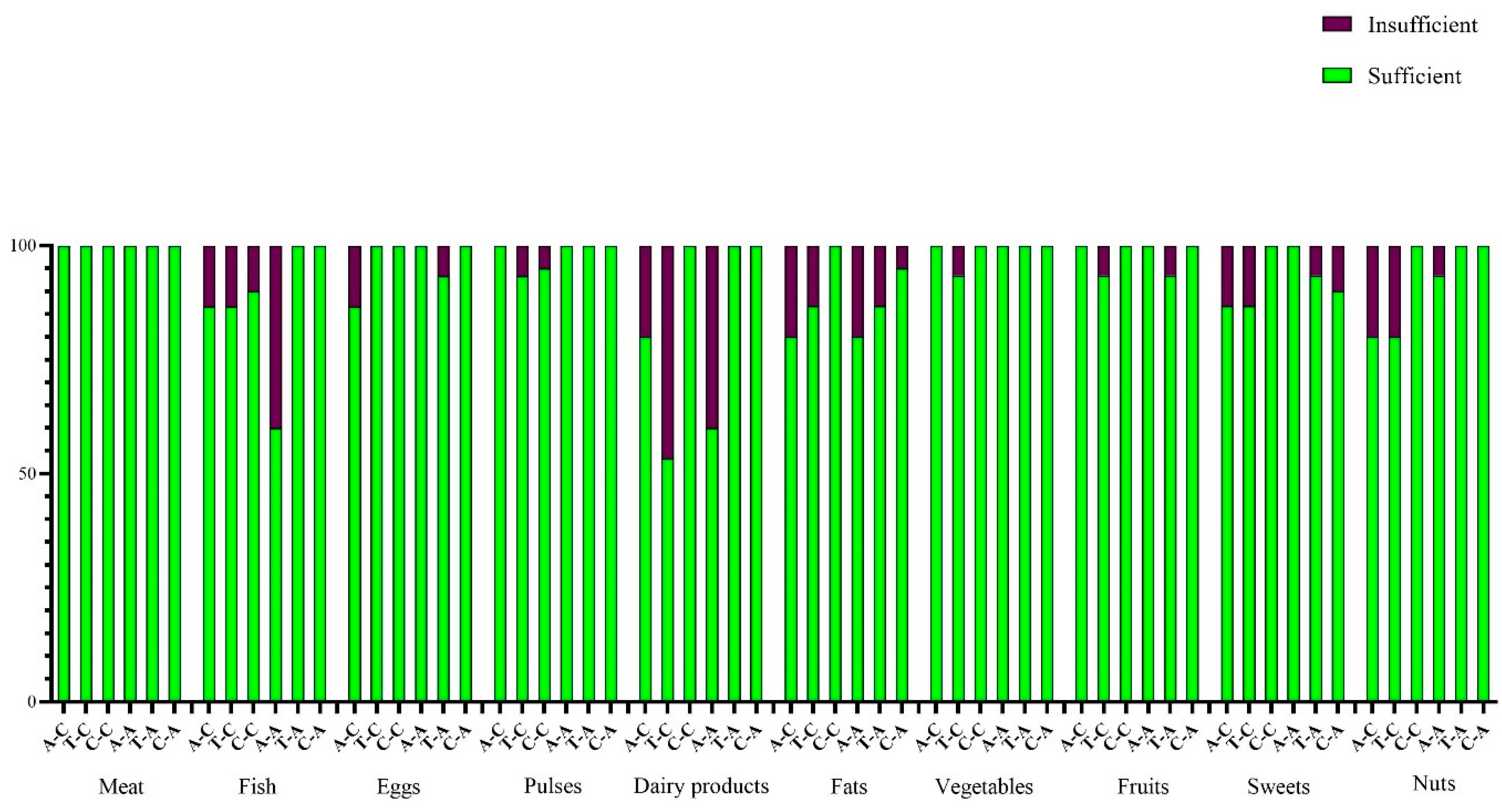
Dietary Habits
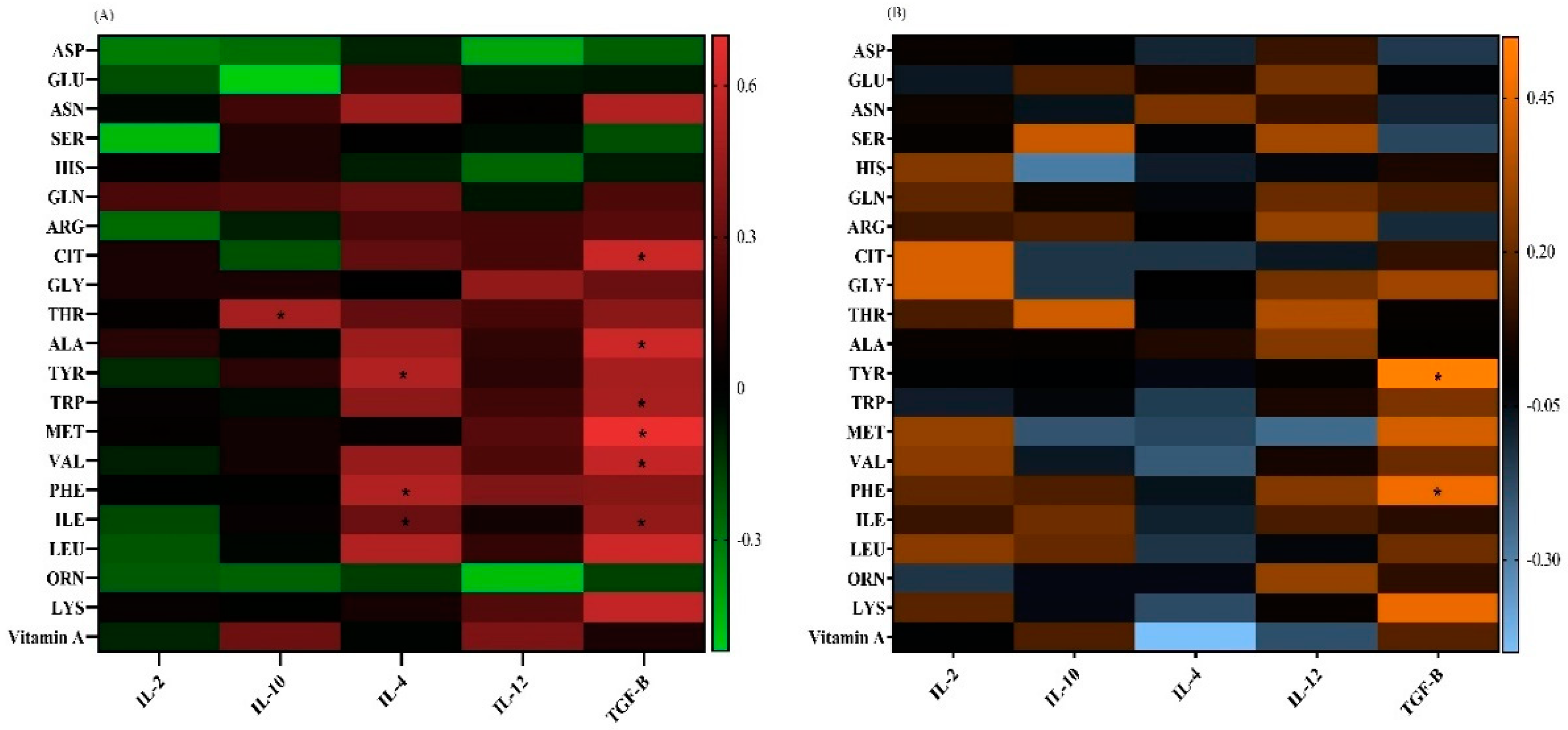
Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Saturni, L. Celiac disease, inflammation and oxidative damage: A nutrigenetic approach. Nutrients 2012, 4, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Rostami-Nejad, M.; Amani, D.; Rezaei-Tavirani, M.; Mohaghegh-Shalmani, H.; Zali, M.R. A rapid and sensitive assay to identify HLA-DQ2/8 risk alleles for celiac disease using real-time PCR method. Gastroenterol. Hepatol. Bed Bench 2018, 11, 250–258. [Google Scholar] [PubMed]
- Parzanese, I.; Qehajaj, D.; Patrinicola, F.; Aralica, M.; Chiriva-Internati, M.; Stifter, S.; Elli, L.; Grizzi, F. Celiac disease: From pathophysiology to treatment. World J. Gastrointest. Pathophysiol. 2017, 8, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-Free Diet. in Celiac Disease-Forever and for All? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef]
- Barone, M.V.; Auricchio, R.; Nanayakkara, M.; Greco, L.; Troncone, R.; Auricchio, S. Pivotal Role of Inflammation in Celiac Disease. Int. J. Mol. Sci. 2022, 23, 7177. [Google Scholar] [CrossRef]
- Mazzarella, G.; Aufiero, V. Immunoregulation in celiac disease. Gastroenterol. Hepatol. Endosc. 2016, 1, 13–17. [Google Scholar] [CrossRef]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef]
- Tran, T.H.; Smith, C.; Mangione, R.A. Drug absorption in celiac disease. Am. J. Health-Syst. Pharm. 2013, 70, 2199–2206. [Google Scholar] [CrossRef]
- Unalp-Arida, A.; Liu, R.; Ruhl, C.E. Nutrient intake differs among persons with celiac disease and gluten-related disorders in the United States. Sci. Rep. 2022, 12, 5566. [Google Scholar] [CrossRef]
- McGrogan, L.; Mackinder, M.; Stefanowicz, F.; Aroutiounova, M.; Catchpole, A.; Wadsworth, J.; Buchanan, E.; Cardigan, T.; Duncan, H.; Hansen, R.; et al. Micronutrient deficiencies in children with coeliac disease; a double-edged sword of both untreated disease and treatment with gluten-free diet. Clin. Nutr. 2021, 40, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Krupa-Kozak, U.; Ciska, E.; Jarocka-Cyrta, E. Plasma profile and urine excretion of amino acids in children with celiac disease on gluten-free diet after oligofructose-enriched inulin intervention: Results of a randomised placebo-controlled pilot study. Amino Acids 2018, 50. [Google Scholar] [CrossRef] [PubMed]
- Chea, E.P.; Lopez, M.J.; Milstein, H.; Vitamin, A. In StatPearls; Treasure Island, FL, USA, 2022.
- Dzopalic, T.; Bozic-Nedeljkovic, B.; Jurisic, V. The role of vitamin A and vitamin D in modulation of the immune response with a focus on innate lymphoid cells. Cent. Eur. J. Immunol. 2021, 46, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Cannons, J.L.; Grainger, J.R.; Dos Santos, L.M.; Hand, T.W.; Naik, S.; Wohlfert, E.A.; Chou, D.B.; Oldenhove, G.; Robinson, M.; et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 2011, 34, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Raverdeau, M.; Mills, K.H. Modulation of T cell and innate immune responses by retinoic Acid. J. Immunol. 2014, 192, 2953–2958. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Awasthi, A. Vitamin A and the Immune System; 2019; pp. 53–73.
- Cassani, B.; Villablanca, E.J.; De Calisto, J.; Wang, S.; Mora, J.R. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Asp. Med. 2012, 33, 63–76. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Rasooly, R.; Jiang, X.; Ceddia, M.A.; Weaver, C.T.; Chandraratna, R.A.; Bucy, R.P. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J. Immunol. 2002, 168, 4495–4503. [Google Scholar] [CrossRef]
- Stephensen, C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001, 21, 167–192. [Google Scholar] [CrossRef]
- DePaolo, R.W.; Abadie, V.; Tang, F.; Fehlner-Peach, H.; Hall, J.A.; Wang, W.; Marietta, E.V.; Kasarda, D.D.; Waldmann, T.A.; Murray, J.A.; et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 2011, 471, 220–224. [Google Scholar] [CrossRef]
- Hall, Jason, A. ; Grainger, John, R.; Spencer, Sean, P.; Belkaid, Y. The Role of Retinoic Acid in Tolerance and Immunity. Immunity 2011, 35, 13–22. [Google Scholar] [CrossRef]
- Oliveira, L.d.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Important roles of amino acids in immune responses. Br. J. Nutr. 2022, 127, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. Tryptophan catabolism and T-cell tolerance: Immunosuppression by starvation? Immunol. Today 1999, 20, 469–473. [Google Scholar] [CrossRef]
- Torres, M.I.; López-Casado, M.A.; Lorite, P.; Ríos, A. Tryptophan metabolism and indoleamine 2,3-dioxygenase expression in coeliac disease. Clin. Exp. Immunol. 2007, 148, 419–424. [Google Scholar] [CrossRef]
- Upadhyay, D.; Das, P.; Dattagupta, S.; Makharia, G.K.; Jagannathan, N.R.; Sharma, U. NMR based metabolic profiling of patients with potential celiac disease elucidating early biochemical changes of gluten-sensitivity: A pilot study. Clin. Chim. Acta Int. J. Clin. Chem. 2022, 531, 291–301. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, C.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 3183. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J.; Lu, R.; Ji, Q.; Xu, J.G. Effect of glutamate on inflammatory responses of intestine and brain after focal cerebral ischemia. World J. Gastroenterol. 2005, 11, 733–736. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Zhao, X.; Yang, Y.; Tian, C. Antioxidant and anti-inflammatory peptide fraction from oyster soft tissue by enzymatic hydrolysis. Food Sci. Nutr. 2020, 8, 3947–3956. [Google Scholar] [CrossRef]
- van Hees, N.J.; Giltay, E.J.; Tielemans, S.M.; Geleijnse, J.M.; Puvill, T.; Janssen, N.; van der Does, W. Essential amino acids in the gluten-free diet and serum in relation to depression in patients with celiac disease. PLoS ONE 2015, 10, e0122619. [Google Scholar] [CrossRef]
- Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A.; Berkenpas, M.; Mulder, C.J.; van Bodegraven, A.A. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef] [PubMed]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch. Dis. Child. 1990, 65, 909–911. [Google Scholar] [CrossRef]
- AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology 2006, 131, 1977–1980. [CrossRef]
- Marcus, J.B. Chapter 12—Global Food and Nutrition: World Food, Health and the Environment: Practical Applications for Nutrition, Food Science and Culinary Professionals. In Culinary Nutrition; Marcus, J.B., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 545–605. [Google Scholar]
- Weintraub, Y.; Ben-Tov, A.; Dotan, G.; Yerushalmy-Feler, A.; Weiner, D.; Levy, D.; Lubetzky, R.; Cohen, S. Vitamin A levels are comparable between children with newly diagnosed coeliac disease and non-coeliac controls. Acta Paediatr. 2019, 108, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Khalkhal, E.; Rezaei-Tavirani, M.; Fathi, F.; Nobakht, M.G.B.F.; Taherkhani, A.; Rostami-Nejad, M.; Asri, N.; Haidari, M.H. Screening of Altered Metabolites and Metabolic Pathways in Celiac Disease Using NMR Spectroscopy. Biomed. Res. Int. 2021, 2021, 1798783. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Ektefa, F.; Arefi Oskouie, A.; Rostami, K.; Rezaei-Tavirani, M.; Mohammad Alizadeh, A.H.; Tafazzoli, M.; Rostami Nejad, M. NMR based metabonomics study on celiac disease in the blood serum. Gastroenterol. Hepatol. Bed Bench 2013, 6, 190–194. [Google Scholar]
- Rémond, D.; Buffière, C.; Godin, J.P.; Mirand, P.P.; Obled, C.; Papet, I.; Dardevet, D.; Williamson, G.; Breuillé, D.; Faure, M. Intestinal inflammation increases gastrointestinal threonine uptake and mucin synthesis in enterally fed minipigs. J. Nutr. 2009, 139, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, Y.; Ma, J.; Yin, J.; Chen, S. The Effects of Dietary Glycine on the Acetic Acid-Induced Mouse Model of Colitis. Mediat. Inflamm. 2020, 2020, 5867627. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Kamada, N. 10 the Role of Dietary L-Serine in the Regulation of Intestinal Mucus Barrier during Inflammation. Gastroenterology 2020, 158, S70. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Eicher, S.D.; Ajuwon, K.M.; Applegate, T.J. Effect of threonine on secretory immune system using a chicken intestinal ex vivo model with lipopolysaccharide challenge. Poult. Sci. 2017, 96, 3043–3051. [Google Scholar] [CrossRef]
- Gaifem, J.; Gonçalves, L.G.; Dinis-Oliveira, R.J.; Cunha, C.; Carvalho, A.; Torrado, E.; Rodrigues, F.; Saraiva, M.; Castro, A.G.; Silvestre, R. L-Threonine Supplementation During Colitis Onset Delays Disease Recovery. Front. Physiol. 2018, 9, 1247. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-B.; Choi, B.-C.; Baek, K.-H. PGK1 modulates balance between pro- and anti-inflammatory cytokines by interacting with ITI-H4. Biomed. Pharmacother. 2023, 161, 114437. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Bertazzo, A.; Ragazzi, E.; Visioli, F. Evolution of tryptophan and its foremost metabolites’ concentrations in milk and fermented dairy products. PharmaNutrition 2016, 4, 62–67. [Google Scholar] [CrossRef]
- Zingone, F.; Iovino, P.; Bucci, C.; Ciacci, C. Coeliac disease: No difference in milk and dairy products consumption in comparison with controls. BMJ Nutr. Prev. Health 2019, 2, 39–42. [Google Scholar] [CrossRef]
- Lamas, B.; Hernandez-Galan, L.; Galipeau, H.J.; Constante, M.; Clarizio, A.; Jury, J.; Breyner, N.M.; Caminero, A.; Rueda, G.; Hayes, C.L.; et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Ray, K. Connecting coeliac disease to the AhR pathway. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 6–6. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Wang, Y.; Fu, L. D-tryptophan triggered epithelial-mesenchymal transition by activating TGF-β signaling pathway. Food Sci. Hum. Wellness 2022, 11, 1215–1221. [Google Scholar] [CrossRef]
- Del Zotto, B.; Mumolo, G.; Pronio, A.M.; Montesani, C.; Tersigni, R.; Boirivant, M. TGF-beta1 production in inflammatory bowel disease: Differing production patterns in Crohn’s disease and ulcerative colitis. Clin. Exp. Immunol. 2003, 134, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Sun, R.; Wang, Q.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; Luo, Y.; Yan, H.; et al. l-Isoleucine Administration Alleviates DSS-Induced Colitis by Regulating TLR4/MyD88/NF-κB Pathway in Rats. Front. Immunol. 2021, 12, 817583. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gu, C.; Ren, M.; Chen, D.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, J.; Luo, Y.; et al. l-Isoleucine Administration Alleviates Rotavirus Infection and Immune Response in the Weaned Piglet Model. Front. Immunol. 2018, 9, 1654. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.C.; Maes, M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J. Psychiatry Neurosci. Jpn. 2004, 29, 11–17. [Google Scholar] [PubMed]
- Meysam, Z.; Mina, A.; Ali Akbar Saboor, Y.; Sama, B.; Fariba, K.; Mohammad Hossein, H.; Mohammad Ali, S.; Niaz Mohammadzadeh, H. Does vitamin A supplementation affect GATA3 and IL-4 genes expression in TCD4+ cell culture? A double blind randomized clinical trial on MS patients. J. Nutr. Sci. Diet. 2018, 4, 8–14. [Google Scholar]
- Manavalan, J.S.; Hernandez, L.; Shah, J.G.; Konikkara, J.; Naiyer, A.J.; Lee, A.R.; Ciaccio, E.; Minaya, M.T.; Green, P.H.; Bhagat, G. Serum cytokine elevations in celiac disease: Association with disease presentation. Hum. Immunol. 2010, 71, 50–57. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Skodje, G.I.; Sarna, V.K.; Dzuris, J.L.; Russell, A.K.; Goel, G.; Wang, S.; Goldstein, K.E.; Williams, L.J.; Sollid, L.M.; et al. Cytokine release after gluten ingestion differentiates coeliac disease from self-reported gluten sensitivity. United Eur. Gastroenterol. J. 2020, 8, 108–118. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Daveson, A.J.M.; Ee, H.C.; Goel, G.; MacDougall, J.; Acaster, S.; Goldstein, K.E.; Dzuris, J.L.; Neff, K.M.; Truitt, K.E.; et al. Elevated serum interleukin-2 after gluten correlates with symptoms and is a potential diagnostic biomarker for coeliac disease. Aliment. Pharmacol. Ther. 2019, 50, 901–910. [Google Scholar] [CrossRef]
- Hamza, T.; Barnett, J.B.; Li, B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int. J. Mol. Sci. 2010, 11, 789–806. [Google Scholar] [CrossRef]
- Balasubbramanian, D.; Goodlett, B.L.; Mitchell, B.M. Is IL-12 pro-inflammatory or anti-inflammatory? Depends on the blood pressure. Cardiovasc. Res. 2019, 115, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Björck, S.; Lindehammer, S.R.; Fex, M.; Agardh, D. Serum cytokine pattern in young children with screening detected coeliac disease. Clin. Exp. Immunol. 2015, 179, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Erben, U.; Kühl, A.A. The Role of T-Cell Subsets in Chronic Inflammation in Celiac Disease and Inflammatory Bowel Disease Patients: More Common Mechanisms or More Differences? Inflamm Intest. Dis. 2016, 1, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Georgy, J.; Arlt, Y.; Moll, J.; Ouzin, M.; Weitz, H.; Gremer, L.; Willbold, D.; Grötzinger, J.; Thives-Kurenbach, F.; Scheller, J.; et al. Tryptophan (W) at position 37 of murine IL-12/IL-23 p40 is mandatory for binding to IL-12Rβ1 and subsequent signal transduction. J. Biol. Chem. 2021, 297, 101295. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.L.; Miyajima, A.; Zurawski, G. Identification of three adjacent amino acids of interleukin-2 receptor beta chain which control the affinity and the specificity of the interaction with interleukin-2. EMBO J. 1992, 11, 2047–2053. [Google Scholar] [CrossRef]
| Adults | ||||||
|---|---|---|---|---|---|---|
|
Variables Groups |
Number | Gender | Age | BMI | ||
| Female | Male | |||||
| Controls | 20 | 10 (50%) | 10 (50%) | 35.25 ± 10.7 | 22.06 ± 8.33 | |
| Treated | 15 | 8 (53.3%) | 7 (46.7%) | 39.0 ± 8.87 | 26.28 ± 4.57 | |
| Active | 15 | 10 (66.6%) | 5 (33.3%) | 31.8 ± 12.71 | 21.68 ± 4.54 | |
| P-value | 0.49 | 0.34 | 0.12 | |||
| Children | ||||||
|
Variables Groups |
Number | Gender | Age | BMI | ||
| Girl | Boy | |||||
| controls | 20 | 10 (50%) | 10 (50%) | 10.9±3.97 | 19.69±4.45 | |
| Treated | 15 | 8 (53.3%) | 7 (46.7%) | 10.60±2.92 | 133.77±12.63 | |
| Active | 15 | 9 (60%) | 6 (40%) | 9.20±3.27 | 14.75±3.81 | |
| P-value | 0.92 | 0.63 | 0.16 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





