Submitted:
14 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Ice Nucleation and Its Hindering in the Presence of an Ice-Binding Protein
2.2. Ice Nucleation: A Theoretical Consideration
2.2.1. Ice Nucleation in Bulk Water Is Only Possible at Rather Low Temperatures
2.2.2. Ice Nucleation on the Ice-Binding Surfaces at High Subzero Temperatures
2.2.3. Ice-Binding Surfaces
2.2.4. Can an Antifreeze Protein Bind to Something That Was Not Evolved to Be an Ice Nucleator?
3. Discussion
3.1. Notes on Antifreeze Protein Functions
3.2. Notes on Ice Nucleators
3.3. Ice Nucleators and Antifreeze Proteins
4. Materials and Methods
4.1. Freezing Experiments Equipment
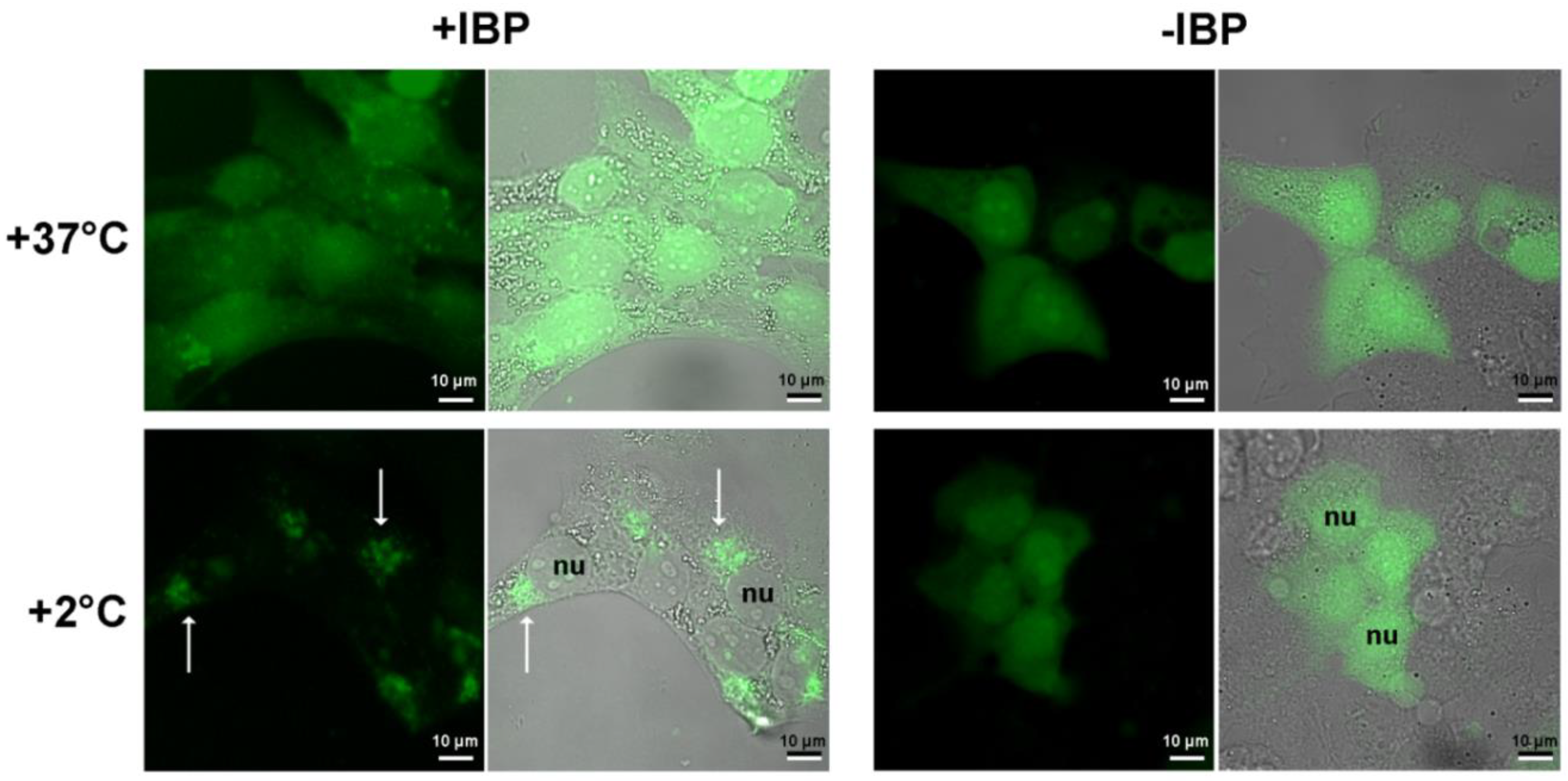
4.2. Experiments with the Human Cell Culture
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salt, R.W. Principles of insect cold-hardiness. Annu. Rev. Entomol. 1961, 6, 55–74. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Biochemistry of cryoprotectants. In Insects at Low Temperatures. Lee, R.E., Denlinger, D., Eds.; Chapman & Hall: New York, USA, 1991; pp. 64–93. [Google Scholar]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem Sci. 2014, 39, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Bar Dolev, M.; Braslavsky, I.; Davies, P.L. Ice-binding proteins and their function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Białkowska, A.; Majewska, E. , Olczak, A.; Twarda-Clapa, A. Ice binding proteins: Diverse biological roles and applications in different types of industry. Biomolecules 2020, 10, 274. [Google Scholar] [CrossRef]
- Duman, J.G. The role of macromolecular antifreeze in the darkling beetle, Meracantha contracta. J. Comp. Physiol. B 1977, 115, 279–286. [Google Scholar] [CrossRef]
- Harding, M.M.; Ward, L.G.; Haymet, A.D. Type I ‘antifreeze’ proteins. Structure–activity studies and mechanisms of ice growth inhibition. Eur. J. Biochem. 1999, 264, 653–665. [Google Scholar] [CrossRef]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef]
- DeVries, A.L. Glycoproteins as biological antifreeze agents in Antarctic fishes. Science 1971, 172, 1152–1155. [Google Scholar] [CrossRef]
- Knight, C.A.; Duman, J.G. Inhibition of recrystallization of ice by insect thermal hysteresis proteins: A possible cryoprotective role. Cryobiology, 1986, 23, 256–262. [Google Scholar] [CrossRef]
- Drori, R.; Celik, Y.; Davies, P.L.; Braslavsky, I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J. R. Soc. Interface 2014, 11, 20140526. [Google Scholar] [CrossRef]
- Rahman, A.T.; Arai, T.; Yamauchi, A.; Miura, A.; Kondo, H.; Ohyama, Y.; Tsuda, S. Ice recrystallization is strongly inhibited when antifreeze proteins bind to multiple ice planes. Sci. Rep. 2019, 9, 2212. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antarctic fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L.; Komatsu, S.K.; Feeney, R.E. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J. Biol. Chem. 1970, 245, 2901–2908. [Google Scholar] [CrossRef]
- Theede, H.; Schneppenheim, R.; Béress, L. Frostschutz-Glykoproteine bei Mytilus edulis? Mar. Biol. 1976, 36, 183–189, [in German]. [Google Scholar] [CrossRef]
- Duman, J.G. Thermal-hysteresis-factors in overwintering insects. J. Insect Physiol. 1979, 25, 805–810. [Google Scholar] [CrossRef]
- Duman, J.G. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu. Rev. Physiol. 2001, 63, 327–357. [Google Scholar] [CrossRef]
- Duman, J.G.; Olsen, T.M. Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. Cryobiology 1993, 30, 322–328. [Google Scholar] [CrossRef]
- Sun, X.; Griffith, M.; Pasternak, J.J.; Glick, B.R. Low temperature growth, freezing survival, and production of antifreeze protein by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 1995, 41, 776–784. [Google Scholar] [CrossRef]
- Bayer-Giraldi, M.; Uhlig, C.; John, U.; Mock, T.; Valentin, K. Antifreeze proteins in polar sea ice diatoms: Diversity and gene expression in the genus Fragilariopsis. Environ. Microbiol. 2010, 12, 1041–1052. [Google Scholar] [CrossRef]
- Gwak, I.G.; Jung, W.S.; Kim, H.J.; Kang, S.H.; Jin, E. Antifreeze protein in Antarctic marine diatom, Chaetoceros neogracile. Mar. Biotechnol. (NY) 2010, 12, 630–639. [Google Scholar] [CrossRef]
- Hoshino, T.; Kiriaki, M.; Ohgiya, S.; Fujiwara, M.; Kondo, H.; Nishimiya, Y.; Yumoto, I.; Tsuda, S. Antifreeze proteins from snow mold fungi. Canad. J. Bot. 2003, 81, 1175–1181. [Google Scholar] [CrossRef]
- Griffith, M.; Ala, P.; Yang, D.S.; Hon, W.C.; Moffatt, B.A. Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol. 1992, 100, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, M.E.; Duman, J.G.; Knight, C.A. Plant thermal hysteresis proteins. Biochim. Biophys. Acta 1992, 1121, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hudait, A.; Moberg, D.R.; Qiu, Y.; Odendahl, N.; Paesani, F.; Molinero, V. Preordering of water is not needed for ice recognition by hyperactive antifreeze proteins. Proc. Natl Acad. Sci. USA 2018, 115, 8266–8271. [Google Scholar] [CrossRef]
- Sun, Y.; Maltseva, D.; Liu, J.; Hooker II, T.; Mailänder, V.; Ramløv, H.; DeVries, A.L.; Bonn, M.; Meister, K. Ice recrystallization inhibition is insufficient to explain cryopreservation abilities of antifreeze proteins. Biomacromolecules 2022, 23, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Tas, R.P.; Hendrix, M.M.R.M.; Voets, I.K. Nanoscopy of single antifreeze proteins reveals that reversible ice binding is sufficient for ice recrystallization inhibition but not thermal hysteresis. Proc. Natl Acad. Sci. USA 2023, 120, e2212456120. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Drori, R.; Pertaya-Braun, N.; Altan, A.; Barton, T.; Bar-Dolev, M.; Groisman, A.; Davies, P.L.I.; Braslavsky, I. Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. Proc. Natl Acad. Sci. USA 2013, 110, 1309–1314. [Google Scholar] [CrossRef]
- Kuramochi, M.; Takanashi, C.; Yamauchi, A.; Doi, M.; Mio, K.; Tsuda, S.; Sasaki, Y.C. Expression of ice-binding proteins in Caenorhabditis elegans improves the survival rate upon cold shock and during freezing. Sci. Rep. 2019, 9, 6246. [Google Scholar] [CrossRef]
- Scholander, P.F.; Dam, L.V.; Kanwisher, J.W.; Hammel, H.T.; Gordon, M.S. Supercooling and osmoregulation in arctic fish. J. Cell. Comp. Physiol. 1957, 49, 5–24. [Google Scholar] [CrossRef]
- Frisbie, M. P.; Lee, R.E., Jr. Inoculative freezing and the problem of winter survival for freshwater macroinvertebrates. J. North Am. Benthol. Soc. 1997, 16, 635–650. [Google Scholar] [CrossRef]
- Praebel, K.; Hunt, B.; Hunt, L.H.; DeVries, A.L. The presence and quantification of splenic ice in the McMurdo Sound notothenioid fish, Pagothenia borchgrevinki (Boulenger, 1902). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, N.E. The freezing of supercooled water. Trans. Am. Phil. Soc. 1948, 38, 247–328. [Google Scholar] [CrossRef]
- Langham, E.J.; Mason, B.J. The heterogeneous and homogeneous nucleation of supercooled water. Proc. R. Soc. Lond. A 1958, 247, 493–504. [Google Scholar] [CrossRef]
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation; Springer: New York, USA, 2010; Chapter 7. [Google Scholar]
- Murray, B.J.; Broadley, S.L.; Wilson, T.W.; Bull, S.J.; Wills, R.H.; Christenson, H,K.; Murray, E.J. Kinetics of the homogeneous freezing of water. Phys. Chem. Chem. Phys. 2010, 12, 10380–10387. [Google Scholar] [CrossRef]
- Zeldovich, J.B. Toward the theory of formation of a new phase. Cavitation. J. Exp. Theor. Phys. 1942, 12, 525–538. [Google Scholar]
- Ubbelohde, A.R. Melting and Crystal Structure; Clarendon Press: Oxford, UK, 1965; Chapter 14. [Google Scholar]
- Chernov, A.A. Modern Crystallography III. Springer Berlin: Heidelberg, Germany, 1984. [CrossRef]
- Slezov, V.V. Kinetics of First Order Phase Transitions, 1st ed.; Wiley-VCH: Weinheim, Germany, 2009; Chapter 3. [Google Scholar]
- Ruckenstein, E.; Berim, G. Kinetic Theory of Nucleation; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Finkelstein, A.V. Some peculiarities of water freezing at small sub-zero temperatures. arXiv:2008.13682v1 [Preprint], 2020. Available at: https://doi.org/10.48550/arXiv.2008.13682 (Accessed on August 29, 2023). [CrossRef]
- Finkelstein, A.V.; Garbuzynskiy, S.O.; Melnik, B.S. How can ice emerge at 0 °C? Biomolecules 2022, 12, 981, Correction - in Biomolecules, 2023, in press. [Google Scholar] [CrossRef]
- Du, N.; Liu, X.Y.; Hew, C.L. Ice nucleation inhibition: Mechanism of antifreeze by antifreeze protein. J. Biol. Chem. 2003, 278, 36000–36004. [Google Scholar] [CrossRef]
- Inada, T.; Koyama, T.; Goto, F.; Seto, T. Inactivation of ice nucleating activity of silver iodide by antifreeze proteins and synthetic polymers. J. Phys. Chem. B 2012, 116, 5364–5371. [Google Scholar] [CrossRef]
- Glukhova, K.A.; Okulova, J.D.; Melnik, B.S. Designing and studying a mutant form of the ice-binding protein from Choristoneura fumiferana. bioRxiv [Preprint], 2020. Available at: https://doi.org/10.1101/2020.08.31.275651 (Accessed on August 29, 2023). [CrossRef]
- Deeva, A.A.; Glukhova, K.A.; Isoyan, L.S.; Okulova, Y.D.; Uversky, V.N.; Melnik, B.S. Design and analysis of a mutant form of the ice-binding protein from Choristoneura fumiferana. Protein J. 2022, 41, 304–314. [Google Scholar] [CrossRef]
- Tyshenko, M.G.; Doucet, D.; Davies, P.L.; Walker, V.K. The antifreeze potential of the spruce budworm thermal hysteresis protein. Nature Biotechnol. 1997, 15, 887–890. [Google Scholar] [CrossRef]
- Leinala, E.K.; Davies, P.L.; Doucet, D.; Tyshenko, M.G.; Walker, V.K.; Jia, Z. A β-helical antifreeze protein isoform with increased activity. J. Biol. Chem. 2002, 277, 33349–33352. [Google Scholar] [CrossRef] [PubMed]
- Leinala, E.K.; Davies, P.L.; Jia, Z. Crystal structure of β-helical antifreeze protein points to a general ice binding model. Structure 2002, 10, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.J. Biology of North American spruce budworms. In Tortricid Pests, their Biology, Natural Enemies and Control; van der Geest, L.P.S., Evenhuis, H.H., Eds.; Elsevier Science Publishers B.V.: Amsterdam, the Netherlands, 1991. [Google Scholar]
- Fukuda, H.; Arai, M.; Kuwajima, K. Folding of green fluorescent protein and the cycle3 mutant. Biochemistry 2000, 39, 12025–12032. [Google Scholar] [CrossRef]
- Glukhova, K.F.; Marchenkov, V.V.; Melnik, T.N.; Melnik, B.S. Isoforms of green fluorescent protein differ from each other in solvent molecules “trapped” inside this protein. J. Biomol. Struct. Dyn. 2017, 35, 1215–1225. [Google Scholar] [CrossRef]
- Melnik, B.S.; Finkelstein, A.V. Physical basis of functioning of antifreeze protein. Mol. Biol. (Moscow) 2022, 56, 297–305. [Google Scholar] [CrossRef]
- Doucet, D.; Tyshenko, M.G.; Kuiper, M.J.; Graether, S.P.; Sykes, B.D.; Daugulis, A.J.; Davies, P.L.; Walker, V.K. Structure-function relationships in spruce budworm antifreeze protein revealed by isoform diversity. Eur. J. Biochem. 2000, 267, 6082–6088. [Google Scholar] [CrossRef]
- Veselova, V.R.; Majorina, M.A.; Melnik, B.S. An experimental technique for accurate measurement of the freezing point of solutions and ice melting in the presence of biological objects on the examples of P. syringae and E. coli. Opera Med. Physiol. 2022, 9, 19–30. [Google Scholar] [CrossRef]
- Pawlowicz, R. Key physical variables in the ocean: temperature, salinity, and density. Nature Education Knowledge 2013, 4, 13. [Google Scholar]
- Melnik, B.S.; Glukhova, K.A.; Sokolova, E.A.; Balalaeva, I.V.; Finkelstein, A.V. A novel view on the mechanism of biological activity of antifreeze proteins. bioRxiv [Preprint], 2021. Available at: https://doi.org/10.1101/2021.09.22.461391 (Accessed on August 29, 2023). [CrossRef]
- Koop, T.; Murray, B.J. A physically constrained classical description of the homogeneous nucleation of ice in water. J. Chem. Phys. 2016, 145, 211915. [Google Scholar] [CrossRef]
- Gibbs, J.W. Graphical methods in the thermodynamics of fluids. Trans. Conn. Acad. 1873, 2, 309–342. [Google Scholar]
- Becker, R.; Döring, W. Kinetic treatment of grain-formation in super-saturated vapors. Ann. Phys. 1935, 416, 719–752. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics on CD; CRC Press: Boca Raton, FL, USA, 2005; Section 6. [Google Scholar]
- Hillig, W.B. Measurement of interfacial free energy for ice/water system. J. Cryst. Growth 1998, 183, 463–468. [Google Scholar] [CrossRef]
- Zaragoza, A.; Conde, M.M.; Espinosa, J.R.; Valeriani, C.; Vega, C.; Sanz, E. Competition between ices Ih and Ic in homogeneous water freezing. J. Chem. Phys. 2015, 143, 134504. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Corem, G.; Godsi, O.; Alexandrowicz, G.; Darling, G.R.; Hodgson, A. Ice nucleation on a corrugated surface. J. Am. Chem. Soc. 2018, 140, 15804–15811. [Google Scholar] [CrossRef]
- Lindow, S.E.; Arny, D.C.; Upper, C.D. Bacterial ice nucleation: A factor in frost injury to plants. Plant Physiol. 1982, 70, 1084–1089. [Google Scholar] [CrossRef]
- Krog, J.; Zachariassen, K.E.; Larsen, B.; Smidsrød, O. Thermal buffering in Afro-alpine plants due to nucleating agent-induced water freezing. Nature 1979, 282, 300–301. [Google Scholar] [CrossRef]
- Heisig, M.; Mattessich, S.; Rembisz, A.; Acar, A.; Shapiro, M.; Booth, C.J.; Neelakanta, G.; Fikrig, E. Frostbite protection in mice expressing an antifreeze glycoprotein. PLoS One 2015, 10, e0116562. [Google Scholar] [CrossRef]
- Raraty, L.E.; Tabor, D. The adhesion and strength properties of ice. Proc. R. Soc. Lond. A 1958, 245, 184–201. [Google Scholar] [CrossRef]
- Work, A.; Lian, Y. A critical review of the measurement of ice adhesion to solid substrates. Prog. Aerosp. Sci. 2017, 98, 1–26. [Google Scholar] [CrossRef]
- Antson, A.A.; Smith, D.J.; Roper, D.I.; Lewis, S.; Caves, L.S.; Verma, C.S.; Buckley, S.L.; Lillford, P.J.; Hubbard, R.E. Understanding the mechanism of ice binding by type III antifreeze proteins. J. Mol. Biol. 2001, 305, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Schwidetzky, R.; Kunert, A.T.; Bonn, M.; Pöschl, U.; Ramløv, H.; DeVries, A.L.; Fröhlich-Nowoisky, J.; Meister, K. Inhibition of bacterial ice nucleators is not an intrinsic property of antifreeze proteins. J. Phys. Chem. B. 2020, 124, 4889–4895. [Google Scholar] [CrossRef] [PubMed]
- Packard, G.C.; Packard, M.J. Cold acclimation enhances cutaneous resistance to inoculative freezing in hatchling painted turtles, Chrysemys picta. Funct. Ecol. 2003, 17, 94–100. [Google Scholar] [CrossRef]
- Cziko, P.A.; DeVries, A.L.; Evans, C.W.; Cheng, C.-H.C. Antifreeze protein-induced superheating of ice inside Antarctic notothenioid fishes inhibits melting during summer warming. Proc. Natl Acad. Sci. USA 2014, 111, 14583–14588. [Google Scholar] [CrossRef]
- Hirano, Y.; Nishimiya, Y.; Kowata, K.; Mizutani, F.; Tsuda, S. Construction of time-lapse scanning electrochemical microscopy with temperature control and its application to evaluate the preservation effects of antifreeze proteins on living cells. Anal. Chem. 2008, 80, 9349–9354. [Google Scholar] [CrossRef] [PubMed]
- Neelakanta, G.; Hudson, A.M.; Sultana, H.; Cooley, L.; Fikrig, E. Expression of Ixodes scapularis antifreeze glycoprotein enhances cold tolerance in Drosophila melanogaster. PLoS One 2012, 7, e33447. [Google Scholar] [CrossRef] [PubMed]
- Bissoyi, A.; Reicher, N.; Chasnitsky, M.; Arad, S.; Koop, T.; Rudich, Y.; Braslavsky, I. Ice nucleation properties of ice-binding proteins from snow fleas. Biomolecules 2019, 9, 532. [Google Scholar] [CrossRef]
- Xu, H.; Griffith, M.; Patten, C.L.; Glick, B. R. Isolation and characterization of an antifreeze protein with ice nucleation activity from the plant growth promoting rhizobacterium Pseudomonas putida GR12−2. Can. J. Microbiol. 1998, 44, 64–73. [Google Scholar] [CrossRef]
- Govindarajan, A.G.; Lindow, S.E. Size of bacterial ice-nucleation sites measured in situ by radiation inactivation analysis. Proc. Natl Acad. Sci. USA 1988, 85, 1334–1338. [Google Scholar] [CrossRef]
- Ling, M.L.; Wex, H.; Grawe, S.; Jakobsson, J.; Löndahl, J.; Hartmann, S.; et al. Effects of ice nucleation protein repeat number and oligomerization level on ice nucleation activity. J. Geophys. Res. Atmos. 2018, 123, 1802–1810. [Google Scholar] [CrossRef]
- Eickhoff, L.; Dreischmeier, L.; Zipori, A.; Sirotinskaya, V.; Adar, C.; Reicher, N.; Braslavsky, I.; Rudich, Y.; Koop, T. Contrasting behavior of antifreeze proteins: Ice growth inhibitors and ice nucleation promoters. J. Phys. Chem. Lett. 2019, 10, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, Y.; Nishimiya, Y.; Miura, K.; Ohgiya, S.; Miura, A.; Tsuda, S. A part of ice nucleation protein exhibits the ice-binding ability. FEBS Lett. 2005, 579, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Vonnegut, B. The nucleation of ice formation by silver iodide. J. Appl. Phys. 1947, 18, 593–595. [Google Scholar] [CrossRef]
- Marcolli, C.; Nagare, B.; Welti, A.; Lohmann, U. Ice nucleation efficiency of AgI: Review and new insights. Atmos. Chem. Phys. 2016, 16, 8915–8937. [Google Scholar] [CrossRef]
- Head, R. Steroids as ice nucleators. Nature 1961, 191, 1058–1059. [Google Scholar] [CrossRef]
- Popovitz-Biro, R.; Wang, J.L.; Majewski, J.; Shavit, E.; Leiserowitz, L.; Lahav, M. Induced freezing of supercooled water into ice by self-assembled crystalline monolayers of amphiphilic alcohols at the air-water interface. J. Am. Chem. Soc. 1994, 116, 1179–1191. [Google Scholar] [CrossRef]
- Power, B.; Power, R. Some amino-acids as ice nucleators. Nature 1962, 194, 1170–1171. [Google Scholar] [CrossRef]
- Gute, E.; Abbatt, J.P.D. Ice nucleating behavior of different tree pollen in the immersion mode. Atmos. Environ. 2020, 231, 117488. [Google Scholar] [CrossRef]
- Maki, L.R.; Galyan, E.L.; Chang-Chien, M.M.; Caldwell, D.R. Ice nucleation induced by Pseudomonas syringae. Appl. Microbiol. 1974, 28, 456–459. [Google Scholar] [CrossRef]
- Gurian-Sherman, D.; Lindow, S.E. Bacterial ice nucleation: Significance and molecular basis. FASEB J. 1993, 7, 1338–1343. [Google Scholar] [CrossRef]
- Roeters, S.J.; Golbek, T.W.; Bregnhøj, M.; Drace, T.; Alamdari, S.; Roseboom, W.; Kramer. G.; Šantl-Temkiv, T.; Finster, K.; Pfaendtner, J.; Woutersen, S.; Boesen, T.; Weidner, T. Ice-nucleating proteins are activated by low temperatures to control the structure of interfacial water. Nature Commun. 2021, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.M.; Duman, J.G. Maintenance of the supercooled state in overwintering pyrochroid beetle larvae, Dendroides canadensis: Role of hemolymph ice nucleators and antifreeze proteins. J. Comp. Physiol. B 1997, 167, 105–113. [Google Scholar] [CrossRef]
- Packard, G.C.; Packard, M.J. To freeze or not to freeze: Adaptations for overwintering by hatchlings of the North American painted turtle. J. Exp. Biol. 2004, 207, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.L.; Wolf, E.E.; Duman, J.G. A scanning tunneling microscopy study of an insect lipoprotein ice nucleator. J. Vac. Sci. Technol. B 1991, 9, 1197–1201. [Google Scholar] [CrossRef]
- Hartmann, S.; Ling, M.; Dreyer, L.S.A.; Zipori, A.; Finster, K.; Grawe, S.; Jensen, L.Z.; Borck, S.; Reicher, N.; Drace, T.; Niedermeier, D.; Jones, N.C.; Hoffmann, S.V.; Wex, H.; Rudich, Y.; Boesen, T.; Šantl-Temkiv, T. Structure and protein-protein interactions of ice nucleation proteins drive their activity. Front. Microbiol. 2022, 13, 872306. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Lee, J.C.; Reicher, N.; Ovadia, G.; Guo, S.; Guo, W.; Liu, J.; Braslavsky, I.; Rudich, Y.; Davies, P.L. Ice nucleation proteins self-assemble into large fibers to trigger freezing at near 0 °C. bioRxiv [Preprint], 2023. Available at: https://doi.org/10.1101/2023.08.03.551873 (Accessed on September 11, 2023). [CrossRef]
- Brush, R. A.; Griffith, M.; Mlynarz, A. Characterization and quantification of intrinsic ice nucleators in winter rye. Plant. Physiol. 1994, 104, 725–735. [Google Scholar] [CrossRef]
- Parody-Morreale, A.; Murphy, K.P.; Di Cera, E.; Fall, R.; DeVries, A.L.; Gill, S.J. Inhibition of bacterial ice nucleators by fish antifreeze glycoproteins. Nature 1988, 333, 782–783. [Google Scholar] [CrossRef]
- Sosso, G.C.; Whale, T.F.; Holden, M.A.; Pedevilla, P.; Murray, B.J.; Michaelides, A. Unravelling the origins of ice nucleation on organic crystals. Chem. Sci. 2018, 9, 8077–8088. [Google Scholar] [CrossRef]
- Fukuta, N.; Mason, B.J. Epitaxial growth of ice on organic crystals. J. Phys. Chem. Solids 1963, 24, 715–718. [Google Scholar] [CrossRef]
- Hiranuma, N.; Möhler, O.; Yamashita, K.; Tajiri, T.; Saito, A.; Kiselev, A.; Hoffmann, N.; Hoose, C.; Jantsch, E.; Koop, T.; Murakami, M. Ice nucleation by cellulose and its potential contribution to ice formation in clouds. Nature Geosci. 2015, 8, 273–277. [Google Scholar] [CrossRef]
- Duman, J.G.; Xu, L.; Neven, L.G.; Tursman, D.; Wu, D.W. Hemolymph proteins involved in insect low temperature tolerance: Ice nucleators and antifreeze proteins. In Insects at Low Temperatures. Lee, R.E., Denlinger, D., Eds.; Chapman & Hall: New York, USA, 1991; pp. 94–127. [Google Scholar]
- Olsen, T.M.; Duman, J.G. Maintenance of the supercooled state in the gut fluid of overwintering pyrochroid beetle larvae, Dendroides canadensis : Role of ice nucleators and antifreeze proteins. J. Comp. Physiol. B 1997, 167, 114–122. [Google Scholar] [CrossRef]
- Kawahara, H.; Nagae, I.; Obata, H. Purification and characterization of a new anti-nucleating protein isolated from Acinetobacter calcoaceticus KINI-1. Biocontrol Sci. 1996, 1, 11–17. [Google Scholar] [CrossRef]
- Tomalty, H.E.; Walker, V.K. Perturbation of bacterial ice nucleation activity by a grass antifreeze protein. Biochem. Biophys. Res. Commun. 2014, 452, 636–641. [Google Scholar] [CrossRef] [PubMed]
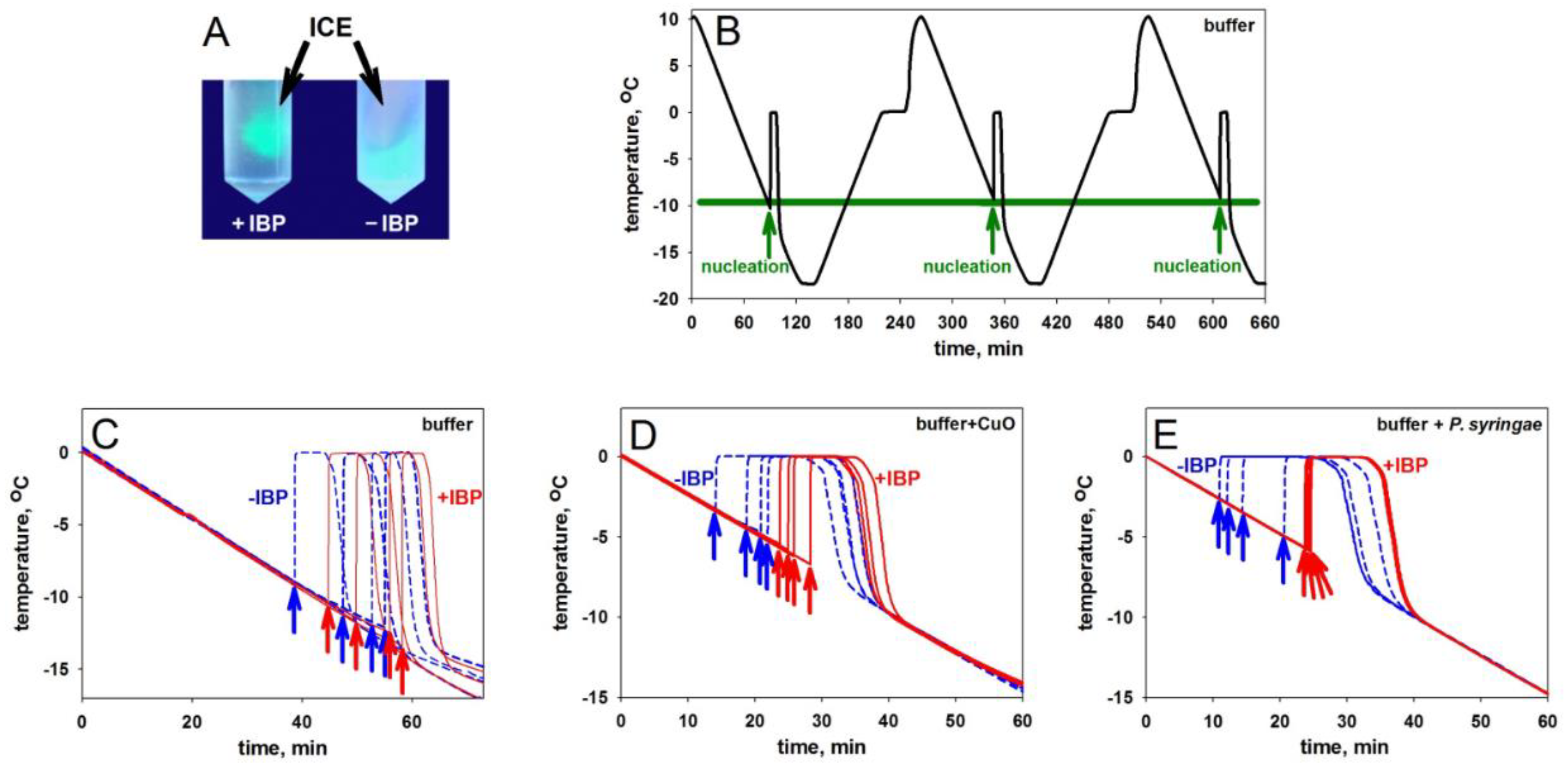

| Sample | Range of nucleation temperatures, °C | Number of measurements |
|---|---|---|
| Sodium phosphate buffer | from –8 to –13 | 30 |
| mIBP83 † in phosphate buffer | from –11 to –14 | 15 |
| CuO* in the buffer | from –3 to –6 | 15 |
| CuO* + mIBP83 † in the buffer | from –6 to –8 | 15 |
| P. syringae* in the buffer | from –2 to –5 | 15 |
| P. syringae* + mIBP83 † in the buffer | from –5 to –7 | 15 |
| Carbonic anhydrase B in the buffer | –10.0±0.4 & –10.5±0.3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





