Submitted:
17 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Prevention through room and surface disinfection.
1.2. From empirical findings to science.
2. Results
2.1. Preclinical trials in animal and cell models
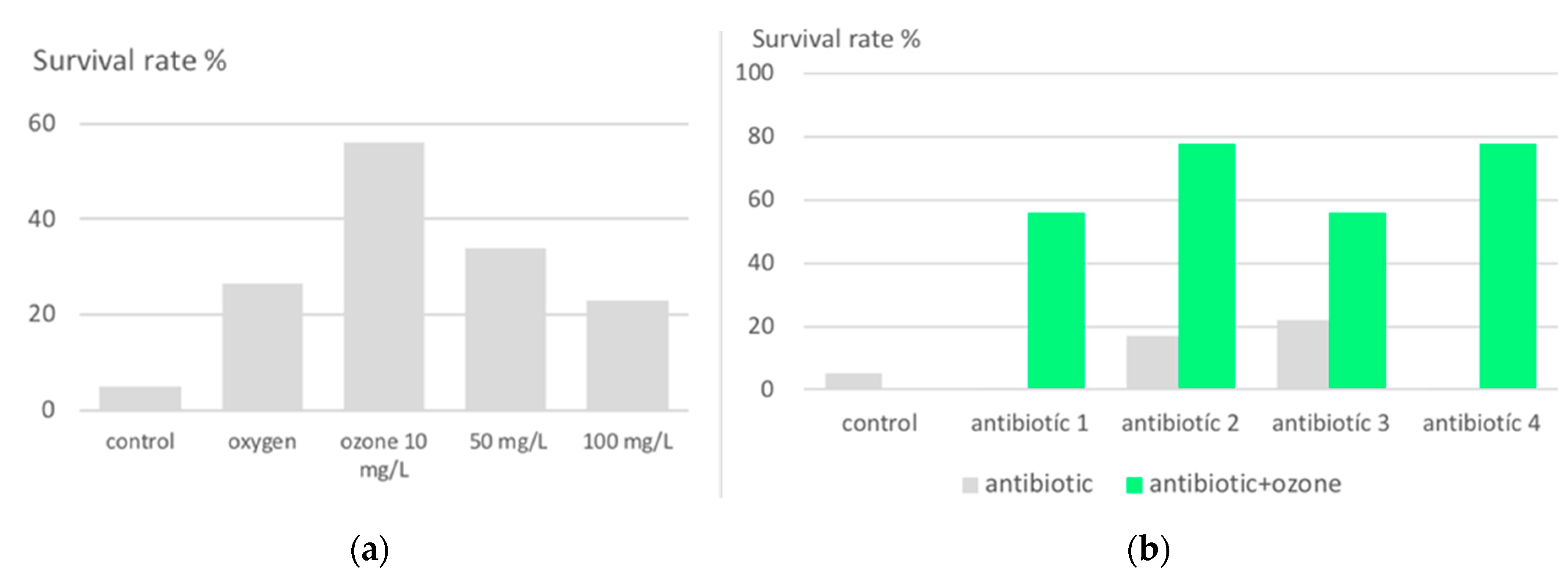
2.1.1. Growth inhibition of plasmodium falciparum in ozone-pretreated Red Blood Cells.
2.1.2. Protection against lethal peritonitis by oxidative preconditioning with ozone in animal models.
2.2. Clinical trials and reports
2.2.1. Prevention from Covid-19
| Control group, n=52 | Ozone group, n=57 |
|---|---|
| 3x vaccinated: n=33 (63,5%); 2x vaccinated: n=19 (36,5%) |
3x vaccinated: n=31 (54,4%); 2x vaccinated: n=26 (45,6%) |
| female n=33, male n=19 age 20 to 55 |
female n=24, male n=33 age 20 to 55 |
| MAH: 1x per week, 8 treatments RI: 2x per week during 2 weeks, then 1x per week; 15-16 treatments |
3. Discussion
4. Material and methods
| Type of study | Results | Application route | References |
|---|---|---|---|
| Medical ozone promotes Nrf2 phosphorylation reducing oxidative stress and proinflammatory cytokines in multiple sclerosis patients | Secondary prevention GSH increase. |
Rectal insufflation 20mg/L | Livan Delgado-Roche, Mario Riera-Romo, Fernando Mesta, Yanet Hernández-Matos, Juan M. Barrios, Gregorio Martínez-Sánchez, Said M. Al-Dalaien. European Journal of Pharmacology, 2017, 811, 148-154 |
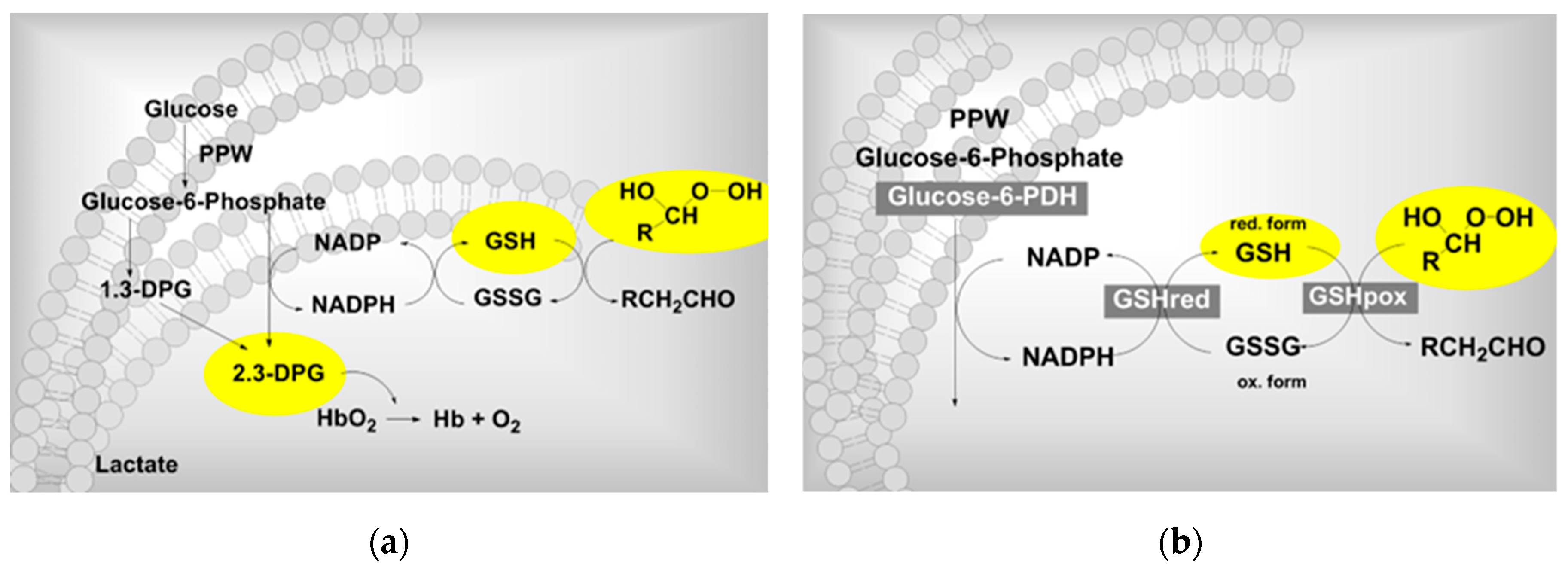
| Ozone influence on the RBC metabolism” (german Einfluss auf den Erythrozyten-Stoffwechsel). Clinical trial . | Metabolic activation in RBC´s: 2,3-DPG and ATP increase. See text. | Rectal insufflation | Viebahn-Hänsler, R. Washüttl. J. Wasser, G. Gutzen, U.1995 [15] |
| Medical ozone arrests oxidative damage progression and regulates vasoactive mediator levels in older patients (60-70 years) with oxidative etiology diseases. Controlled clinical study. | Positive influence on aging process. See text. | Rectal insufflation | León Fernández, O. S., Takon Oru, G., Viebahn-Hänsler, R., López Cabreja, G., Serrano Espinosa, I., García Fernández, E. 2022 [19] |
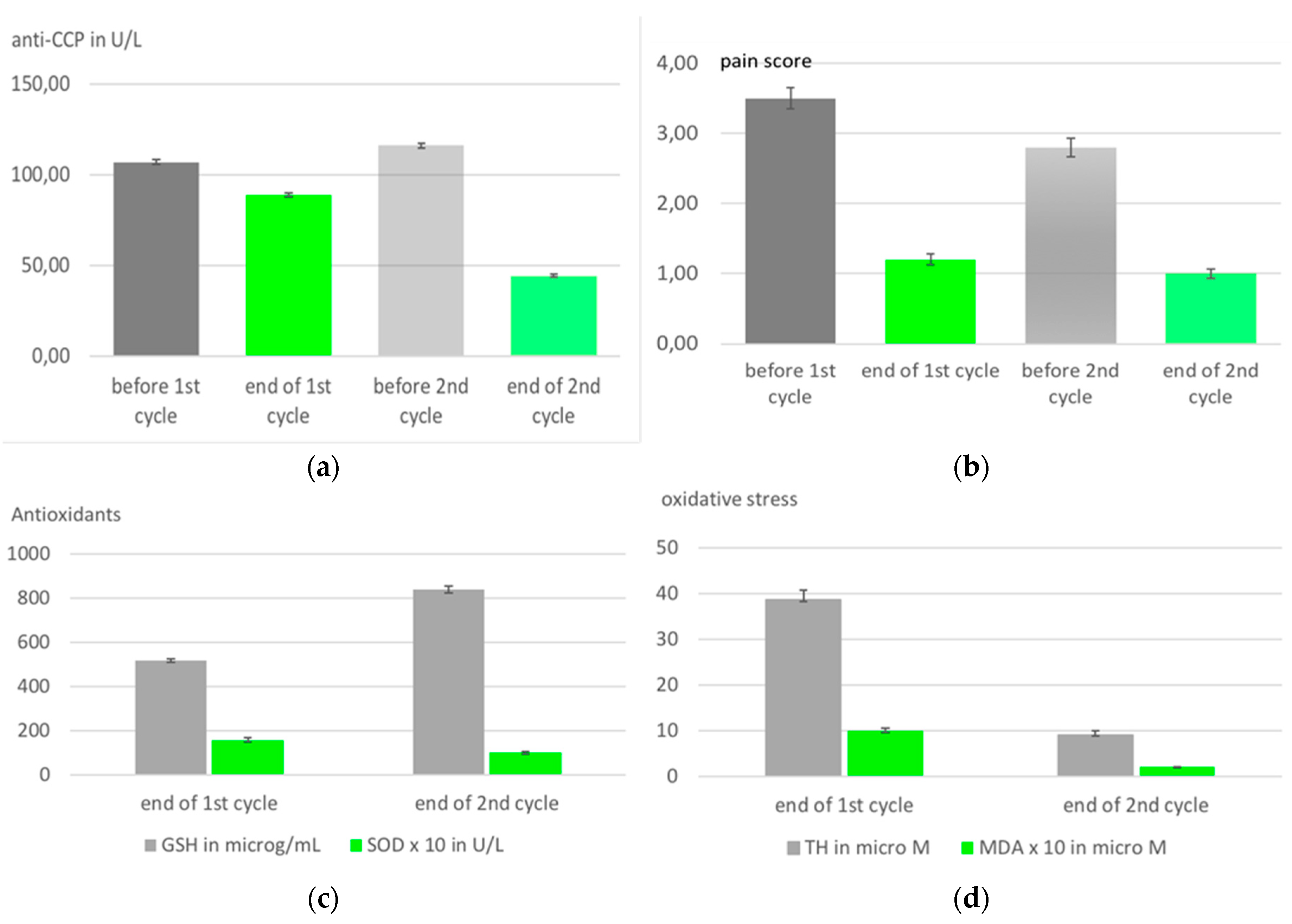
| Medical ozone increases methotrexate clinical response and improves cellular redox balance in patients with rheumatoid arthritis. Clinical trial. |
Statistical significant reduction of liver toxicity in patients with rheumatoid arthritis during MTX treatment. See text. | 10 rectal insufflations in 10 days | León, O.S., Viebahn-Haensler, R., López, C.G., Serrano, E.I., Hernández, M.Y., Delgado, R.L., Tamargo, S.B., Takon, O.G., Polo, V.J.C., 2016. [27] |
| Modulation of Oxidative Stress by Ozone Therapy in the Prevention and Treatment of Chemotherapy-Induced Toxicity: Review and Prospects. |
Prevention from toxicity, mainly in animal models | systemic applications | Bernardino Clavo, Francisco Rodríguez-Esparragó , Delvys Rodríguez-Abreu,Gregorio Martínez-Sánchez, Pedro Llontop, David Aguiar-Bujanda,Leandro Fernández-Pérez and Norberto Santana-Rodríguez Antioxidants 2019, 8, 588; doi:10.3390/antiox8120588 |
| Type of study | Results | Application route | References |
|---|---|---|---|
| Ozone Therapy for Prevention and Treatment of COVID-19. Review. | 4 publications in prevention. | Different forms of application. | Gregorio Martínez-Sánchez1Journal of Exploratory Research in Pharmacology, April 2022. DOI: 10.14218/JERP.2022.00015 |
| Intravenous ozonized saline therapy as prophylaxis for healthcare workers (HCWs) in a dedicated COVID-19 hospital in India. A retrospective study | Less infections in the ozone group (4.6%) than in the control (14.03%). N=64 (235). | Ozonized saline. | A. Sharma, M. Shah, H. Sane, N. Gokulchandran, A. Paranjape, P. Khubchandani, J. Captain, S. Shirke, P. Kulkarni. European Review for Medical and Pharmacological Sciences, 2021; 25, 3632-3639 |
| Immunity Prophylaxis With Ozone Therapy Review Report. | 2.19 % incidence rate (n=320). | Minor autohemotherapy. | Dr. Mili Shah, Jignasha Captain and Gayatri Ganu. ejbps, 2020, 7, 86-88. |
| Could the minor autohemotherapy be a complementary therapy for healthcare professionals to prevent COVID-19 infection? | (n=73) No infection or positive test. |
Minor autohemotherapy. | Aydan Orscelik, Burak Karaaslan, Betul Agiragac, Ilker Solmaz, Murat Parpucu. Ann Med Res 2021, 28, 1863-9 DOI:10.5455/annalsmedres.2020.11.1133 |
| Could ozone therapy be used to prevent COVID-19? Clinical Trial. | 2 of 71 persons were tested positive.Retrospective, no control. 45 % medical professionals. See text. | Major autohemotherapy 10 treatments. | Kardelen Gencer-Atalay1 , Tulay Sahin-Marmara Med J 2022;35(2): 196-201. http://doi.org/10.5472/marumj.1121363. [22] |
| COVID-19 profilaxis with ozone therapy | n=9, good effect but no protection from further infection. | Rectal insufflation. | Falzoni, W.; Senvaitis, M. I.; Iwasa, S. Acupuncture and Electro-TherapeuticsResearch,2021,46,3 5-36,. |
| Comparative analysis of 2 groups of people according to age and sex, vaccinated triple versus covid-19, were subjected to quantitative analysis of antibodies and B lymphocytes after ozone therapy. Clinical trial. | See text. | Major autohemotherapy . |
Medina, José German, Journal of Ozone Therapy, 2022. 6, 11-12. [23] |
| The Role of Ozone Therapy as Adjuvant in the Management of Covid-19 in Indonesia. Clinical trial. | See text. | Major autohemotherapy, Rectal insufflation, vaginal insufflation. | Dian Chaijadi, Asep Hendradiana, Peter Djoko Tjahjono, Endang Kusumaningsih, Hariyanto, Carles Siagian, Djaja Surya Atmadja, Renate Viebahn-Hänsler. Indonesian Police Central Hospital and Faculty of Medicine, Indonesia University, Jakarta, proceedings Association IOA in Milan 2023. [21] |
5. Conclusion
References
- Schalekamp, M. Alles über Ozon, seine Vor- und Nachteile bei der Trinkwasserversorgung. (All about ozone, its advantages and disadvantages in drinking water supply)). OzoNachrichten 1983, 2, 74-82. http://ozongesellschaft.de/ozone-society.html.
- Alimohammadi, M.; Naderia, M. Effectiveness of Ozone Gas on Airborne Virus Inactivation in Enclosed Spaces: A Review Study. Ozone Sci. Eng. 2021, 43, 21–31. [CrossRef]
- Loeb. B. Ozone: Sci. & Engineering. Thirty Years of Progress. Ozone Sci. Eng. 2009, 31, 379–392. [CrossRef]
- Martins, R. B.; Castro, I. A.; Pontelli, M.; Souza, J.P.; Lima, T.M.; Rezende Melo, R.S.; Zen Siqueira, J. P.; Caetano, M.H.; Arruda, E.; Gottardo de Almeida. M.T. SARS-CoV-2 Inactivation by Ozonated Water: A Preliminary Alternative for Environmental Disinfection. Ozone Sci. Eng. 2021, 43, 108–111. [CrossRef]
- Inagaki, H. Akatsuki, S.; Sudaryatma, P.E.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid Inactivation of SARS-CoV-2 with Ozonated Water. Ozone Sci. Eng. 2021, 43, 208–212. [CrossRef]
- Tizaoui, C. Ozone: A Potential Oxidant for COVID-19 Virus (SARS-CoV-2)Ozone Sci. Eng 2020, 42, 378–385. [CrossRef]
- Hudson, J.B.; Sharma, M.; Vimalanathan, S. Development of a Practical Method for Using Ozone Gas as a Virus Decontaminating Agent Ozone Sci. Eng. 2009 31, 216–223. [CrossRef]
- Morrison, C.; Atkinson, A.; Zamyadi, A.; Kibuye, F.; McKie, M.; Hogard, S.; Mollica, P.; Jasim, S.; Wert, E.C. Critical Review and Research Needs of Ozone Applications Related to Virus Inactivation: Potential Implications for SARS-CoV-2 Ozone Sci. Eng. 2021, 43. 2–20. [CrossRef]
- Hoigne, L.; Bader.H. Ozonation of Water: Selectivity and Rate of Oxidation of Solutes. Ozone Sci. Eng. 1979, 1, 73-85. [CrossRef]
- Viebahn-Haensler, R.; León Fernández, O.S. Ozone In Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action In Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7890. [CrossRef]
- Schulz, S.; Rodriguez, Z.; Mutters, R.; Bette M.; Hoffmann, S. Die lethale postoperative Peritonitis im Tiermodell. Infektprophylaxe und Therapie durch Kombination von Ozon und Antibiotika. (The lethal post-operative peritonitis in an animal model. Infection prophylaxis and therapy by combination of ozone and antibiotics) in Viebahn-Hänsler, R.; Knoch, H.G. (Eds.) Ozon-Handbuch. Grundlagen-Prävention-Therapie (Ozone-Handbook, Basics-Prevention-Therapy) ecomed, Landsberg, 2000 (1995-2006).
- Viebahn-Haensler, R.; León Fernández, O.S. The low-dose ozone concept and its pharmacologyin prevention and convalescence. Babacan, C.A. (ed.). Ozone and Neural Therapy. 1st ed. Ankara: Türkiye Klinikleri; 2022, 19-29.
- Krishna, S. Bustamante, L. Haynes R.K. Staines, H.M. Artemisinins: their growing importance in medicine. Trends in Pharmacological Sciences 2008. 29, 520-527. [CrossRef]
- Lell, B.; Viebahn, R.; Kremsner, P. The activity of ozone against plasmodium falciparum. Ozone: Sci Eng 2001 23: 89-93. [CrossRef]
- Viebahn, R. Washüttl, J. Wasser, G. Gutzen, U. Influence of ozone on RBC metabolism“ referring a pilot study: „Rectal ozone insufflation in elderly“ in Viebahn-Haensler, R.; Knoch, H.G. (eds.) Ozone Handbook 1995. Ecomed, Landsberg 1995-2006.
- Schulz,S.; Rodriguez, Z.Z.; Mutters, R.; Menendez, S.; Bette, M. Repetitive pneumoperitoneum with ozonized oxygen as a preventive in lethal polymicrobial sepsis in rats. Eur Surg Res. 2003 Jan-Feb;35(1):26-34. [CrossRef]
- Zamora, Z.B.; Borrego, A.; Lopez, O. Y.; Delgado, R.; Gonzalez, R.; Menendez, S.; Hernandez, F.; Schulz, S. Effects of Ozone Oxidative Preconditioning on TNF- α Release and Antioxidant-Prooxidant Intracellular Balance in Mice During Endotoxic Shock. Mediators of Inflammation, 2005. 1–7. PII. S0962935104309257 http://mi.hindawi.com.
- Bette, M.; Nuessing, R.M.; Mutters,R.; Zamora, Z.B.; Menendez, S.; Schulz, S.; Efficiency of tazobactam/piperacillin in lethal peritonitis is enhanced after preconditioning of rats with O3/O2-pneumoperitoneum. SHOCK, 2006. 25, 23–29. [CrossRef]
- León Fernández, O. S.; Takon Oru, G.; Viebahn-Hänsler, R.; López Cabreja, L.; Serrano Espinosa, I.; García Fernández, E. Medical ozone arrests oxidative damage progression and regulates vasoactive mediator levels in older patients (60-70 years) with oxidative etiology diseases. Front. Physiol. 03 November 2022, 13. https://www.frontiersin.org/articles/10.3389/fphys.2022.1029805/full.
- Viebahn-Haensler, R.; León Fernández, O.S.; Fahmy, Z. Ozone in medicine: The low-dose ozone concept. Guidelines and treatment strategies. Ozone: Sci. Eng. 2012, 34, 408–424. [CrossRef]
- Chaijadi, D.; Hendradiana, A.; Tjahjono, P.D.; Kusumaningsih, E.; Hariyanto.; Siagian, C.; Atmadja, D.S.; Viebahn-Hänsler, R. The Role of Ozone Therapy as Adjuvant in the Management of Covid-19 in Indonesia. Proceedings 26 Ozone World Congress of the International Ozone Association IOA, 2023.
- Gencer-Aalay, K.; Sahin, T.; Could ozone therapy be used to prevent COVID-19? Marmara Med J- .2022; 35, 196-201. https://dergipark.org.tr/tr/pub/marumj.
- Medina, J. G. Journal of Ozone Therapy. 2022. 6, 11-12. [CrossRef]
- Togi, S.; Togo, M.; Nagashima, S.; Kitai, Y.; Muromoto, R.; Kashiwakura, J.; Miura, T.; Matsuda, T. Implication of NFkB Activation of Ozone-Induced HO-1 Activation. BRP Reports 2021. 4: 59-63. http://hdl.handle.net/2115/82070.
- Wolff, Hans 1979 “Das Medizinische Ozon” Verlag für Medizin vfm Heidelberg/Thieme.
- Takon Oru, G.; Viebahn-Haensler, R.; García Fernández, E.; Alvarez Almiñaque, D.; Polo Vega, J.C.; Tamargo Santos, B.; López Cabreja, G.; Serrano Espinosa, I.; Tabares Nápoles, N.; León Fernández, O.S. Medical Ozone Effects and Innate Immune Memory in Rheumatoid Arthritis Patients Treated with Methotrexate+Ozone After a Second Cycle of Ozone Exposure. Chronic Pain Manag 2019, 2, 114. [CrossRef]
- León, F.O.S.; Viebahn-Haensler, R.; López, C.G.; Serrano, E.I.; Hernández, M.Y.; Delgado,R.L.; Tamargo, S.B.; Takon, O.G.; Polo, V.J.C. Medical ozone increases methotrexate clinical response and improves cellular redox balance in patients with rheumatoid arthritis. Eur. J.Pharmacol. 2016, 789, 313–318. [CrossRef]

| Injury biomarkers: Oxidative stress markers | Protective markers: antioxidants | Clinical parameters |
|---|---|---|
| TH (total hydroperoxides) | total SOD (superoxide dismutase) CAT (catalase) |
DAS (disease activity score) PAIN |
| MDA (malondialdehyde) AOOP (advanced oxidation protein products) |
GSH (reduced glutathione) | HAQ: DI, (health Assessment disability questionnaire) |
| NO (nitrogen monoxide) | GGT (gamma glutamyl transferase) | Auto antibodies CCP (anti-cyclic citrullinated peptides) |
| Application | Ozone conc. | Ozone volume | Treatment frequency |
|---|---|---|---|
| Rectal ozone-insufflation | 15 - 25 µg/ml In general: 10 to max. 40 µg/ml |
150–300 ml |
2x to 3x per week, if possible prior to chemotherapy or antibiotics. At least 1x per week as adjuvant therapy or 2 to 3 series per year with 10 treatments each |
| Major autohemotherapy | 15 - 25 µg/ml In general: 10 to max. 40 µg/ml |
50 ml | 2x to 3x per week, if possible prior to chemotherapy or antibiotics. At least 1x per week as adjuvant therapy or 2 to 3 series per year with 10 treatments each |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




