Submitted:
17 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
Introduction
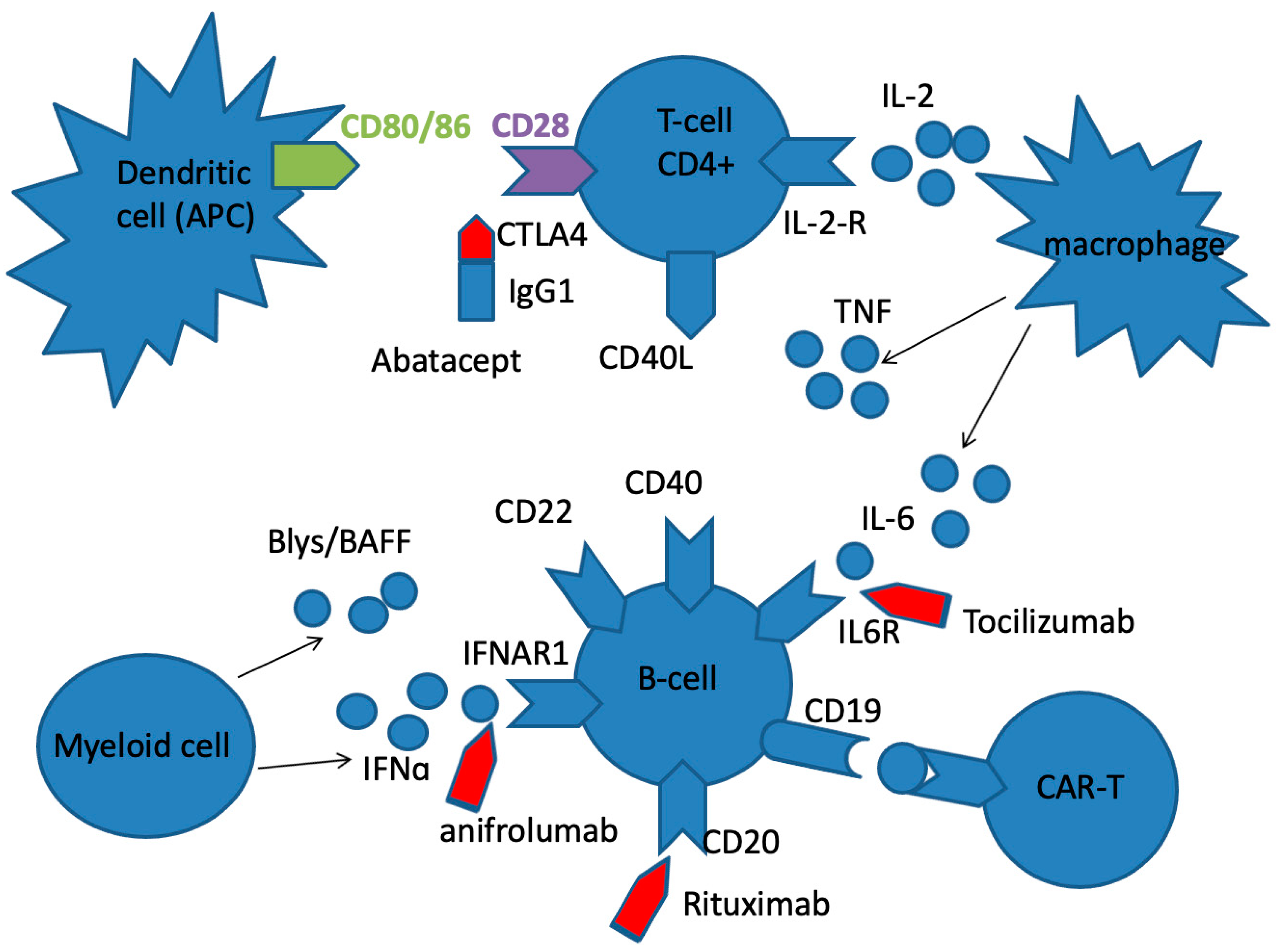
Systemic lupus erythematosus (SLE)
Systemic Sclerosis (SSc)
Rheumatoid arthritis (RA)
Conclusion and Perspective
Funding
Acknowledgments
List of abbreviations
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| ACPA | Anticitrullinated protein antibody |
| APRIL | A proliferating inducing ligand |
| BAFF | B cell activating factor |
| BsAb | Bispecific antibody |
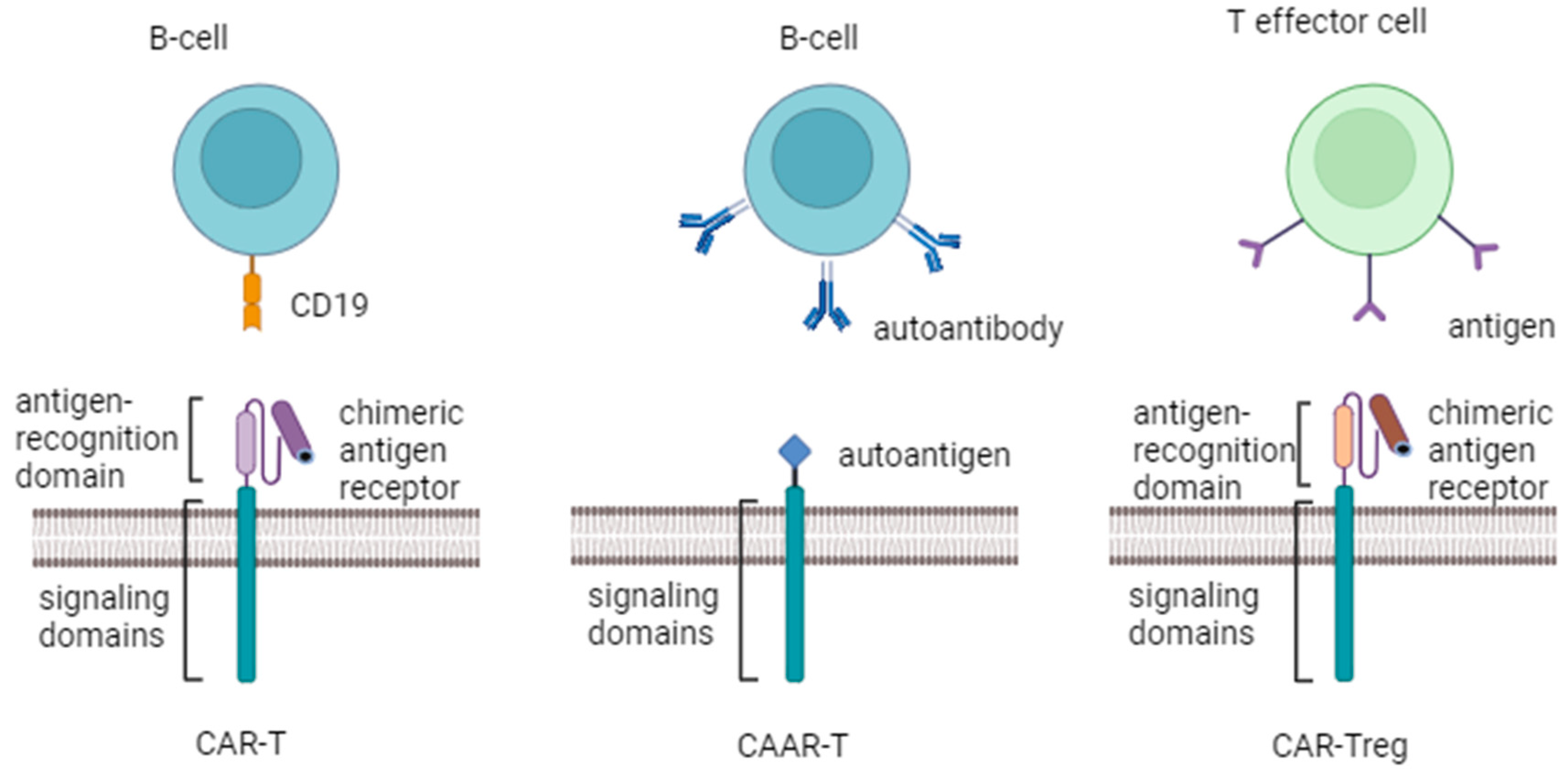
| CAR-T cell | Chimeric antigen receptor T cell |
| CAAR-T cell | Chimeric autoantibody receptor T cell |
| CCLE | Chronic cutaneous lupus erythematosus |
| CDC | Complement-dependent cytotoxicity |
| CLE | Cutaneous lupus erythematosus |
| CTLA-4 | Cytotoxic T lymphocyte-associated protein 4 |
| DLE | Discoid lupus erythematosus |
| FDA US | Food and Drug Administration |
| FITC | Fluorescein isothiocyanate |
| FLS | Fibroblast-like synoviocytes |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| JAK | Janus kinase family |
| mAb | Monoclonal antibody |
| SAIDs | Systemic autoimmune diseases |
| SLE | Systemic lupus erythematosus |
| TNF | Tumor necrosis factor |
| RA | КRheumatoid arthritis |
References
- Wang, L.; Wang, F.; Gershwin, M.E. Human Autoimmune Diseases: A Comprehensive Update. J. Intern. Med. 2015, 278, 369–395. [CrossRef]
- Dima, A.; Jurcut, C.; Arnaud, L. Hydroxychloroquine in Systemic and Autoimmune Diseases: Where Are We Now? Jt. Bone Spine 2021, 88, 105143. [CrossRef]
- Emamikia, S.; Gentline, C.; Chatzidionysiou, K.; Arnaud, L.; Vollenhoven, R. van Relationship between Glucocorticoid Dose and Adverse Events in Systemic Lupus Erythematosus: Data from a Randomized Clinical Trial. Scand. J. Rheumatol. 2018, 47, 131–140. [CrossRef]
- Dubey, A.K.; Handu, S.S.; Dubey, S.; Sharma, P.; Sharma, K.K.; Ahmed, Q.M. Belimumab: First Targeted Biological Treatment for Systemic Lupus Erythematosus. J. Pharmacol. Pharmacother. 2011, 2, 317–319. [CrossRef]
- Felten, R.; Dervovic, E.; Chasset, F.; Gottenberg, J.-E.; Sibilia, J.; Scher, F.; Arnaud, L. The 2018 Pipeline of Targeted Therapies under Clinical Development for Systemic Lupus Erythematosus: A Systematic Review of Trials. Autoimmun. Rev. 2018, 17, 781–790. [CrossRef]
- Hafeez, U.; Gan, H.K.; Scott, A.M. Monoclonal Antibodies as Immunomodulatory Therapy against Cancer and Autoimmune Diseases. Curr. Opin. Pharmacol. 2018, 41, 114–121. [CrossRef]
- Zhao, Q. Bispecific Antibodies for Autoimmune and Inflammatory Diseases: Clinical Progress to Date. BioDrugs 2020, 34, 111–119. [CrossRef]
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T Cell Therapy for Refractory Systemic Lupus Erythematosus. Nat. Med. 2022, 28, 2124–2132. [CrossRef]
- Bergmann, C.; Müller, F.; Jörg, D.; Györfi, D.M.H.; Völkl, S.; Aigner, M.; Harrer, T.; Bayerl, N.; Atzinger, A.; Taubmann, J.; et al. AB0816 TREATMENT OF A PATIENT WITH SEVERE DIFFUSE SYSTEMIC SCLEROSIS (SSC) USING CD19-TARGETING CAR-T-CELLS. Ann. Rheum. Dis. 2023, 82, 1621.1-1621. [CrossRef]
- Ganeeva, I.; Zmievskaya, E.; Valiullina, A.; Kudriaeva, A.; Miftakhova, R.; Rybalov, A.; Bulatov, E. Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies. Bioengineering 2022, 9, 808. [CrossRef]
- Yaniv, G.; Twig, G.; Shor, D.B.-A.; Furer, A.; Sherer, Y.; Mozes, O.; Komisar, O.; Slonimsky, E.; Klang, E.; Lotan, E.; et al. A Volcanic Explosion of Autoantibodies in Systemic Lupus Erythematosus: A Diversity of 180 Different Antibodies Found in SLE Patients. Autoimmun. Rev. 2015, 14, 75–79. [CrossRef]
- Mohan, C.; Putterman, C. Genetics and Pathogenesis of Systemic Lupus Erythematosus and Lupus Nephritis. Nat. Rev. Nephrol. 2015, 11, 329–341. [CrossRef]
- Mok, M.Y.; Shoenfeld, Y. Recent Advances and Current State of Immunotherapy in Systemic Lupus Erythematosus. Expert Opin. Biol. Ther. 2016, 16, 927–939. [CrossRef]
- Tanaka, Y.; Kubo, S.; Iwata, S.; Yoshikawa, M.; Nakayamada, S. B Cell Phenotypes, Signaling and Their Roles in Secretion of Antibodies in Systemic Lupus Erythematosus. Clin. Immunol. 2018, 186, 21–25. [CrossRef]
- Yang, B.; Zhao, M.; Wu, H.; Lu, Q. A Comprehensive Review of Biological Agents for Lupus: Beyond Single Target. Front. Immunol. 2020, 11, 539797. [CrossRef]
- Bossen, C.; Schneider, P. BAFF, APRIL and Their Receptors: Structure, Function and Signaling. Semin. Immunol. 2006, 18, 263–275. [CrossRef]
- Zollars, E.; Bienkowska, J.; Czerkowicz, J.; Allaire, N.; Ranger, A.M.; Magder, L.; Petri, M. BAFF (B Cell Activating Factor) Transcript Level in Peripheral Blood of Patients with SLE Is Associated with Same-Day Disease Activity as Well as Global Activity over the next Year. Lupus Sci. Med. 2015, 2, e000063. [CrossRef]
- Mackay, F.; Ambrose, C. The TNF Family Members BAFF and APRIL: The Growing Complexity. Cytokine Growth Factor Rev. 2003, 14, 311–324. [CrossRef]
- Vincent, F.B.; Morand, E.F.; Schneider, P.; Mackay, F. The BAFF/APRIL System in SLE Pathogenesis. Nat. Rev. Rheumatol. 2014, 10, 365–373. [CrossRef]
- Baker, K.P.; Edwards, B.M.; Main, S.H.; Choi, G.H.; Wager, R.E.; Halpern, W.G.; Lappin, P.B.; Riccobene, T.; Abramian, D.; Sekut, L.; et al. Generation and Characterization of LymphoStat-B, a Human Monoclonal Antibody That Antagonizes the Bioactivities of B Lymphocyte Stimulator. Arthritis Rheum. 2003, 48, 3253–3265. [CrossRef]
- Navarra, S.V.; Guzmán, R.M.; Gallacher, A.E.; Hall, S.; Levy, R.A.; Jimenez, R.E.; Li, E.K.-M.; Thomas, M.; Kim, H.-Y.; León, M.G.; et al. Efficacy and Safety of Belimumab in Patients with Active Systemic Lupus Erythematosus: A Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2011, 377, 721–731. [CrossRef]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A Phase III, Randomized, Placebo-controlled Study of Belimumab, a Monoclonal Antibody That Inhibits B Lymphocyte Stimulator, in Patients with Systemic Lupus Erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [CrossRef]
- Dörner, T.; Lipsky, P.E. Beyond Pan-B-Cell-Directed Therapy — New Avenues and Insights into the Pathogenesis of SLE. Nat. Rev. Rheumatol. 2016, 12, 645–657. [CrossRef]
- Macauley, M.S.; Pfrengle, F.; Rademacher, C.; Nycholat, C.M.; Gale, A.J.; Drygalski, A. von; Paulson, J.C. Antigenic Liposomes Displaying CD22 Ligands Induce Antigen-Specific B Cell Apoptosis. J. Clin. Investig. 2013, 123, 3074–3083. [CrossRef]
- Du, F.H.; Mills, E.A.; Mao-Draayer, Y. Next-Generation Anti-CD20 Monoclonal Antibodies in Autoimmune Disease Treatment. Autoimmun. Highlights 2017, 8, 12. [CrossRef]
- Daridon, C.; Blassfeld, D.; Reiter, K.; Mei, H.E.; Giesecke, C.; Goldenberg, D.M.; Hansen, A.; Hostmann, A.; Frölich, D.; Dörner, T. Epratuzumab Targeting of CD22 Affects Adhesion Molecule Expression and Migration of B-Cells in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2010, 12, R204. [CrossRef]
- Valentine, K.M.; Hoyer, K.K. CXCR5+ CD8 T Cells: Protective or Pathogenic? Front. Immunol. 2019, 10, 1322. [CrossRef]
- Da, Z.; Li, L.; Zhu, J.; Gu, Z.; You, B.; Shan, Y.; Shi, S. CXCL13 Promotes Proliferation of Mesangial Cells by Combination with CXCR5 in SLE. J. Immunol. Res. 2016, 2016, 2063985. [CrossRef]
- Bao, Y.-Q.; Wang, J.-P.; Dai, Z.-W.; Mao, Y.-M.; Wu, J.; Guo, H.-S.; Xia, Y.-R.; Ye, D.-Q. Increased Circulating CXCL13 Levels in Systemic Lupus Erythematosus and Rheumatoid Arthritis: A Meta-Analysis. Clin. Rheumatol. 2020, 39, 281–290. [CrossRef]
- Klimatcheva, E.; Pandina, T.; Reilly, C.; Torno, S.; Bussler, H.; Scrivens, M.; Jonason, A.; Mallow, C.; Doherty, M.; Paris, M.; et al. CXCL13 Antibody for the Treatment of Autoimmune Disorders. BMC Immunol. 2015, 16, 6. [CrossRef]
- Schoenberger, S.P.; Toes, R.E.M.; Voort, E.I.H. van der; Offringa, R.; Melief, C.J.M. T-Cell Help for Cytotoxic T Lymphocytes Is Mediated by CD40–CD40L Interactions. Nature 1998, 393, 480–483. [CrossRef]
- McCoy, K.D.; Gros, G.L. The Role of CTLA-4 in the Regulation of T Cell Immune Responses. Immunol. Cell Biol. 1999, 77, 1–10. [CrossRef]
- Sharabi, A.; Tsokos, G.C. T Cell Metabolism: New Insights in Systemic Lupus Erythematosus Pathogenesis and Therapy. Nat. Rev. Rheumatol. 2020, 16, 100–112. [CrossRef]
- Koenig, K.F.; Groeschl, I.; Pesickova, S.S.; Tesar, V.; Eisenberger, U.; Trendelenburg, M. Serum Cytokine Profile in Patients with Active Lupus Nephritis. Cytokine 2012, 60, 410–416. [CrossRef]
- MOK, M.Y.; WU, H.J.; LO, Y.; LAU, C.S. The Relation of Interleukin 17 (IL-17) and IL-23 to Th1/Th2 Cytokines and Disease Activity in Systemic Lupus Erythematosus. J. Rheumatol. 2010, 37, 2046–2052. [CrossRef]
- Mok, M.Y.; Huang, F.P.; Ip, W.K.; Lo, Y.; Wong, F.Y.; Chan, E.Y.T.; Lam, K.F.; Xu, D. Serum Levels of IL-33 and Soluble ST2 and Their Association with Disease Activity in Systemic Lupus Erythematosus. Rheumatology 2010, 49, 520–527. [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S.; et al. Anifrolumab, an Anti–Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The Making of Bispecific Antibodies. mAbs 2017, 9, 182–212. [CrossRef]
- Spiess, C.; Zhai, Q.; Carter, P.J. Alternative Molecular Formats and Therapeutic Applications for Bispecific Antibodies. Mol. Immunol. 2015, 67, 95–106. [CrossRef]
- Baeuerle, P.A.; Kufer, P.; Bargou, R. BiTE: Teaching Antibodies to Engage T-Cells for Cancer Therapy. Curr Opin Mol Ther 2009, 11, 22–30.
- Nagorsen, D.; Baeuerle, P.A. Immunomodulatory Therapy of Cancer with T Cell-Engaging BiTE Antibody Blinatumomab. Exp Cell Res 2011, 317, 1255–1260. [CrossRef]
- Merrill, J.T.; Neuwelt, C.M.; Wallace, D.J.; Shanahan, J.C.; Latinis, K.M.; Oates, J.C.; Utset, T.O.; Gordon, C.; Isenberg, D.A.; Hsieh, H.; et al. Efficacy and Safety of Rituximab in Moderately-to-severely Active Systemic Lupus Erythematosus: The Randomized, Double-blind, Phase Ii/Iii Systemic Lupus Erythematosus Evaluation of Rituximab Trial. Arthritis Rheum. 2010, 62, 222–233. [CrossRef]
- Kamburova, E.G.; Koenen, H.J.P.M.; Borgman, K.J.E.; Berge, I.J. ten; Joosten, I.; Hilbrands, L.B. A Single Dose of Rituximab Does Not Deplete B Cells in Secondary Lymphoid Organs but Alters Phenotype and Function. Am. J. Transplant. 2013, 13, 1503–1511. [CrossRef]
- Tedder, T.F.; Engel, P. CD20: A Regulator of Cell-Cycle Progression of B Lymphocytes. Immunol. Today 1994, 15, 450–454. [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive Cell Transfer as Personalized Immunotherapy for Human Cancer. Science 2015, 348, 62–68. [CrossRef]
- Valiullina, A.Kh.; Zmievskaya, E.A.; Ganeeva, I.A.; Zhuravleva, M.N.; Garanina, E.E.; Rizvanov, A.A.; Petukhov, A.V.; Bulatov, E.R. Evaluation of CAR-T Cells’ Cytotoxicity against Modified Solid Tumor Cell Lines. Biomed 2023, 11, 626. [CrossRef]
- Kansal, R.; Richardson, N.; Neeli, I.; Khawaja, S.; Chamberlain, D.; Ghani, M.; Ghani, Q.; Balazs, L.; Beranova-Giorgianni, S.; Giorgianni, F.; et al. Sustained B Cell Depletion by CD19-Targeted CAR T Cells Is a Highly Effective Treatment for Murine Lupus. Sci. Transl. Med. 2019, 11. [CrossRef]
- Jin, X.; Xu, Q.; Pu, C.; Zhu, K.; Lu, C.; Jiang, Y.; Xiao, L.; Han, Y.; Lu, L. Therapeutic Efficacy of Anti-CD19 CAR-T Cells in a Mouse Model of Systemic Lupus Erythematosus. Cell. Mol. Immunol. 2021, 18, 1896–1903. [CrossRef]
- Mougiakakos, D.; Krönke, G.; Völkl, S.; Kretschmann, S.; Aigner, M.; Kharboutli, S.; Böltz, S.; Manger, B.; Mackensen, A.; Schett, G. CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. N. Engl. J. Med. 2021, 385, 567–569. [CrossRef]
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [CrossRef]
- Sato, S.; Fujimoto, M.; Hasegawa, M.; Takehara, K. Altered Blood B Lymphocyte Homeostasis in Systemic Sclerosis: Expanded Naive B Cells and Diminished but Activated Memory B Cells. Arthritis Rheum. 2004, 50, 1918–1927. [CrossRef]
- Maher, T.M.; Tudor, V.A.; Saunders, P.; Gibbons, M.A.; Fletcher, S.V.; Denton, C.P.; Hoyles, R.K.; Parfrey, H.; Renzoni, E.A.; Kokosi, M.; et al. Rituximab versus Intravenous Cyclophosphamide in Patients with Connective Tissue Disease-Associated Interstitial Lung Disease in the UK (RECITAL): A Double-Blind, Double-Dummy, Randomised, Controlled, Phase 2b Trial. Lancet Respir. Med. 2023, 11, 45–54. [CrossRef]
- Ebata, S.; Yoshizaki, A.; Oba, K.; Kashiwabara, K.; Ueda, K.; Uemura, Y.; Watadani, T.; Fukasawa, T.; Miura, S.; Yoshizaki-Ogawa, A.; et al. Safety and Efficacy of Rituximab in Systemic Sclerosis (DESIRES): A Double-Blind, Investigator-Initiated, Randomised, Placebo-Controlled Trial. Lancet Rheumatol. 2021, 3, e489–e497. [CrossRef]
- Sakkas, L.I.; Chikanza, I.C.; Platsoucas, C.D. Mechanisms of Disease: The Role of Immune Cells in the Pathogenesis of Systemic Sclerosis. Nat. Clin. Pr. Rheumatol. 2006, 2, 679–685. [CrossRef]
- Ntelis, K.; Solomou, E.E.; Sakkas, L.; Liossis, S.-N.; Daoussis, D. The Role of Platelets in Autoimmunity, Vasculopathy, and Fibrosis: Implications for Systemic Sclerosis. Semin. Arthritis Rheum. 2017, 47, 409–417. [CrossRef]
- Bergmann, C.; Müller, F.; Distler, J.H.W.; Györfi, A.-H.; Völkl, S.; Aigner, M.; Kretschmann, S.; Reimann, H.; Harrer, T.; Bayerl, N.; et al. Treatment of a Patient with Severe Systemic Sclerosis (SSc) Using CD19-Targeted CAR T Cells. Ann. Rheum. Dis. 2023, 82, 1117–1120. [CrossRef]
- Huang, J.; Fu, X.; Chen, X.; Li, Z.; Huang, Y.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [CrossRef]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring Synovial Homeostasis in Rheumatoid Arthritis by Targeting Fibroblast-like Synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [CrossRef]
- Barnas, J.L.; Looney, R.J.; Anolik, J.H. B Cell Targeted Therapies in Autoimmune Disease. Curr. Opin. Immunol. 2019, 61, 92–99. [CrossRef]
- Edwards, J.C.W.; Szczepański, L.; Szechiński, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-Cell–Targeted Therapy with Rituximab in Patients with Rheumatoid Arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [CrossRef]
- Kaplanski, G.; Marin, V.; Montero-Julian, F.; Mantovani, A.; Farnarier, C. IL-6: A Regulator of the Transition from Neutrophil to Monocyte Recruitment during Inflammation. Trends Immunol. 2003, 24, 25–29. [CrossRef]
- Hashizume, M.; Hayakawa, N.; Mihara, M. IL-6 Trans-Signalling Directly Induces RANKL on Fibroblast-like Synovial Cells and Is Involved in RANKL Induction by TNF-α and IL-17. Rheumatology 2008, 47, 1635–1640. [CrossRef]
- Maini, R.N.; Taylor, P.C.; Szechinski, J.; Pavelka, K.; Bröll, J.; Balint, G.; Emery, P.; Raemen, F.; Petersen, J.; Smolen, J.; et al. Double-blind Randomized Controlled Clinical Trial of the Interleukin-6 Receptor Antagonist, Tocilizumab, in European Patients with Rheumatoid Arthritis Who Had an Incomplete Response to Methotrexate. Arthritis Rheum. 2006, 54, 2817–2829. [CrossRef]
- Gabay, C.; Emery, P.; Vollenhoven, R. van; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab Monotherapy versus Adalimumab Monotherapy for Treatment of Rheumatoid Arthritis (ADACTA): A Randomised, Double-Blind, Controlled Phase 4 Trial. Lancet 2013, 381, 1541–1550. [CrossRef]
- Genovese, M.C.; Fleischmann, R.; Kivitz, A.J.; Rell-Bakalarska, M.; Martincova, R.; Fiore, S.; Rohane, P.; Hoogstraten, H. van; Garg, A.; Fan, C.; et al. Sarilumab Plus Methotrexate in Patients With Active Rheumatoid Arthritis and Inadequate Response to Methotrexate: Results of a Phase III Study. Arthritis Rheumatol. 2015, 67, 1424–1437. [CrossRef]
- Schaible, T.F. Long Term Safety of Infliximab. Can. J. Gastroenterol. 2020, 14, 29C-32C. [CrossRef]
- Nam, J.L.; Ramiro, S.; Gaujoux-Viala, C.; Takase, K.; Leon-Garcia, M.; Emery, P.; Gossec, L.; Landewe, R.; Smolen, J.S.; Buch, M.H. Efficacy of Biological Disease-Modifying Antirheumatic Drugs: A Systematic Literature Review Informing the 2013 Update of the EULAR Recommendations for the Management of Rheumatoid Arthritis. Ann. Rheum. Dis. 2014, 73, 516. [CrossRef]
- Santamaria-Alza, Y.; Vasquez, G. Are Chimeric Antigen Receptor T Cells (CAR-T Cells) the Future in Immunotherapy for Autoimmune Diseases? Inflamm. Res. 2021, 70, 651–663. [CrossRef]
- Ellebrecht, C.T.; Bhoj, V.G.; Nace, A.; Choi, E.J.; Mao, X.; Cho, M.J.; Zenzo, G.D.; Lanzavecchia, A.; Seykora, J.T.; Cotsarelis, G.; et al. Reengineering Chimeric Antigen Receptor T Cells for Targeted Therapy of Autoimmune Disease. Science 2016, 353, 179–184. [CrossRef]
- Li, Y.-J.; Chen, Z. Cell-Based Therapies for Rheumatoid Arthritis: Opportunities and Challenges. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221100294. [CrossRef]
- Tenspolde, M.; Zimmermann, K.; Weber, L.C.; Hapke, M.; Lieber, M.; Dywicki, J.; Frenzel, A.; Hust, M.; Galla, M.; Buitrago-Molina, L.E.; et al. Regulatory T Cells Engineered with a Novel Insulin-Specific Chimeric Antigen Receptor as a Candidate Immunotherapy for Type 1 Diabetes. J. Autoimmun. 2019, 103, 102289. [CrossRef]
- Kaminskiy, Y.; Kuznetsova, V.; Kudriaeva, A.; Zmievskaya, E.; Bulatov, E. Neglected, yet Significant Role of FOXP1 in T-Cell Quiescence, Differentiation and Exhaustion. Front. Immunol. 2022, 13, 971045. [CrossRef]
- Zmievskaya, E.; Valiullina, A.; Ganeeva, I.; Petukhov, A.; Rizvanov, A.; Bulatov, E. Application of CAR-T Cell Therapy beyond Oncology: Autoimmune Diseases and Viral Infections. Biomedicines 2021, 9. [CrossRef]
- Boroughs, A.C.; Larson, R.C.; Choi, B.D.; Bouffard, A.A.; Riley, L.S.; Schiferle, E.; Kulkarni, A.S.; Cetrulo, C.L.; Ting, D.; Blazar, B.R.; et al. Chimeric Antigen Receptor Costimulation Domains Modulate Human Regulatory T Cell Function. JCI Insight 2019, 4. [CrossRef]
- Zhang, B.; Wang, Y.; Yuan, Y.; Sun, J.; Liu, L.; Huang, D.; Hu, J.; Wang, M.; Li, S.; Song, W.; et al. In Vitro Elimination of Autoreactive B Cells from Rheumatoid Arthritis Patients by Universal Chimeric Antigen Receptor T Cells. Ann. Rheum. Dis. 2021, 80, 176–184. [CrossRef]
- Minutolo, N.G.; Hollander, E.E.; Powell, D.J. The Emergence of Universal Immune Receptor T Cell Therapy for Cancer. Front. Oncol. 2019, 9, 176. [CrossRef]
- Sakkas, L.I.; Bogdanos, D.P.; Katsiari, C.; Platsoucas, C.D. Anti-Citrullinated Peptides as Autoantigens in Rheumatoid Arthritis—Relevance to Treatment. Autoimmun. Rev. 2014, 13, 1114–1120. [CrossRef]
- Kim, M.S.; Ma, J.S.Y.; Yun, H.; Cao, Y.; Kim, J.Y.; Chi, V.; Wang, D.; Woods, A.; Sherwood, L.; Caballero, D.; et al. Redirection of Genetically Engineered CAR-T Cells Using Bifunctional Small Molecules. J. Am. Chem. Soc. 2015, 137, 2832–2835. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




