Submitted:
16 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
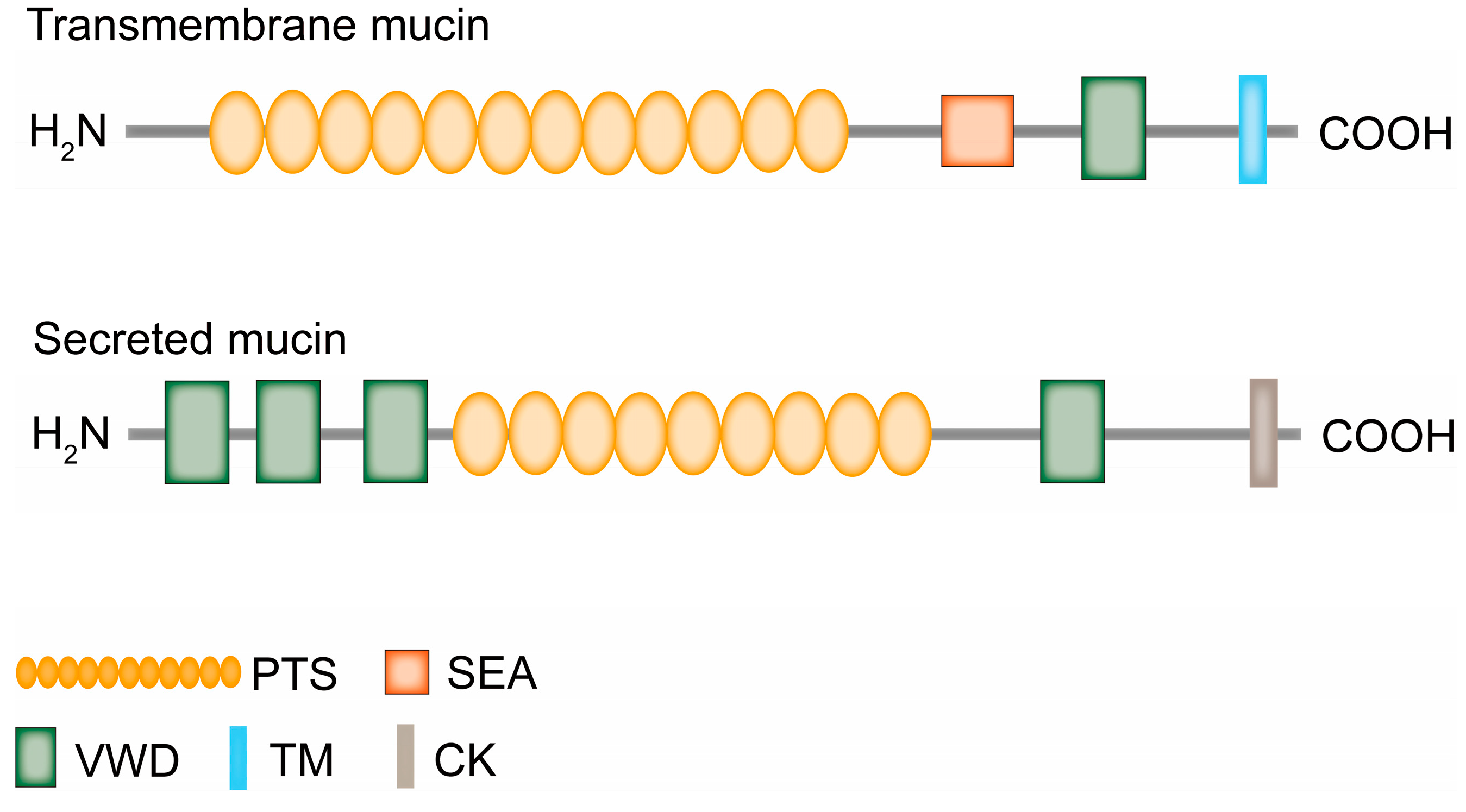
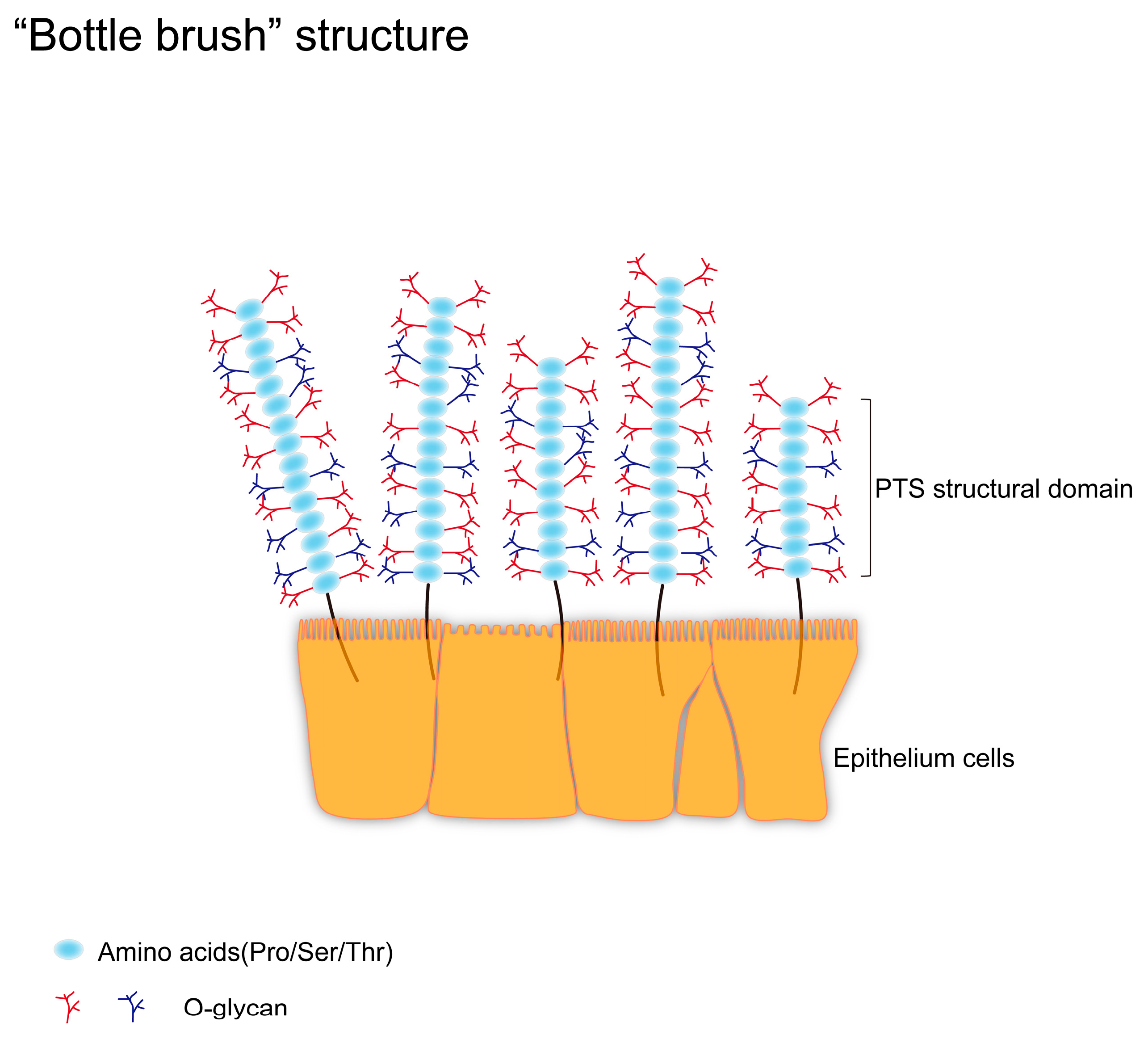
2. Major Types of Glycosylation on Mucins
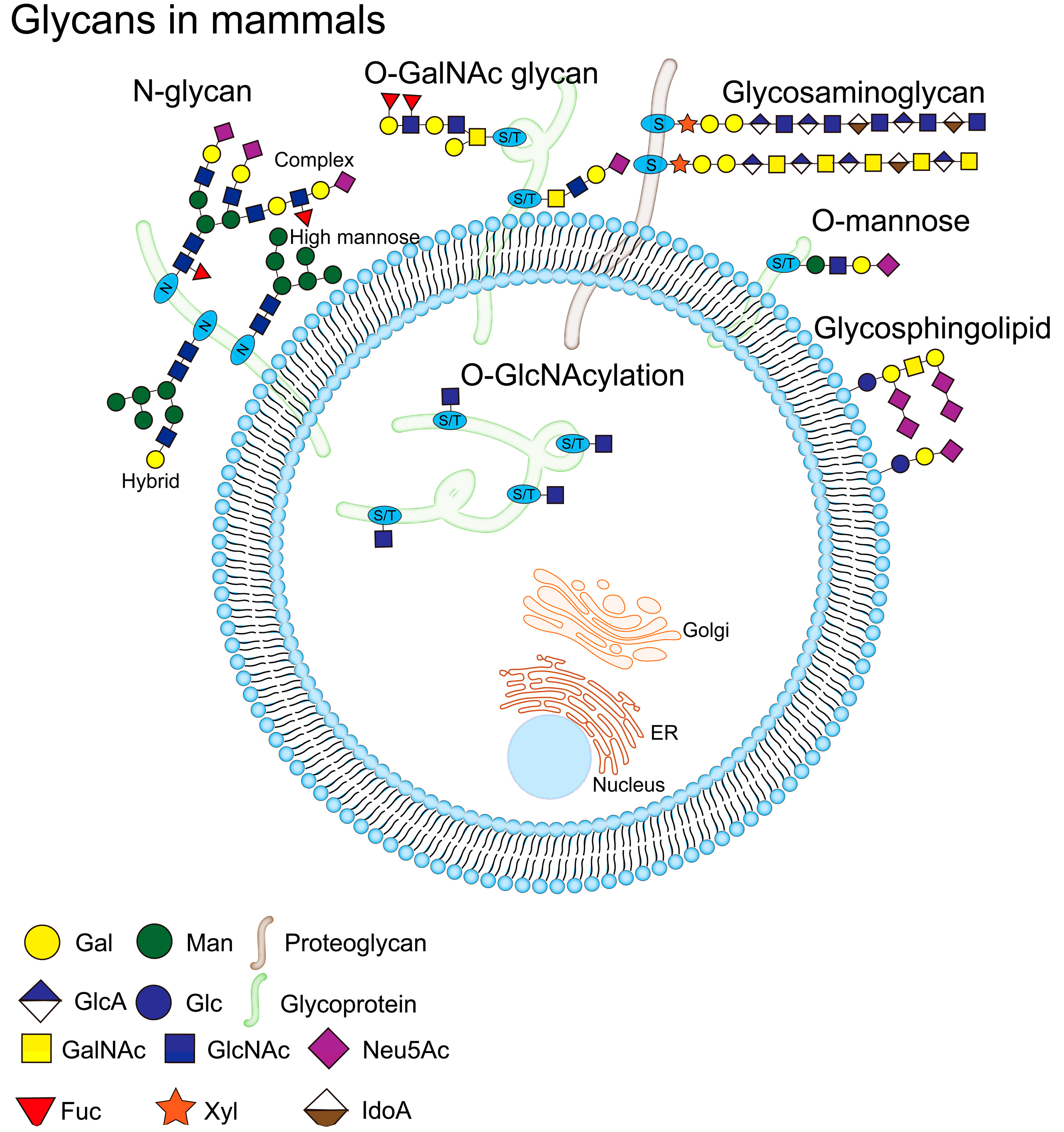
2.1. O-Glycosylation
2.2. N-Glycosylation
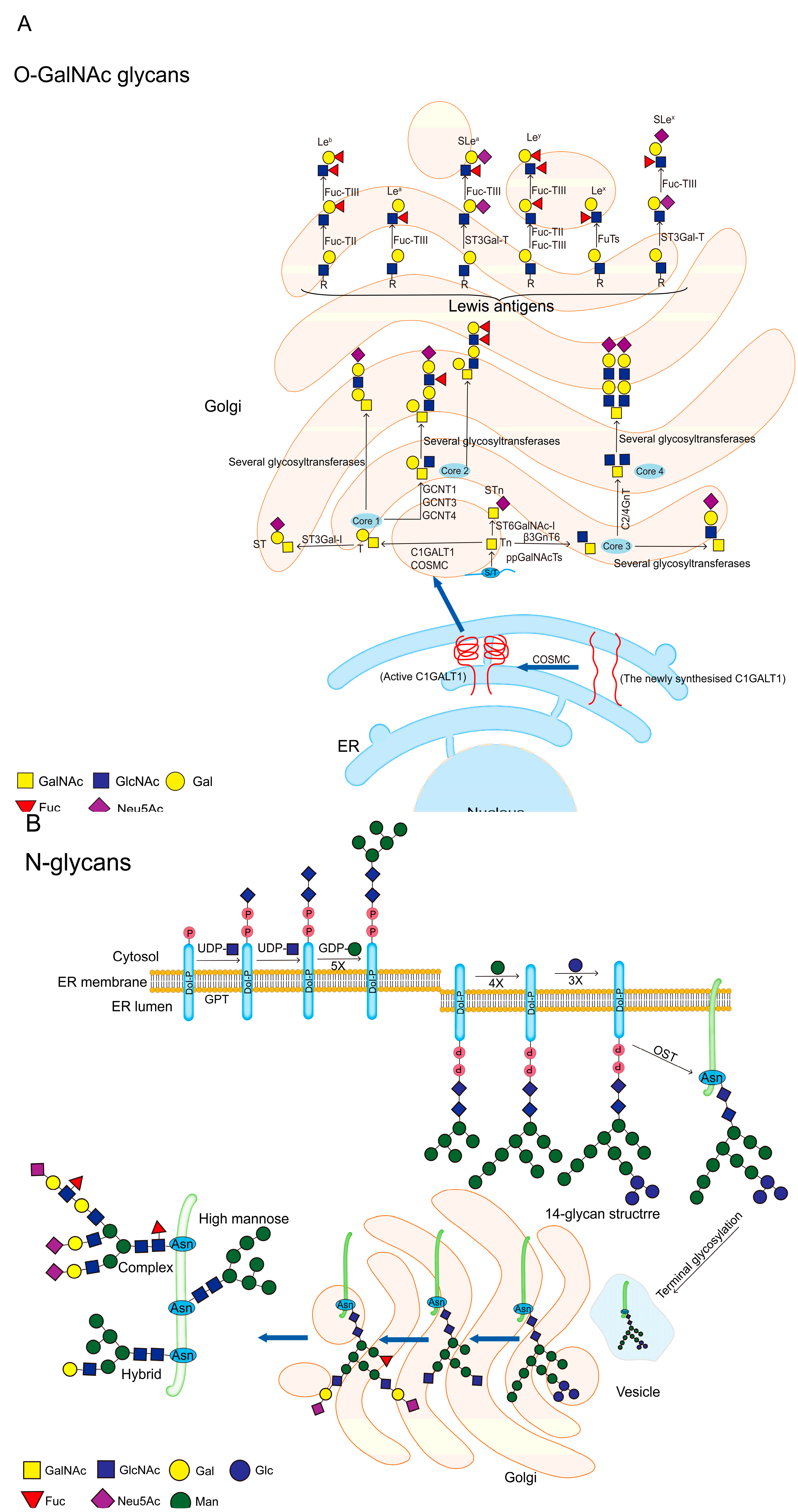
3. The Mechanisms of Abnormal Mucin Glycans Production
3.1. The Activities and Localizations of Glycosyltransferases
3.2. Changes of Golgi pH
3.3. Efficiency of Nucleotide Transporters
4. The Role of Abnormal Mucin Glycans in Cancer Development
4.1. Proliferative Capacity
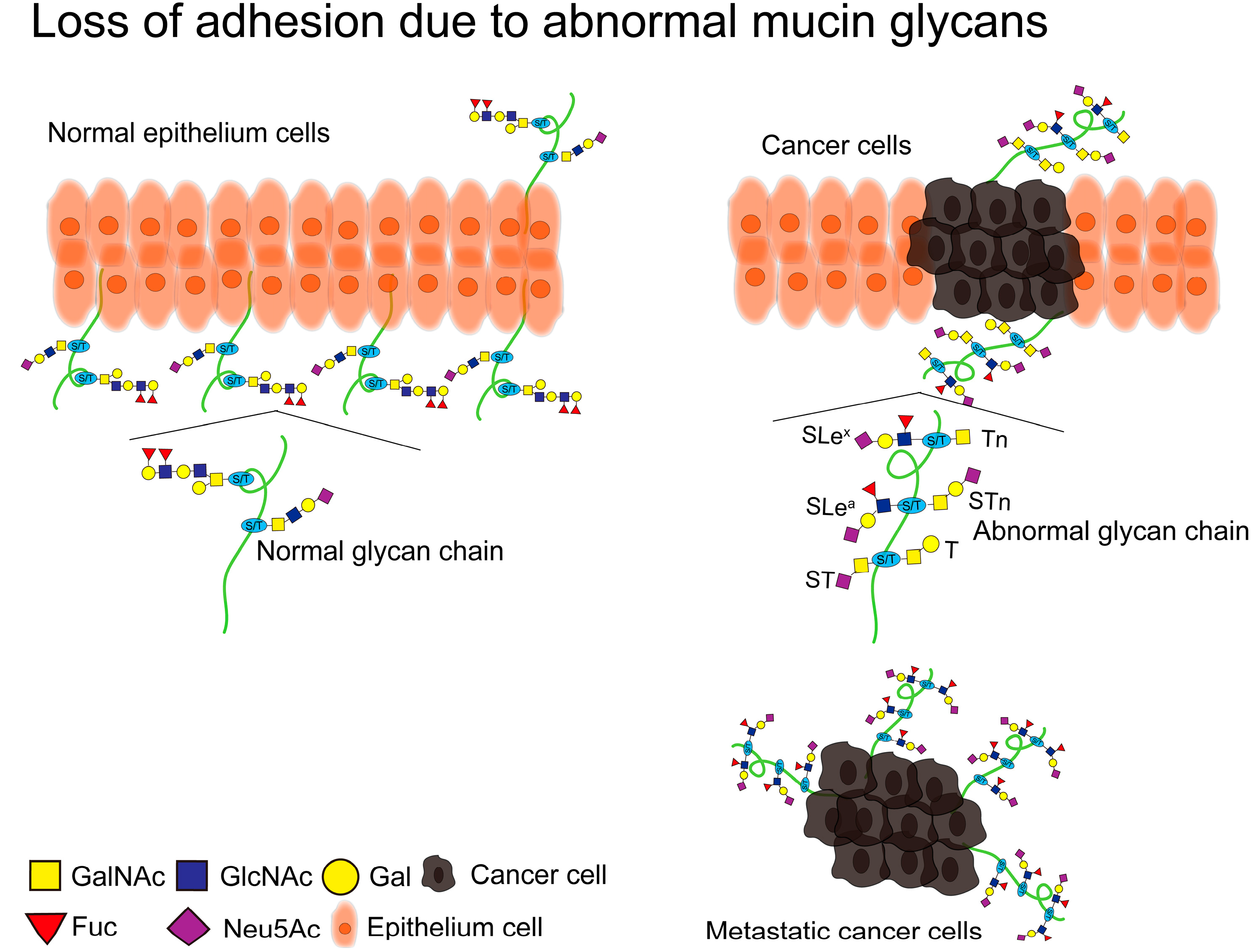
4.2. Loss of Adhesion
4.3. Cancer Metastasis
4.4. Cancer Immune Escape
4.5. Carcinogenic Pathogens
5. MUC1-Based Cancer Diagnosis and Targeted Therapy
5.1. Antibodies
5.2. Radiopharmaceuticals
5.3. Vaccines
5.4. CAR-T
| Type of therapy | Designation | Antigenic epitope/target | Type of cancer | Ref. |
|---|---|---|---|---|
|
Antibodies |
SM3 5E10 5E5 TAB004 hMUC1 HzMUC1 |
PDTRP MUC1-N MUC1-Tn/STn MUC1(STAPPAHGV) MUC1-C MUC1-N and MUC1-C |
Breast cancer Prostate cancer Breast cancer Pancreatic cancer Breast cancer Pancreatic cancer |
[126,127] [128] [129,131] [133] [134] [135] |
|
Radiopharmac-euticals |
99mTc-HYNIC-PR81 DDS MUC1-FA- [18F] SFB |
MUC1(AVGLSPDGSRGV) MUC1 MUC1 |
Breast cancer Triple-negative breast cancer Breast cancer |
[138] [139] [140] |
|
Vaccines |
CTB-MUC1 pcDNA3.1-VNTR TG4010 DC-based vaccine MUC1-glycopeptide vaccines |
VNTR VNTR MUC1 MUC1 MUC1-Tn/STn |
Breast cancer Pancreatic cancer Non-small-cell lung cancer Non-small-cell lung cancer Breast cancer, Pancreatic cancer |
[147] [148] [149] [150] [151] |
|
CAR-T |
Anti-MUC1-Tn-CAR-T Anti-MUC1-Tn-CAR-T MUC28z CAR-T Enhanced MUC1-CAR-T ICR |
MUC1-Tn MUC1-Tn MUC1 MUC1 --- |
Leukemia, pancreatic cancer Intrahepatic cholangiocarcinoma Triple-negative breast cancer Esophageal cancer Prostate cancer |
[154] [156] [153] [157] [158] |
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Behera, S.K.; Praharaj, A.B.; Dehury, B.; Negi, S. Exploring the role and diversity of mucins in health and disease with special insight into non-communicable diseases. Glycoconj. J. 2015, 32, 575–613. [CrossRef]
- Wi, D.-H.; Cha, J.-H.; Jung, Y.-S. Mucin in cancer: a stealth cloak for cancer cells. BMB Rep. 2021, 54, 344–355. [CrossRef]
- Van Seuningen, P. Pigny, M. Perrais, N. Porchet, J.P. Aubert, Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer?, Frontiers in bioscience : a journal and virtual library 6 (2001) D1216-34. [CrossRef]
- Tailford, L.E.; Crost, E.H.; Kavanaugh, D.; Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 2015, 6, 81–81. [CrossRef]
- Jonckheere, N.; Vincent, A.; Neve, B.; Van Seuningen, I. Mucin expression, epigenetic regulation and patient survival: A toolkit of prognostic biomarkers in epithelial cancers. Biochim. et Biophys. Acta (BBA) - Rev. Cancer 2021, 1876, 188538. [CrossRef]
- S. Pinzón Martín, P.H. Seeberger, D. Varón Silva, Mucins and Pathogenic Mucin-Like Molecules Are Immunomodulators During Infection and Targets for Diagnostics and Vaccines, Frontiers in chemistry 7 (2019) 710. [CrossRef]
- Buscaglia, C.A.; Campo, V.A.; Frasch, A.C.C.; Di Noia, J.M. Trypanosoma cruzi surface mucins: host-dependent coat diversity. Nat. Rev. Genet. 2006, 4, 229–236. [CrossRef]
- Szafranski-Schneider, E.; Swidergall, M.; Cottier, F.; Tielker, D.; Román, E.; Pla, J.; Ernst, J.F. Msb2 Shedding Protects Candida albicans against Antimicrobial Peptides. PLOS Pathog. 2012, 8, e1002501. [CrossRef]
- G.C. Hansson, Mucins and the Microbiome, Annual review of biochemistry 89 (2020) 769-793. [CrossRef]
- Syed, Z.A.; Zhang, L.; Hagen, K.G.T. In vivo models of mucin biosynthesis and function. Adv. Drug Deliv. Rev. 2022, 184, 114182–114182. [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [CrossRef]
- Jonckheere, N.; Van Seuningen, I. The membrane-bound mucins: From cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie 2010, 92, 1–11. [CrossRef]
- G. Radicioni, A. Ceppe, A.A. Ford, N.E. Alexis, R.G. Barr, E.R. Bleecker, S.A. Christenson, C.B. Cooper, M.K. Han, N.N. Hansel, A.T. Hastie, E.A. Hoffman, R.E. Kanner, F.J. Martinez, E. Ozkan, R. Paine, 3rd, P.G. Woodruff, W.K. O'Neal, R.C. Boucher, M. Kesimer, Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort, The Lancet. Respiratory medicine 9(11) (2021) 1241-1254. [CrossRef]
- Terada, T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases.. Int. J. Clin. Exp. Pathol. 2013, 6, 613–21.
- Burcham, L.R.; Bath, J.R.; Werlang, C.A.; Lyon, L.M.; Liu, N.; Evans, C.; Ribbeck, K.; Doran, K.S. Role of MUC5B during Group B Streptococcal Vaginal Colonization. Mbio 2022, 13, e0003922. [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [CrossRef]
- D. Jüngst, A. Niemeyer, I. Müller, B. Zündt, G. Meyer, M. Wilhelmi, R. del Pozo, Mucin and phospholipids determine viscosity of gallbladder bile in patients with gallstones, World journal of gastroenterology 7(2) (2001) 203-7. [CrossRef]
- Kohout, V.R.; Wardzala, C.L.; Kramer, J.R. Synthesis and biomedical applications of mucin mimic materials. Adv. Drug Deliv. Rev. 2022, 191, 114540. [CrossRef]
- Brockhausen, Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions, EMBO reports 7(6) (2006) 599-604. [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [CrossRef]
- Larsson, J.M.H.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.V.; Eklund, L.; Sjövall, H.; Hansson, G.C. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 2011, 17, 2299–2307. [CrossRef]
- Matsuura, N.; Narita, T.; Hiraiwa, N.; Hiraiwa, M.; Murai, H.; Iwase, T.; Funahashi, H.; Imai, T.; Takagi, H.; Kannagi, R. Gene expression of fucosyl- and sialyl-transferases which synthesize sialyl Lewisx, the carbohydrate ligands for E-selectin, in human breast cancer.. Int. J. Oncol. 1998, 12, 1157–1221. [CrossRef]
- Varki, Biological roles of glycans, Glycobiology 27(1) (2017) 3-49. [CrossRef]
- B.M. Harvey, R.S. Haltiwanger, Regulation of Notch Function by O-Glycosylation, Advances in experimental medicine and biology 1066 (2018) 59-78. [CrossRef]
- B.C. Holdener, R.S. Haltiwanger, Protein O-fucosylation: structure and function, Current opinion in structural biology 56 (2019) 78-86. [CrossRef]
- Larsen, I.S.B.; Narimatsu, Y.; Clausen, H.; Joshi, H.J.; Halim, A. Multiple distinct O-Mannosylation pathways in eukaryotes. Curr. Opin. Struct. Biol. 2019, 56, 171–178. [CrossRef]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [CrossRef]
- Yu, H.; Takeuchi, H. Protein O-glucosylation: another essential role of glucose in biology. Curr. Opin. Struct. Biol. 2019, 56, 64–71. [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [CrossRef]
- Cummings, R.D. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 2009, 5, 1087–1104. [CrossRef]
- A.V. Nairn, W.S. York, K. Harris, E.M. Hall, J.M. Pierce, K.W. Moremen, Regulation of glycan structures in animal tissues: transcript profiling of glycan-related genes, The Journal of biological chemistry 283(25) (2008) 17298-313. [CrossRef]
- L.A. Tabak, In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins, Annual review of physiology 57 (1995) 547-64.
- R.A. Cone, Barrier properties of mucus, Advanced drug delivery reviews 61(2) (2009) 75-85. [CrossRef]
- Chugh, S.; Gnanapragassam, V.S.; Jain, M.; Rachagani, S.; Ponnusamy, M.P.; Batra, S.K. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim. et Biophys. Acta (BBA) - Rev. Cancer 2015, 1856, 211–225. [CrossRef]
- in: A. Varki, R.D. Cummings, J.D. Esko, P. Stanley, G.W. Hart, M. Aebi, D. Mohnen, T. Kinoshita, N.H. Packer, J.H. Prestegard, R.L. Schnaar, P.H. Seeberger (Eds.), Essentials of Glycobiology, Cold Spring Harbor Laboratory Press Copyright © 2022 by the Consortium of Glycobiology Editors, La Jolla, California. Published by Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. All rights reserved., Cold Spring Harbor (NY), 2022.
- R. Kornfeld, S. Kornfeld, Assembly of asparagine-linked oligosaccharides, Annual review of biochemistry 54 (1985) 631-64. [CrossRef]
- P. Van den Steen, P.M. Rudd, R.A. Dwek, G. Opdenakker, Concepts and principles of O-linked glycosylation, Critical reviews in biochemistry and molecular biology 33(3) (1998) 151-208. [CrossRef]
- Parry, S.; Hanisch, F.G.; Leir, S.-H.; Sutton-Smith, M.; Morris, H.R.; Dell, A.; Harris, A. N-Glycosylation of the MUC1 mucin in epithelial cells and secretions. Glycobiology 2006, 16, 623–634. [CrossRef]
- Blanas, N.M. Sahasrabudhe, E. Rodríguez, Y. van Kooyk, S.J. van Vliet, Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy, Frontiers in oncology 8 (2018) 39. [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [CrossRef]
- T. Ju, R.D. Cummings, A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase, Proceedings of the National Academy of Sciences of the United States of America 99(26) (2002) 16613-8. [CrossRef]
- T. Ju, R.P. Aryal, M.R. Kudelka, Y. Wang, R.D. Cummings, The Cosmc connection to the Tn antigen in cancer, Cancer biomarkers : section A of Disease markers 14(1) (2014) 63-81. [CrossRef]
- Bergstrom, K.S.B.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037. [CrossRef]
- Breloy, I.; Hanisch, F.-G. Functional Roles of O-Glycosylation. Molecules 2018, 23, 3063. [CrossRef]
- Werlang, C.A.; Chen, W.G.; Aoki, K.; Wheeler, K.M.; Tymm, C.; Mileti, C.J.; Burgos, A.C.; Kim, K.; Tiemeyer, M.; Ribbeck, K. Mucin O-glycans suppress quorum-sensing pathways and genetic transformation in Streptococcus mutans. Nat. Microbiol. 2021, 6, 574–583. [CrossRef]
- B. Nichols, C.R. Dawson, B. Togni, Surface features of the conjunctiva and cornea, Investigative ophthalmology & visual science 24(5) (1983) 570-6.
- Argüeso, P.; Guzman-Aranguez, A.; Mantelli, F.; Cao, Z.; Ricciuto, J.; Panjwani, N. Association of Cell Surface Mucins with Galectin-3 Contributes to the Ocular Surface Epithelial Barrier. J. Biol. Chem. 2009, 284, 23037–23045. [CrossRef]
- Seelenmeyer, C.; Wegehingel, S.; Lechner, J.; Nickel, W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J. Cell Sci. 2003, 116, 1305–1318. [CrossRef]
- Brockhausen, Pathways of O-glycan biosynthesis in cancer cells, Biochimica et biophysica acta 1473(1) (1999) 67-95. [CrossRef]
- S. Hakomori, Glycosylation defining cancer malignancy: new wine in an old bottle, Proceedings of the National Academy of Sciences of the United States of America 99(16) (2002) 10231-3. [CrossRef]
- D.F. Hayes, Serum (circulating) tumor markers for breast cancer, Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer 140 (1996) 101-13.
- Beckwith, D.M.; Cudic, M. Tumor-associated O-glycans of MUC1: Carriers of the glyco-code and targets for cancer vaccine design. Semin. Immunol. 2020, 47, 101389–101389. [CrossRef]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.-D.; Klausing, S.; Hillier, M.; Maher, D.A.J.; Noll, S.K.T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [CrossRef]
- Liu, F.; Fu, J.; Bergstrom, K.; Shan, X.; McDaniel, J.M.; McGee, S.; Bai, X.; Chen, W.; Xia, L. Core 1–derived mucin-type O-glycosylation protects against spontaneous gastritis and gastric cancer. J. Exp. Med. 2019, 217. [CrossRef]
- Baldus, S.E.; Zirbes, T.K.; Engel, S.; Hanisch, F.-G.; Mönig, S.P.; Lorenzen, J.; Glossmann, J.; Fromm, S.; Thiele, J.; Pichlmaier, H.; et al. Correlation of the immunohistochemical reactivity of mucin peptide cores MUC1 and MUC2 with the histopathological subtype and prognosis of gastric carcinomas. Int. J. Cancer 1998, 79, 133–138. [CrossRef]
- Carvalho, F.; Seruca, R.; David, L.; Amorim, A.; Seixas, M.; Bennett, E.; Clausen, H.; Sobrinho-Simoes, M. MUC1 gene polymorphism and gastric cancer–an epidemiological study. Glycoconj. J. 1997, 14, 107–111. [CrossRef]
- Malaby, H.L.H.; Kobertz, W.R. Molecular determinants of co- and post-translational N-glycosylation of type I transmembrane peptides. Biochem. J. 2013, 453, 427–434. [CrossRef]
- Taniguchi, T.; Woodward, A.M.; Magnelli, P.; McColgan, N.M.; Lehoux, S.; Jacobo, S.M.P.; Mauris, J.; Argüeso, P. N-Glycosylation affects the stability and barrier function of the MUC16 mucin. J. Biol. Chem. 2017, 292, 11079–11090. [CrossRef]
- Bell, S.L.; Xu, G.; Khatri, I.A.; Wang, R.; Rahman, S.; Forstner, J.F. N-linked oligosaccharides play a role in disulphide-dependent dimerization of intestinal mucin Muc2. Biochem. J. 2003, 373, 893–900. [CrossRef]
- Asker, N.; Axelsson, M.A.B.; Olofsson, S.-O.; Hansson, G.C. Dimerization of the Human MUC2 Mucin in the Endoplasmic Reticulum Is Followed by a N-Glycosylation-dependent Transfer of the Mono- and Dimers to the Golgi Apparatus. J. Biol. Chem. 1998, 273, 18857–18863. [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [CrossRef]
- T. Lange, S. Ullrich, I. Müller, M.F. Nentwich, K. Stübke, S. Feldhaus, C. Knies, O.J. Hellwinkel, R.L. Vessella, C. Abramjuk, M. Anders, J. Schröder-Schwarz, T. Schlomm, H. Huland, G. Sauter, U. Schumacher, Human prostate cancer in a clinically relevant xenograft mouse model: identification of β(1,6)-branched oligosaccharides as a marker of tumor progression, Clinical cancer research : an official journal of the American Association for Cancer Research 18(5) (2012) 1364-73.
- Gubbels, J.A.; Belisle, J.; Onda, M.; Rancourt, C.; Migneault, M.; Ho, M.; Bera, T.K.; Connor, J.; Sathyanarayana, B.K.; Lee, B.; et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 2006, 5, 1–50. [CrossRef]
- Liu, Y.; Lan, L.; Li, Y.; Lu, J.; He, L.; Deng, Y.; Fei, M.; Lu, J.-W.; Shangguan, F.; Lu, J.-P.; et al. N-glycosylation stabilizes MerTK and promotes hepatocellular carcinoma tumor growth. Redox Biol. 2022, 54, 102366. [CrossRef]
- Zhu, L.; Chen, Y.; Du, H.; Cong, Y.; Yan, W.; Ma, K.; Huang, X. N-glycosylation of CD82 at Asn157 is required for suppressing migration and invasion by reversing EMT via Wnt/β-catenin pathway in colon cancer. Biochem. Biophys. Res. Commun. 2022, 629, 121–127. [CrossRef]
- Varki, A. Factors controlling the glycosylation potential of the Golgi apparatus. Trends Cell Biol. 1998, 8, 34–40. [CrossRef]
- Scott, E.; Hodgson, K.; Calle, B.; Turner, H.; Cheung, K.; Bermudez, A.; Marques, F.J.G.; Pye, H.; Yo, E.C.; Islam, K.; et al. Upregulation of GALNT7 in prostate cancer modifies O-glycosylation and promotes tumour growth. Oncogene 2023, 42, 926–937. [CrossRef]
- M. Prorok-Hamon, F. Notel, S. Mathieu, C. Langlet, M. Fukuda, A. El-Battari, N-glycans of core2 beta(1,6)-N-acetylglucosaminyltransferase-I (C2GnT-I) but not those of alpha(1,3)-fucosyltransferase-VII (FucT-VII) are required for the synthesis of functional P-selectin glycoprotein ligand-1 (PSGL-1): effects on P-, L- and E-selectin binding, The Biochemical journal 391(Pt 3) (2005) 491-502.
- Ruggiero, F.M.; Vilcaes, A.A.; Iglesias-Bartolomé, R.; Daniotti, J.L. Critical role of evolutionarily conserved glycosylation at Asn211 in the intracellular trafficking and activity of sialyltransferase ST3Gal-II. Biochem. J. 2015, 469, 83–95. [CrossRef]
- Skrincosky, D.; Kain, R.; El-Battari, A.; Exner, M.; Kerjaschki, D.; Fukuda, M. Altered Golgi Localization of Core 2 β-1,6-N-Acetylglucosaminyltransferase Leads to Decreased Synthesis of Branched O-Glycans. J. Biol. Chem. 1997, 272, 22695–22702. [CrossRef]
- G. Egea, C. Francí, G. Gambús, T. Lesuffleur, A. Zweibaum, F.X. Real, cis-Golgi resident proteins and O-glycans are abnormally compartmentalized in the RER of colon cancer cells, Journal of cell science 105 ( Pt 3) (1993) 819-30.
- Rivinoja, A.; Hassinen, A.; Kokkonen, N.; Kauppila, A.; Kellokumpu, S. Elevated Golgi pH impairs terminalN-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J. Cell. Physiol. 2009, 220, 144–154. [CrossRef]
- Rivinoja, A.; Kokkonen, N.; Kellokumpu, I.; Kellokumpu, S. Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell. Physiol. 2006, 208, 167–174. [CrossRef]
- Axelsson, M.A.; Karlsson, N.G.; Steel, D.M.; Ouwendijk, J.; Nilsson, T.; Hansson, G.C. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 2001, 11, 633–644. [CrossRef]
- B.J. Campbell, G.E. Rowe, K. Leiper, J.M. Rhodes, Increasing the intra-Golgi pH of cultured LS174T goblet-differentiated cells mimics the decreased mucin sulfation and increased Thomsen-Friedenreich antigen (Gal beta1-3GalNac alpha-) expression seen in colon cancer, Glycobiology 11(5) (2001) 385-93.
- Hadley, B.; Maggioni, A.; Ashikov, A.; Day, C.J.; Haselhorst, T.; Tiralongo, J. Structure and function of nucleotide sugar transporters: Current progress. Comput. Struct. Biotechnol. J. 2014, 10, 23–32. [CrossRef]
- Nji, E.; Gulati, A.; Qureshi, A.A.; Coincon, M.; Drew, D. Structural basis for the delivery of activated sialic acid into Golgi for sialyation. Nat. Struct. Mol. Biol. 2019, 26, 415–423. [CrossRef]
- Takeshima-Futagami, T.; Sakaguchi, M.; Uehara, E.; Aoki, K.; Ishida, N.; Sanai, Y.; Sugahara, Y.; Kawakita, M. Amino acid residues important for CMP-sialic acid recognition by the CMP-sialic acid transporter: analysis of the substrate specificity of UDP-galactose/CMP-sialic acid transporter chimeras. Glycobiology 2012, 22, 1731–1740. [CrossRef]
- Bhatia, R.; Gautam, S.K.; Cannon, A.; Thompson, C.; Hall, B.R.; Aithal, A.; Banerjee, K.; Jain, M.; Solheim, J.C.; Kumar, S.; et al. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 2019, 38, 223–236. [CrossRef]
- S.J. Gendler, MUC1, the renaissance molecule, Journal of mammary gland biology and neoplasia 6(3) (2001) 339-53.
- Burchell, J.M.; Mungul, A.; Taylor-Papadimitriou, J. O-Linked Glycosylation in the Mammary Gland: Changes that Occur During Malignancy. J. Mammary Gland. Biol. Neoplasia 2001, 6, 355–364. [CrossRef]
- Mungul, A.; Cooper, L.; Brockhausen, I.; Ryder, K.; Mandel, U.; Clausen, H.; Rughetti, A.; Miles, D.W.; Taylor-Papadimitriou, J.; Burchell, J.M. Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice.. Int. J. Oncol. 2004, 25.
- Chou, C.-H.; Huang, M.-J.; Chen, C.-H.; Shyu, M.-K.; Huang, J.; Hung, J.-S.; Huang, C.-S.; Huang, M.-C. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget 2015, 6, 6123–6135. [CrossRef]
- Mao, Y.; Zhang, Y.; Fan, S.; Chen, L.; Tang, L.; Chen, X.; Lyu, J. GALNT6 Promotes Tumorigenicity and Metastasis of Breast Cancer Cell via β-catenin/MUC1-C Signaling Pathway. Int. J. Biol. Sci. 2019, 15, 169–182. [CrossRef]
- Z. Chen, Z.G. Gulzar, C.A. St Hill, B. Walcheck, J.D. Brooks, Increased expression of GCNT1 is associated with altered O-glycosylation of PSA, PAP, and MUC1 in human prostate cancers, The Prostate 74(10) (2014) 1059-67.
- Premaratne, P.; Welén, K.; Damber, J.-E.; Hansson, G.C.; Bäckström, M. O-glycosylation of MUC1 mucin in prostate cancer and the effects of its expression on tumor growth in a prostate cancer xenograft model. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2010, 32, 203–213. [CrossRef]
- Honn, K.V.; Tang, D.G. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992, 11, 353–375. [CrossRef]
- Paschos, K.A.; Canovas, D.; Bird, N.C. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell. Signal. 2009, 21, 665–674. [CrossRef]
- Burdick, M.D.; Harris, A.; Reid, C.J.; Iwamura, T.; Hollingsworth, M.A. Oligosaccharides Expressed on MUC1 Produced by Pancreatic and Colon Tumor Cell Lines. J. Biol. Chem. 1997, 272, 24198–24202. [CrossRef]
- Fernandez-Rodriguez, J.; Dwir, O.; Alon, R.; Hansson, G.C. Tumor cell MUC1 and CD43 are glycosylated differently with sialyl-Lewis a and x epitopes and show variable interactions with E-selectin under physiological flow conditions.. Glycoconj. J. 2001, 18, 925–930. [CrossRef]
- Solatycka, T. Owczarek, F. Piller, V. Piller, B. Pula, L. Wojciech, M. Podhorska-Okolow, P. Dziegiel, M. Ugorski, MUC1 in human and murine mammary carcinoma cells decreases the expression of core 2 β1,6-N-acetylglucosaminyltransferase and β-galactoside α2,3-sialyltransferase, Glycobiology 22(8) (2012) 1042-54.
- J.H. Park, T. Nishidate, K. Kijima, T. Ohashi, K. Takegawa, T. Fujikane, K. Hirata, Y. Nakamura, T. Katagiri, Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis, Cancer research 70(7) (2010) 2759-69.
- Zhang, L.; Gallup, M.; Zlock, L.; Chen, Y.T.F.; E Finkbeiner, W.; A McNamara, N. Pivotal role of MUC1 glycosylation by cigarette smoke in modulating disruption of airway adherens junctions in vitro. J. Pathol. 2014, 234, 60–73. [CrossRef]
- M. Amado, F. Carneiro, M. Seixas, H. Clausen, M. Sobrinho-Simões, Dimeric sialyl-Le(x) expression in gastric carcinoma correlates with venous invasion and poor outcome, Gastroenterology 114(3) (1998) 462-70.
- Rambaruth, N.D.; Dwek, M.V. Cell surface glycan–lectin interactions in tumor metastasis. Acta Histochem. 2011, 113, 591–600. [CrossRef]
- L. Borsig, Selectins in cancer immunity, Glycobiology 28(9) (2018) 648-655.
- Nath, D.; Hartnell, A.; Happerfield, L.; Miles, D.W.; Burchell, J.; Papadimitriou, T.; Crocker, P.R. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology 1999, 98, 213–219. [CrossRef]
- Q. Zhao, X. Guo, G.B. Nash, P.C. Stone, J. Hilkens, J.M. Rhodes, L.G. Yu, Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface, Cancer research 69(17) (2009) 6799-806.
- Piyush, T.; Rhodes, J.M.; Yu, L.-G. MUC1 O-glycosylation contributes to anoikis resistance in epithelial cancer cells. Cell Death Discov. 2017, 3, 17044. [CrossRef]
- X. Jiang, J. Wang, X. Deng, F. Xiong, J. Ge, B. Xiang, X. Wu, J. Ma, M. Zhou, X. Li, Y. Li, G. Li, W. Xiong, C. Guo, Z. Zeng, Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape, Molecular cancer 18(1) (2019) 10.
- Rabinovich, G.A.; Toscano, M.A. Turning 'sweet' on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [CrossRef]
- C.B. Madsen, K. Lavrsen, C. Steentoft, M.B. Vester-Christensen, H. Clausen, H.H. Wandall, A.E. Pedersen, Glycan elongation beyond the mucin associated Tn antigen protects tumor cells from immune-mediated killing, PloS one 8(9) (2013) e72413.
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [CrossRef]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Smits, S.A.; Sonnenburg, E.D.; Costello, E.K.; Higginbottom, S.K.; Domino, S.E.; Holmes, S.P.; Relman, D.A.; et al. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc. Natl. Acad. Sci. 2013, 110, 17059–17064. [CrossRef]
- Lin, B.; Qing, X.; Liao, J.; Zhuo, K. Role of Protein Glycosylation in Host-Pathogen Interaction. Cells 2020, 9, 1022. [CrossRef]
- Owen, C.D.; Tailford, L.E.; Monaco, S.; Šuligoj, T.; Vaux, L.; Lallement, R.; Khedri, Z.; Yu, H.; Lecointe, K.; Walshaw, J.; et al. Unravelling the specificity and mechanism of sialic acid recognition by the gut symbiont Ruminococcus gnavus. Nat. Commun. 2017, 8, 1–15. [CrossRef]
- D.M. Hardbower, R.M. Peek, Jr., K.T. Wilson, At the Bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer, Journal of leukocyte biology 96(2) (2014) 201-12.
- Magalhães, A.; Rossez, Y.; Robbe-Masselot, C.; Maes, E.; Gomes, J.; Shevtsova, A.; Bugaytsova, J.; Borén, T.; Reis, C.A. Muc5ac gastric mucin glycosylation is shaped by FUT2 activity and functionally impacts Helicobacter pylori binding. Sci. Rep. 2016, 6, 25575–25575. [CrossRef]
- Skoog, E.C.; Padra, M.; Åberg, A.; Gideonsson, P.; Obi, I.; Quintana-Hayashi, M.P.; Arnqvist, A.; Lindén, S.K. BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS. Sci. Rep. 2017, 7, srep40656. [CrossRef]
- C. Solórzano, M. Angel Mayoral, M. de los Angeles Carlos, J. Berumen, J. Guevara, F. Raúl Chávez, G. Mendoza-Hernández, C. Agundis, E. Zenteno, Overexpression of glycosylated proteins in cervical cancer recognized by the Machaerocereus eruca agglutinin, Folia histochemica et cytobiologica 50(3) (2012) 398-406.
- W. Ahmad, K. Shabbiri, B. Ijaz, S. Asad, N. Nazar, S. Nazar, K. Fouzia, H. Kausar, S. Gull, M.T. Sarwar, I. Shahid, S. Hassan, Serine 204 phosphorylation and O-β-GlcNAC interplay of IGFBP-6 as therapeutic indicator to regulate IGF-II functions in viral mediated hepatocellular carcinoma, Virology journal 8 (2011) 208.
- M.P. Thompson, R. Kurzrock, Epstein-Barr virus and cancer, Clinical cancer research : an official journal of the American Association for Cancer Research 10(3) (2004) 803-21.
- Liu, J.; Zhang, Y.; Liu, W.; Zhang, Q.; Xiao, H.; Song, H.; Luo, B. MiR-BART1-5p targets core 2β-1,6-acetylglucosaminyltransferase GCNT3 to inhibit cell proliferation and migration in EBV-associated gastric cancer. Virology 2019, 541, 63–74. [CrossRef]
- Kondo, S.; Yoshizaki, T.; Wakisaka, N.; Horikawa, T.; Murono, S.; Jang, K.L.; Joab, I.; Furukawa, M.; Pagano, J.S. MUC1 Induced by Epstein-Barr Virus Latent Membrane Protein 1 Causes Dissociation of the Cell-Matrix Interaction and Cellular Invasiveness via STAT Signaling. J. Virol. 2007, 81, 1554–1562. [CrossRef]
- Springer, G.F. T and Tn, General Carcinoma Autoantigens. Science 1984, 224, 1198–1206. [CrossRef]
- Fuster, M.M.; Esko, J.D. The Sweet and Sour of Cancer: Glycans As Novel Therapeutic Targets. Nat. Rev. Cancer 2005, 5, 526–542. [CrossRef]
- Julien, S.; Picco, G.; Sewell, R.; Vercoutter-Edouart, A.-S.; Tarp, M.; Miles, D.; Clausen, H.; Taylor-Papadimitriou, J.; Burchell, J.M. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer 2009, 100, 1746–1754. [CrossRef]
- Taylor-Papadimitriou, J.; Peterson, J.A.; Arklie, J.; Burchell, J.; Ceriani, R.L.; Bodmer, W.F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: Production and reaction with cells in culture. Int. J. Cancer 1981, 28, 17–21. [CrossRef]
- Gendler, S.; Lancaster, C.; Taylor-Papadimitriou, J.; Duhig, T.; Peat, N.; Burchell, J.; Pemberton, L.; Lalani, E.; Wilson, D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin.. J. Biol. Chem. 1990, 265, 15286–15293. [CrossRef]
- Tarp, M.A.; Clausen, H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2008, 1780, 546–563. [CrossRef]
- Wilkinson, R.W.; Ross, E.L.; E Lee-MacAry, A.; Laylor, R.; Burchell, J.; Taylor-Papadimitriou, J.; Snary, D. A transgenic mouse model for tumour immunotherapy: induction of an anti-idiotype response to human MUC1. Br. J. Cancer 2000, 83, 1202–1208. [CrossRef]
- Moore, Z. Medarova, A. Potthast, G. Dai, In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe, Cancer research 64(5) (2004) 1821-7.
- Singh, R.; Bandyopadhyay, D. MUC1: A target molecule for cancer therapy. Cancer Biol. Ther. 2007, 6, 481–486. [CrossRef]
- M.A. Cheever, J.P. Allison, A.S. Ferris, O.J. Finn, B.M. Hastings, T.T. Hecht, I. Mellman, S.A. Prindiville, J.L. Viner, L.M. Weiner, L.M. Matrisian, The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research, Clinical cancer research : an official journal of the American Association for Cancer Research 15(17) (2009) 5323-37.
- H.H. Wandall, O. Blixt, M.A. Tarp, J.W. Pedersen, E.P. Bennett, U. Mandel, G. Ragupathi, P.O. Livingston, M.A. Hollingsworth, J. Taylor-Papadimitriou, J. Burchell, H. Clausen, Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes, Cancer research 70(4) (2010) 1306-13.
- Burchell, J.; Gendler, S.; Taylor-Papadimitriou, J.; Girling, A.; Lewis, A.; Millis, R.; Lamport, D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin.. Cancer Res. 1987, 47, 5476–82.
- Burchell, J.; Taylor-Papadimitriou, J.; Boshell, M.; Gendler, S.; Duhig, T. A short sequence, within the amino acid tandem repeat of a cancer-associated mucin, contains immunodominant epitopes. Int. J. Cancer 1989, 44, 691–696. [CrossRef]
- Rokhlin, O.W.; Weiner, G.J.; Cohen, M.B. 5E10: a prostate-specific surface-reactive monoclonal antibody. Cancer Lett. 1998, 131, 129–136. [CrossRef]
- Gong, Y.; Klein Wolterink, R.G.; Gulaia, V.; Cloosen, S.; Ehlers, F.A.; Wieten, L.; Graus, Y.F.; Bos, G.M.J.; Germeraad, W.T.W. Defucosylation of Tumor-Specific Humanized Anti-MUC1 Monoclonal Antibody Enhances NK Cell-Mediated Anti-Tumor Cell Cytotoxicity. Cancers 2021, 13, 2579. [CrossRef]
- J. Macías-León, I.A. Bermejo, A. Asín, A. García-García, I. Compañón, E. Jiménez-Moreno, H. Coelho, V. Mangini, I.S. Albuquerque, F. Marcelo, J.L. Asensio, G.J.L. Bernardes, H.J. Joshi, R. Fiammengo, O. Blixt, R. Hurtado-Guerrero, F. Corzana, Structural characterization of an unprecedented lectin-like antitumoral anti-MUC1 antibody, Chemical communications (Cambridge, England) 56(96) (2020) 15137-15140.
- Sørensen, A.L.; Reis, C.A.; Tarp, M.A.; Mandel, U.; Ramachandran, K.; Sankaranarayanan, V.; Schwientek, T.; Graham, R.; Taylor-Papadimitriou, J.; Hollingsworth, M.A.; et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology 2005, 16, 96–107. [CrossRef]
- Maleki, F.; Rezazadeh, F.; Varmira, K. MUC1-Targeted Radiopharmaceuticals in Cancer Imaging and Therapy. Mol. Pharm. 2021, 18, 1842–1861. [CrossRef]
- M. Bose, A. Sanders, C. De, R. Zhou, P. Lala, S. Shwartz, B. Mitra, C. Brouwer, P. Mukherjee, Targeting tumor-associated MUC1 overcomes anoikis-resistance in pancreatic cancer, Translational research : the journal of laboratory and clinical medicine 253 (2023) 41-56.
- Wu, G.; Kim, D.; Kim, J.N.; Park, S.; Maharjan, S.; Koh, H.; Moon, K.; Lee, Y.; Kwon, H.-J. A Mucin1 C-terminal Subunit-directed Monoclonal Antibody Targets Overexpressed Mucin1 in Breast Cancer. Theranostics 2018, 8, 78–91. [CrossRef]
- Wu, G.; Li, L.; Liu, M.; Chen, C.; Wang, G.; Jiang, Z.; Qin, Y.; He, L.; Li, H.; Cao, J.; et al. Therapeutic effect of a MUC1-specific monoclonal antibody-drug conjugates against pancreatic cancer model. Cancer Cell Int. 2022, 22, 1–14. [CrossRef]
- Okarvi, S. Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treat. Rev. 2008, 34, 13–26. [CrossRef]
- Bolzati, C.; Refosco, F.; Marchiani, A.; Ruzza, P. 99mTc-Radiolabelled Peptides for Tumour Imaging: Present and Future. Curr. Med. Chem. 2010, 17, 2656–2683. [CrossRef]
- Salouti, M.; Rajabi, H.; Babaei, M.H.; Rasaee, M.J. Breast tumor targeting with 99mTc-HYNIC-PR81 complex as a new biologic radiopharmaceutical. Nucl. Med. Biol. 2008, 35, 763–768. [CrossRef]
- Carmo, F.S.D.; Ricci-Junior, E.; Cerqueira-Coutinho, C.; Albernaz, M.D.S.; Bernardes, E.S.; Missailidis, S.; Santos-Oliveira, R. Anti-MUC1 nano-aptamers for triple-negative breast cancer imaging by single-photon emission computed tomography in inducted animals: initial considerations. Int. J. Nanomed. 2016, ume 12, 53–60. [CrossRef]
- Al Jammaz, I.; Al-Otaibi, B.; Al-Malki, Y.; Abousekhrah, A.; Okarvi, S.M. Fast Fluorine-18 labeling and preclinical evaluation of novel Mucin1 and its Folate hybrid peptide conjugate for targeting breast carcinoma. EJNMMI Radiopharm. Chem. 2021, 6, 1–17. [CrossRef]
- Nabavinia, M.S.; Gholoobi, A.; Charbgoo, F.; Nabavinia, M.; Ramezani, M.; Abnous, K. Anti-MUC1 aptamer: A potential opportunity for cancer treatment. Med. Res. Rev. 2017, 37, 1518–1539. [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [CrossRef]
- Sun, Q.; Li, J.; Ding, Z.; Liu, Z. Radiopharmaceuticals heat anti-tumor immunity. Theranostics 2023, 13, 767–786. [CrossRef]
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R.; et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 2019, 11, eaaw8412. [CrossRef]
- Lakshminarayanan, V.; Thompson, P.; Wolfert, M.A.; Buskas, T.; Bradley, J.M.; Pathangey, L.B.; Madsen, C.S.; Cohen, P.A.; Gendler, S.J.; Boons, G.-J. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. 2011, 109, 261–266. [CrossRef]
- T. Gao, Q. Cen, H. Lei, A review on development of MUC1-based cancer vaccine, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 132 (2020) 110888.
- Pinkhasov, J.; Alvarez, M.L.; Pathangey, L.B.; Tinder, T.L.; Mason, H.S.; Walmsley, A.M.; Gendler, S.J.; Mukherjee, P. Analysis of a cholera toxin B subunit (CTB) and human mucin 1 (MUC1) conjugate protein in a MUC1-tolerant mouse model. Cancer Immunol. Immunother. 2010, 59, 1801–1811. [CrossRef]
- Rong, Y.; Jin, D.; Wu, W.; Lou, W.; Wang, D.; Kuang, T.; Ni, X.; Qin, X. Induction of protective and therapeutic anti-pancreatic cancer immunity using a reconstructed MUC1 DNA vaccine. BMC Cancer 2009, 9, 191–191. [CrossRef]
- Quoix, E.; Lena, H.; Losonczy, G.; Forget, F.; Chouaid, C.; Papai, Z.; Gervais, R.; Ottensmeier, C.; Szczesna, A.; Kazarnowicz, A.; et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2015, 17, 212–223. [CrossRef]
- Teramoto, K.; Ozaki, Y.; Hanaoka, J.; Sawai, S.; Tezuka, N.; Fujino, S.; Daigo, Y.; Kontani, K. Predictive biomarkers and effectiveness of MUC1-targeted dendritic-cell-based vaccine in patients with refractory non-small cell lung cancer. Ther. Adv. Med Oncol. 2016, 9, 147–157. [CrossRef]
- Palitzsch, B.; Gaidzik, N.; Stergiou, N.; Stahn, S.; Hartmann, S.; Gerlitzki, B.; Teusch, N.; Flemming, P.-D.D.P.; Schmitt, E.; Kunz, H. A Synthetic Glycopeptide Vaccine for the Induction of a Monoclonal Antibody that Differentiates between Normal and Tumor Mammary Cells and Enables the Diagnosis of Human Pancreatic Cancer. Angew. Chem. Int. Ed. 2016, 55, 2894–2898. [CrossRef]
- M. Sadelain, R. Brentjens, I. Rivière, The basic principles of chimeric antigen receptor design, Cancer discovery 3(4) (2013) 388-98.
- Zhou, R.; Yazdanifar, M.; Das Roy, L.; Whilding, L.M.; Gavrill, A.; Maher, J.; Mukherjee, P. CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth. Front. Immunol. 2019, 10, 1149. [CrossRef]
- Stanley, P.; Ad, P.; Rd, S.; Ac, B.; C, S.; U, M.; B, E.; Jd, S.; Td, M.; K, S.; et al. Faculty Opinions recommendation of Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma.. 2016, 44. [CrossRef]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [CrossRef]
- Mao, L.; Su, S.; Li, J.; Yu, S.; Gong, Y.; Chen, C.; Hu, Z.; Huang, X. Development of Engineered CAR T Cells Targeting Tumor-Associated Glycoforms of MUC1 for the Treatment of Intrahepatic Cholangiocarcinoma. J. Immunother. 2023, 46, 89–95. [CrossRef]
- Zhang, H.; Zhao, H.; He, X.; Xi, F.; Liu, J. JAK-STAT Domain Enhanced MUC1-CAR-T Cells Induced Esophageal Cancer Elimination. Cancer Manag. Res. 2020, ume 12, 9813–9824. [CrossRef]
- Weimin, S.; Abula, A.; Qianghong, D.; Wenguang, W. Chimeric cytokine receptor enhancing PSMA-CAR-T cell-mediated prostate cancer regression. Cancer Biol. Ther. 2020, 21, 570–580. [CrossRef]
- E. Grishman, Histochemical analysis of mucopolysaccharides occurring in mucus-producing tumors; mixed tumors of the parotid gland, colloid carcinomas of the breast, and myxomas, Cancer 5(4) (1952) 700-7.
- Mei, Z.; Zhang, K.; Lam, A.K.; Huang, J.; Qiu, F.; Qiao, B.; Zhang, Y. MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma. Cancer Med. 2019, 9, 640–652. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





