Submitted:
17 September 2023
Posted:
18 September 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction
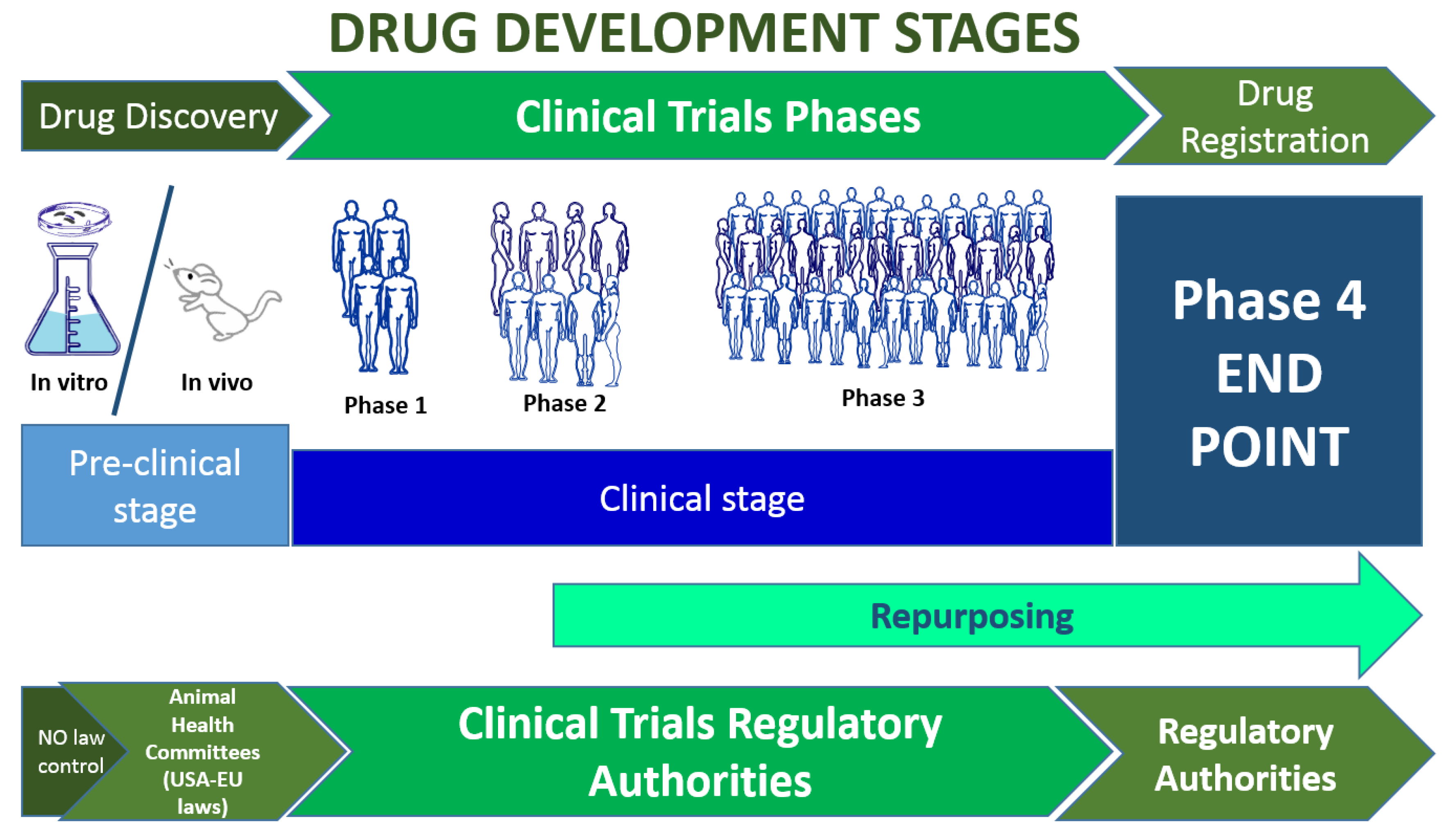
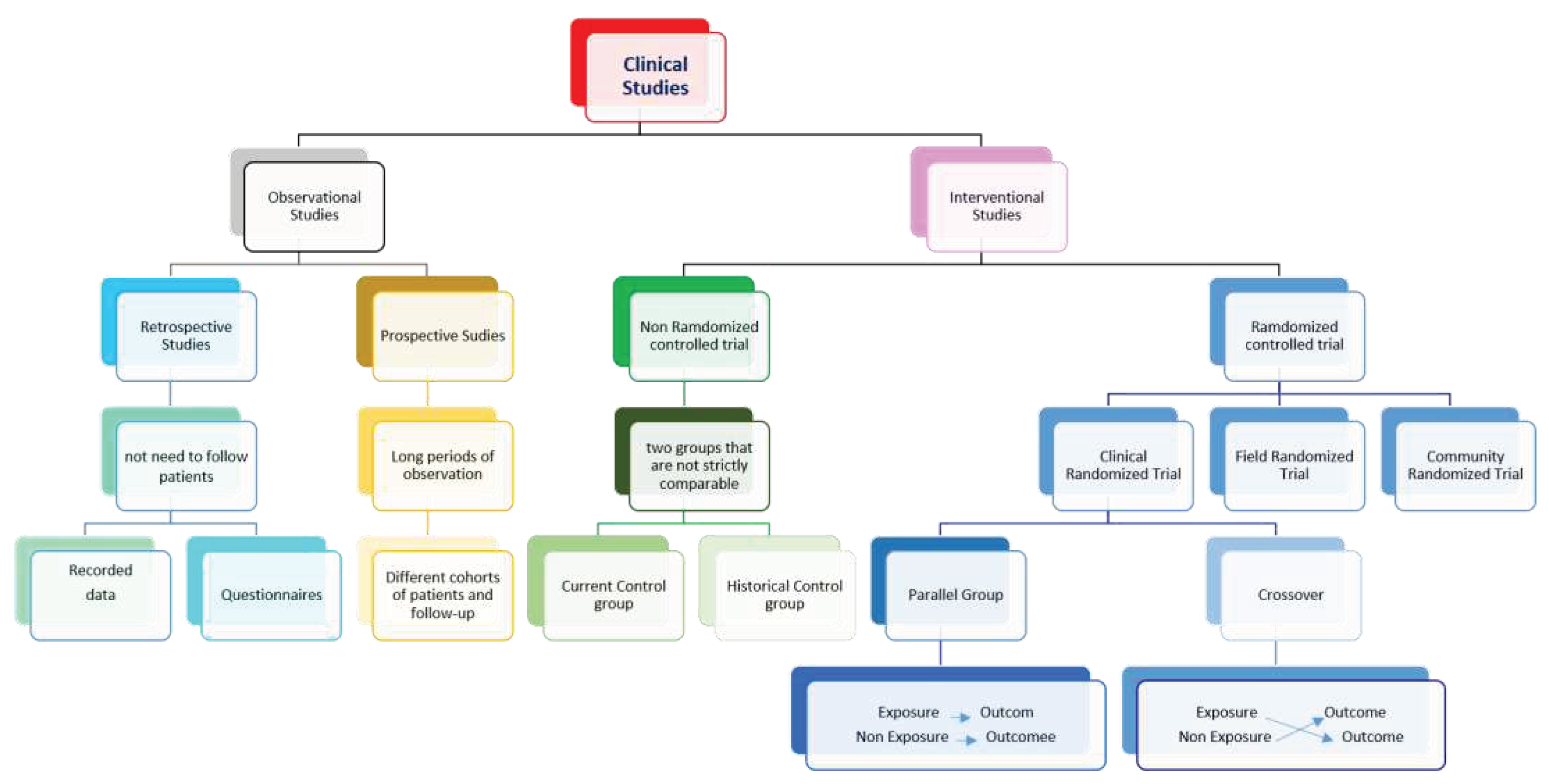
Detailed procedures
Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohs, R.C.; Greig, N.H. Drug Discovery and Development: Role of Basic Biological Research. Alzheimers Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.A. Lost in Translation: The Valley of Death across Preclinical and Clinical Divide – Identification of Problems and Overcoming Obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Torres-Saavedra, P.A.; Winter, K.A. An Overview of Phase 2 Clinical Trial Designs. Int. J. Radiat. Oncol. 2022, 112, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Salloum, N.C.; Fava, M.; Ball, S.; Papakostas, G.I. Success and Efficiency of Phase 2/3 Adjunctive Trials for MDD Funded by Industry: A Systematic Review. Mol. Psychiatry 2020, 25, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Cesana, B.M.; Biganzoli, E.M. Phase IV Studies: Some Insights, Clarifications, and Issues. Curr. Clin. Pharmacol. 2018, 13, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zamami, Y.; Hamano, H.; Niimura, T.; Aizawa, F.; Yagi, K.; Goda, M.; Izawa-Ishizawa, Y.; Ishizawa, K. Drug-Repositioning Approaches Based on Medical and Life Science Databases. Front. Pharmacol. 2021, 12, 752174. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kim, P.Y. Drug Trials. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [Google Scholar]
- Thiese, M.S. Observational and Interventional Study Design Types; an Overview. Biochem. Medica 2014, 24, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Bero, L.; Chartres, N.; Diong, J.; Fabbri, A.; Ghersi, D.; Lam, J.; Lau, A.; McDonald, S.; Mintzes, B.; Sutton, P.; Turton, J.L.; Woodruff, T.J. The Risk of Bias in Observational Studies of Exposures (ROBINS-E) Tool: Concerns Arising from Application to Observational Studies of Exposures. Syst. Rev. 2018, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.R. Retrospective Studies and Chart Reviews. Respir. Care 2004, 49, 1171–1174. [Google Scholar] [PubMed]
- Song, J.W.; Chung, K.C. Observational Studies: Cohort and Case-Control Studies: Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Al-Jundi, A.; Sakka, S. Protocol Writing in Clinical Research. J. Clin. Diagn. Res. JCDR 2016, 10, ZE10–ZE13. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.R. Fundamentals of Clinical Trial Design. J. Exp. Stroke Transl. Med. 2010, 3, 19–27. [Google Scholar] [CrossRef] [PubMed]
- An, M.-W.; Duong, Q.; Le-Rademacher, J.; Mandrekar, S.J. Principles of Good Clinical Trial Design. J. Thorac. Oncol. 2020, 15, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- An, M.-W.; Duong, Q.; Le-Rademacher, J.; Mandrekar, S.J. Principles of Good Clinical Trial Design. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Ravi, R.; Gogtay, N.; Thatte, U. Recruitment and Retention of the Participants in Clinical Trials: Challenges and Solutions. Perspect. Clin. Res. 2020, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug Repositioning: A Brief Overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
| Country | Regulatory Body | Actually under control of |
|---|---|---|
| Austria | Austrian Agency for Health and Food Safety | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Belgium | Federal Agency for Medicines and Health Products (FAMHP) | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Bulgaria | Bulgarian Drug Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Croatia | Agency for medicinal products and medical devices of Croatia | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Cyprus | Ministry of Health - Pharmaceutical Services | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Czechia | State Institute for Drug Control | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Denmark | Danish Medicines Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Estonia | State Agency of Medicines | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Finland | Finnish Medicines Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| France | National Agency for the Safety of Medicine and Health Products | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Germany | Federal Institute for Drugs and Medical Devices | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Greece | National Organization for Medicines | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Hungary | National Institute of Pharmacy and Nutrition | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Iceland | Icelandic Medicines Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Ireland | Health Products Regulatory Authority (HPRA) | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Italy | Italian Medicines Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Latvia | State Agency of Medicines | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Liechtenstein | Office of Health / Department of Pharmaceuticals | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Lithuania | State Medicines Control Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Luxembourg | Ministry of Health | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Malta | Malta Medicines Authority (MMA) | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Netherlands | Medicines Evaluation Board | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Netherlands | Healthcare and Youth Care Inspectorate, Ministry of Health, Welfare and Sport | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Norway | Norwegian Medicines Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Poland | Office for Registration of Medicinal Products, Medical Devices and Biocidal Products | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Portugal | National Authority of Medicines and Health Products | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Romania | National Authority of Medicines and Medical Devices of Romania | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Slovakia | State Institute for Drug Control | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Slovenia | Agency for Medicinal Products and Medical Devices of the Republic of Slovenia | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Spain | Spanish Agency for Medicines and Health Products | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Sweden | Swedish Medical Products Agency | The European Medicines Agency (EMA) 2022 |
| https://www.ema.europa.eu/en | ||
| Albania; | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Bosnia | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Kosovo | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Republic of Macedonia; | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Montenegro; | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Serbia; | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Turkey. | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Herzegovina; | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Yugoslav | The European Medicines Agency (EMA) 2022 | |
| https://www.ema.europa.eu/en | ||
| Bangladesh | Directorate General of Drug Administration (DGDA) | https://dgdagov.info/ |
| China | State Food and Drug Administration (SFDA), National Medical Products Administration (NMPA) | http://www.sfda.com/ |
| http://english.nmpa.gov.cn/ | ||
| Hong Kong | Drug Office - Department of Health | https://www.dh.gov.hk/english/main/main_ps/main_ps.html |
| India | Central Drug Standards Control Organization (CDSCO) | https://cdsco.gov.in/opencms/opencms/en/Home/ |
| Japan | Ministry of Health, Labor and Welfare (MHLW), Japanese Pharmaceuticals and Medical Devices Agency (PMDA) | https://www.mhlw.go.jp/english/ |
| https://cdx-japan.mblbio.com/ | ||
| Malaysia | Ministry of Health (MOH) | https://www.moh.gov.my/ |
| Philippines | Department of Health (DOH) | https://lawphil.net/administ/doh/doh.html |
| Singapore | Health Sciences Authority (HSA) | https://www.hsa.gov.sg/ |
| South Korea | Ministry of Food and Drug Safety (MFDS) | https://www.mfds.go.kr/eng/index.do |
| Thailand | Food and Drug Administration of Thailand (FDATA) | https://en.fda.moph.go.th/ |
| Taiwan | Taiwan Food and Drug Administration (TFDA) | https://www.fda.gov.tw/eng/ |
| Vietnam | Drug Administration of Vietnam | https://dav.gov.vn/dich-vu-cong-ce5.html |
| Brazil | National Health Surveillance Agency (ANVISA) | The HHS Office for Human Research Protections (2021) |
| https://www.hhs.gov/ohrp/index.html | ||
| Colombia | Ministry of Health | The HHS Office for Human Research Protections (2021) |
| https://www.hhs.gov/ohrp/index.html | ||
| Perù | Ministry of Health | The HHS Office for Human Research Protections (2021) |
| https://www.hhs.gov/ohrp/index.html | ||
| Cile | Ministry of Health | The HHS Office for Human Research Protections (2021) |
| https://www.hhs.gov/ohrp/index.html | ||
| Canada | CanadaFDA, Health Canada (HC) reviews | https://www.canada.ca/en/health-canada/services/drugs-health-products |
| USA | The United States Food and Drug Administration (FDA or US FDA) | https://www.fda.gov/ |
| New Zealand | Medsafe is the Medicines and Medical Devices Regulatory Authorit | https://www.medsafe.govt.nz/ |
| Australia | The Therapeutic Goods Administration (TGA) | https://www.tga.gov.au/therapeutic-goods-administration-tga |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





