Submitted:
08 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Results
Discussion
Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Bi J, Garg V, Yates AR. Galectin-3 and sST2 as Prognosticators for Heart Failure Requiring Extracorporeal Life Support: Jack n' Jill. Biomolecules. 2021 Jan 27;11:166. [CrossRef]
- Frunza O, Russo I, Saxena A, et al. Myocardial Galectin-3 Expression Is Associated with Remodeling of the Pressure-Overloaded Heart and May Delay the Hypertrophic Response without Affecting Survival, Dysfunction, and Cardiac Fibrosis. Am J Pathol. 2016;186:1114-27. [CrossRef]
- Suthahar N, Meijers WC, Silljé HHW, Ho JE, Liu FT, de Boer RA. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics. 2018 Jan 1;8(3):593-609. [CrossRef]
- Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2002;19(7-9):527-35. [CrossRef]
- Tang WH, Shrestha K, Shao Z, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficienc and survival. Am J Cardiol. 2011;108:385-390.
- Lin YH, Lin LY, Wu YW, et al. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta 2009;409. [CrossRef]
- Avishay Grupper, Jose Nativi-Nicolau, Joseph J. Maleszewski, Jennifer R. Geske, Walter K. Kremers, Brooks S. Edwards, Sudhir S. Kushwaha, Naveen L. Pereira, Circulating Galectin-3 Levels Are Persistently Elevated After Heart Transplantation and Are Associated With Renal Dysfunction, JACC: Heart Failure, Volume 4, Issue 11, 2016, Pages 847-856, ISSN 2213-1779. [CrossRef]
- Antoni Bayes-Genis, Marta de Antonio, Joan Vila, Judith Peñafiel, Amparo Galán, Jaume Barallat, Elisabet Zamora, Agustin Urrutia, Josep Lupón, Head-to-Head Comparison of 2 Myocardial Fibrosis Biomarkers for Long-Term Heart Failure Risk Stratification: ST2 Versus Galectin-3, Journal of the American College of Cardiology, Volume 63, Issue 2, 2014, Pages 158-166, ISSN 0735-1097. [CrossRef]
- Frunza O, Russo I, Saxena A, et al. Myocardial galectin-3 expression is associated with remodeling of the pressure-overloaded heart and may delay the hypertrophic response without affecting survival, dysfunction, and cardiac fibrosis. Am J Pathol 2016;186:1114–27. [CrossRef]
- Abou Ezzeddine OF, Haines P, Stevens S, et al. Galectin-3 in heart failure with preserved ejection fraction. A RELAX trial substudy (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure). J Am Coll Cardiol HF 2015;3:245–52.
- Maisel A, Kim P, Stendardi W. Galectin-3 After Heart Transplantation: Does it Get Better? JACC Heart Fail. 2016 Nov;4(11):857-859. [CrossRef] [PubMed]
- O’Seaghdha CM, Hwang SJ, Ho JE, et al. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol 2013;24:1470-7. [CrossRef]
- Iacoviello M, Aspromonte N, Leone M, et al. Galectin-3 serum levels are independently associated with microalbuminuria in chronic heart failure outpatients. Res Cardiovasc Med 2015;5:e28952.
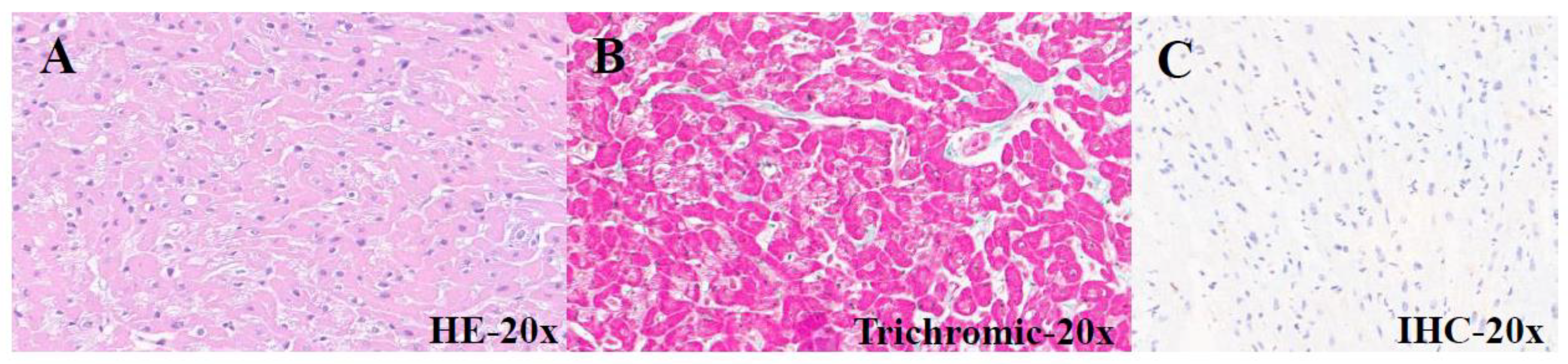
| PATIENTS | AGE (months) | GENDER | CLINICAL DIAGNOSIS |
HISTOLOGY | IHC Gal3 |
|---|---|---|---|---|---|
| 18-I-9862 A1 |
9 | M | Tetralogy of Fallot |
Cardiomyocyte hypertrophy | Negative |
| 18-I-20309 A1 | 11 | M | Tetralogy of Fallot |
Mild interstitial fibrosis | Negative |
| 18-I-20648 A1 | 14 | F | Tetralogy of Fallot |
Interstitial fibrosis and cardiomyocyte hypertrophy |
Negative |
| 18-I-21278 A1-B1 | 13 | M | Tetralogy of Fallot |
Cardiomyocyte hypertrophy | Negative |
| 19-I-2479 A1 |
15 | M | Tetralogy of Fallot |
Hypertrophic-regressive cardiomyocytes and endocardial fibrosis |
Negative |
| 19-I-3718 A1 |
12 | F | Tetralogy of Fallot |
Hypertrophic-regressive cardiomyocytes and subendocardial fibrosis |
Negative |
| 19-I-12929 A1 | 13 | F | Tetralogy of Fallot |
Hypertrophic-regressive cardiomyocytes and endocardial fibrosis |
Negative |
| 19-I-13382 A1 | 11 | M | Tetralogy of Fallot |
Hypertrophic-regressive cardiomyocytes and endocardial fibrosis |
Negative |
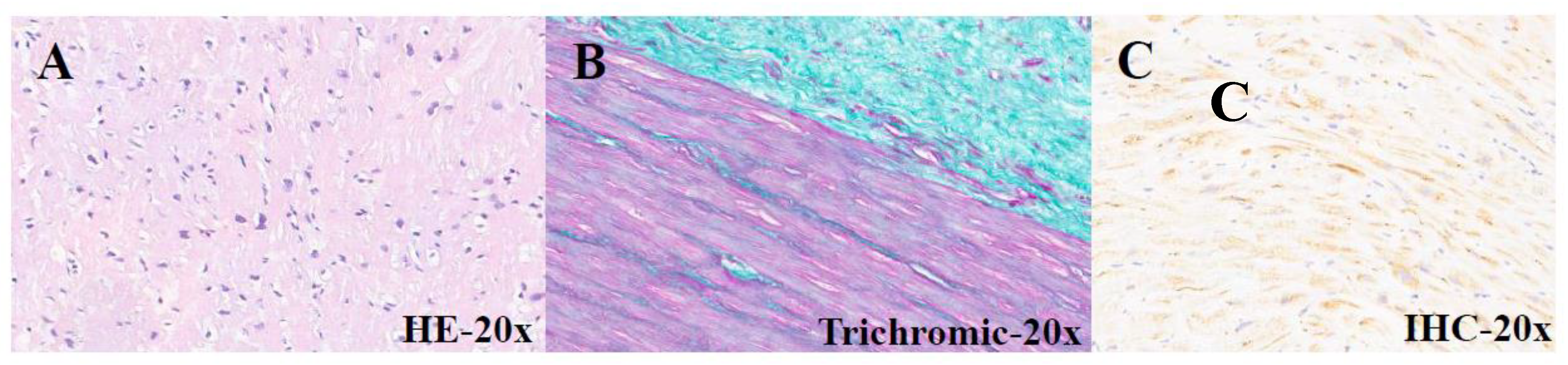
| PATIENTS | AGE (years) | GENDER | CLINICAL DIAGNOSIS |
HISTOLOGY | IHC Gal3 |
|---|---|---|---|---|---|
| 19-I-3851 A1 |
76 | F | Aortic stenosis | Hypertrophic-regressive cardiomyocytes and endocardial fibroelastosis |
Mild band positivity |
| 21-10936 A1 |
48 | F | Aortic stenosis | Hypertrophic-regressive cardiomyocytes and subendocardial fibrosis |
Diffuse subendocardial positivity |
| 21-I-10937 A1 | 48 | F | Aortic stenosis | Hypertrophic-regressive cardiomyocytes and subendocardial fibrosis |
Diffuse subendocardial positivity |
| 21-I-13205 A2 | 55 | M | Cardiac explant (CMD) |
Hypertrophic-regressive cardiomyocytes and subendocardial fibrosis |
Focal subendocardial positivity |
| 21-I-13914 A1 | 75 | F | Subaortic rim | Hypertrophic-regressive cardiomyocytes, endocardial fibroelastosis and interstitial fibrosis |
Mild band positivity |
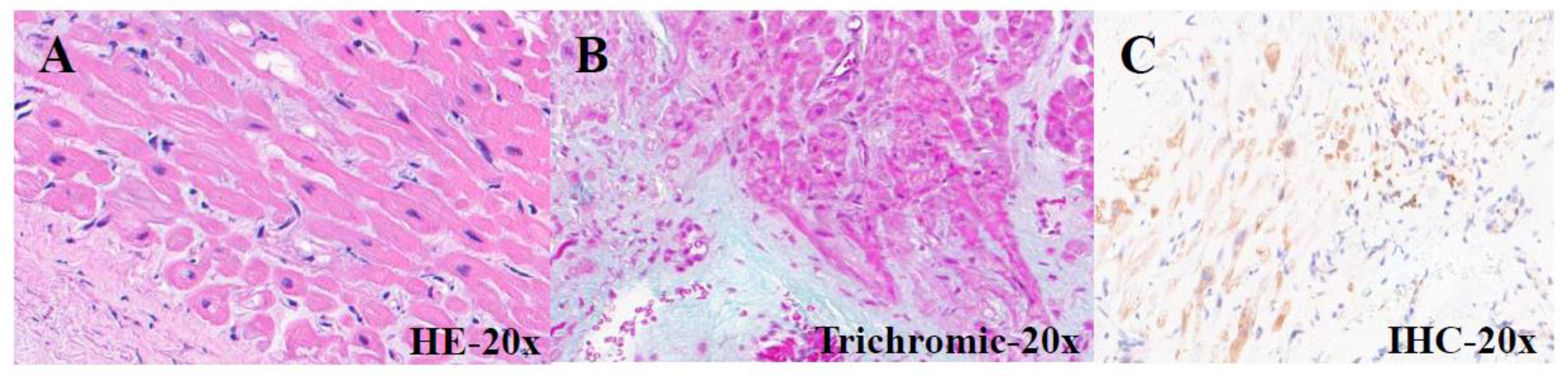
| PATIENTS | AGE (years) | GENDER | CLINICAL DIAGNOSIS |
HISTOLOGY | IHC Gal3 |
Peri-graft serum Gal3 ng/mL |
Post-graft Serum Gal3 ng/mL |
|---|---|---|---|---|---|---|---|
| 21-I-12747 A1 |
71 | M | Heart transplantation (deceased) |
Hypertrophic- regressive cardiomyocytes and subendocardial fibrosis |
Negative | NN | NN |
| 21-I-14228 A1 |
51 | F | Heart transplantation (2021) |
Hypertrophic- regressive cardiomyocytes and subendocardial fibrosis |
Mild and focal positivity |
29 | 22 |
| 21-I-16007 A1 |
77 | M | Heart transplantation (2009) |
Hypertrophic- regressive cardiomyocytes and endocardial fibrosis |
Mild and focal positivity |
NN | 33 |
| 22-I-16044 A1 |
51 | M | Heart transplantation (2022) |
Hypertrophic- regressive cardiomyocytes and discrete subendocardial fibrosis |
Diffuse subendocardial positivity |
45.6 | 26 |
| 22-I-16117 A1 |
59 | M | Heart transplantation (2021) |
Hypertrophic- regressive cardiomyocytes, discrete subendocardial and interstitial fibrosis |
Mild and focal positivity |
65 | 17 |
| 22-I-17480 A1 |
25 | F | Heart transplantation (2019) |
Modest interstitial lymphocytic infiltrate and marked interstitial and subendocardial fibrosis |
Negative | NN | NN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





