1. Introduction
Metabolic surgery is a powerful treatment option
for patients with type 2 diabetes. However, the therapeutic scope is limited
because the underlying mechanism for inconsistent operational outcomes is yet
to be determined. Two distinct factors are associated with the development of
type 2 diabetes. These factors include toxicity from excessive adipose tissue
in overweight individuals and an imbalanced release of gut hormones such as
incretins. The main challenge lies in understanding the mechanism behind the
altered hormonal release from the gastrointestinal tract rather than the issue
of being overweight. Treating imbalances in gut hormones involves rerouting the
intestines and identifying the major target hormones responsible for metabolic
changes.
It is crucial to determine the mechanism
contributing to the recurrence of symptoms several months after surgery [1]. A study examining the density and hormonal gene
expression of small intestinal enteroendocrine cells revealed several changes
in the distribution of these cells following a Roux-en-Y gastric bypass (RYGB) [2]. These findings suggest a link between the
development of postoperative hyperglycemia and the altered distribution of
enteroendocrine cells following surgery. Therefore, we investigated the
mechanism that maintains the spatiotemporal identity of the intestinal
epithelium and the factors contributing to alterations in the distribution of
enteroendocrine cells and the expression of hormonal genes. Various metabolic
surgeries, including modified DJB procedures, were compared based on the
surgical anatomy, clinical course, and outcomes to understand the specific
surgical patterns that lead to conflicting results. Nonobese patients were
included to avoid confounding weight-loss variables from overweight
individuals.
2. Physiology of Intestinal Epithelial Regeneration and Identity Alteration
The intestinal epithelium is continuously replaced
at high rates [3]. Different structures of
epithelial tissue and unique types of epithelial cells within each site enable the
distinct digestive functions necessary for efficient nutritional assimilation [4]. The modeling of the intestinal epithelium
depends on two-way communication between the epithelial and mesenchymal cells,
which is mediated by signaling pathways [3].
Following the loss of the functional epithelial area through resections or
bypasses, the remaining intestine undergoes morphological and functional
adaptive compensatory responses. The distribution of enteroendocrine cells and
their transcriptional activity in the remaining intestine changes [2]. The proximal intestinal epithelial lineage is
transferred to the distal intestine. Recent advances in single-cell RNA
sequencing have revealed that the distal small-intestinal epithelium undergoes
regional reprogramming to acquire a proximal identity after proximal small-bowel
resections [5]. Thus, the general appearance
of the Roux limb in a pylorus-preserving DJB resembles the duodenum [6].
The crosstalk between epithelial and opposing
subepithelial mesenchymal cells in the anastomosis area facilitates reprogramming
the epithelial cellular identity [7]. An intestinal
anastomosis performed to maintain continuity after a bypass or resection
modifies the site-specific epithelial identity. It can be a causal factor for
inconsistent outcomes in metabolic surgery.
The rapid turnover rate of the intestinal
epithelium and compensatory proliferation facilitate the spread of an altered
identity to the distal intestinal epithelium. However, the clinical effect of
epithelial replacement remains unclear. The region-specific functions of
enteroendocrine cells in different parts of the intestine lead to metabolic
changes over time after sufficient proliferation. The type and density of
enteroendocrine cells distributed at the distal end of the preceding bowel
modify the epithelial identity of the subsequent section of the digestive
tract.
3. Altered Identity of the Roux Limb and Common Channel in the Alimentary Tract
As the duodenum is located immediately after the
pyloric sphincter, a small portion of the duodenum connected to the stomach is
always exposed to the nutrients of patients who have undergone a pylorus-preserving
DJB. The pylorus-preserving procedure ensures direct nutrient contact with the
remaining duodenal tissue at the anastomotic site. The counterparts of an
alimentary Roux-limb anastomosis in a DJB are either the pyloric portion of the
stomach or the first duodenal portion. These represent the gastrojejunal and
duodenal–jejunal anastomoses, respectively. A duodenal–jejunal anastomosis is
designed to preserve the pyloric sphincter function, although the postoperative
clinical courses of the two procedures differ. In a pylorus-preserving DJB
surgery group, there was a tendency toward high blood glucose concentrations,
which led to unsatisfactory outcomes [6,8–14].
In contrast, recurrent hyperglycemia was rarely detected in a gastrojejunostomy
group, which yielded favorable outcomes [15–19].
These results suggest that the remaining duodenal
epithelium attached to the pyloric sphincter may trigger recurrent
hyperglycemia. The question remains whether the exposed area of the duodenal
epithelium is too small to induce hyperglycemia. A possible mechanism could be
demonstrated by comparing the different modified forms of DJBs. Commonly
modified DJBs include pylorus-preserving DJBs, conventional (pylorus exclusion)
DJBs, and endoscopic DJB liners (DJBLs); each method exhibits unique
characteristics. Distinct changes in blood glucose levels are observed in
pylorus-preserving DJBs. In most cases, blood glucose levels decrease 2–3
months after surgery; an increase follows this. DJBLs scarcely increase blood
glucose values if a liner is in place, despite exposure to nutrients such as
those from pylorus-preserving DJBs. As exposure to the first portion of the
duodenal epithelium influences inconsistent outcomes, another factor may be
responsible for the recurrence of DJBLs. Different from DJBLs, surgical DJBs
involve an anastomosis procedure. When considering whether an anastomosis is a
determinant of recurrence, another factor may contribute to recurrent
hyperglycemia in conventional DJBs. Based on these results, it can be concluded
that hyperglycemia is associated with the simultaneous nutritional exposure of
the first portion of the duodenal epithelium and the accompanying anastomosis
procedure.
Studies on the outcomes of transpositions elsewhere
in the intestine—such as the transposition of the ileal segment to the jejunal
area, jejunal segment to the ileal site, duodenal segment to the ileal area,
and vice versa—have been conducted to evaluate the epithelial changes in the
intestine. The common features of the adaptations are as follows: when the
ileal segment is transposed to the jejunal area, the sizes of the villi
increase to a similar extent as the jejunal villi; when the jejunal segment is
transposed to the ileal site, the villi shrink to a length likely to be similar
to the ileal villi; and when the duodenal segment is anastomosed with the
ileum, the villus of the ileum increases in size to the extent of the duodenum [20]. There is no mobile mesentery with duodenal
fixations in the retroperitoneum; the distal ileum is brought into the right
upper abdominal cavity, ensuring that the duodenum remains in place before
creating the anastomosis. Incidentally, the final configuration may resemble
that of a pylorus-preserving DJB. Based on these findings, it is evident that
the proximal intestinal epithelium migrates to the opposing distal intestinal
area via the anastomotic site. The underlying mechanisms for these outcomes
likely occur through altered crosstalk between the opposing epithelial and
mesenchymal cells exposed to the cutting edge. A subsequent identity alteration
of the intestinal epithelium is a plausible assumption, although no definite
supporting evidence exists.
4. The Diabetogenic Role of Glucose-Dependent Insulinotropic Peptide (GIP) Compared with the Length of the Biliopancreatic (BP) Limb
Jejunojejunostomy, another surgical anastomosis
used in the DJB procedure, is not exempt from identity changes. Similar to the
mechanism above, the epithelial identity of the common limb is affected by that
of the distal end of the BP limb. The density of GIP-releasing K cells
decreases from the duodenum to the terminal ileum [21].
Therefore, the shorter the BP limb, the higher the density of K-cells at the
cutting end of the BP limb should expected. A recent investigation of GIP
protein contents within the intestine's separated anatomical segments, mediated
by gastrointestinal surgery, characterized significantly increased GIP protein
contents in the mid-jejunum [13]. This was
possibly induced by an insufficient length of the BP limb, which provoked
K-cell proliferation.
Although there is ongoing debate regarding the role
of GIP in diabetes, an increase in GIP concentration after surgery is inversely
correlated with diabetes remission [22]. The
upregulation of Glucagon-like peptide-1(GLP-1) and downregulation of GIP are
typical features of the biliopancreatic diversion (BPD) procedure, which is
most effective in improving type 2 diabetes. Notably, the surgical aspect of
the BPD procedure is characterized by the complete exclusion of the duodenum
and the long BP limb [23]. Although the
primary function of the long BP limb is to separate nutrients from the BP limb
to the high-density K-cell area, another function is to reduce K-cell
proliferation in the subsequent common-channel limb. According to recent
reports on the importance of the BP limb in metabolic surgery, the beneficial
effects of DJBs disappeared after BP limb excisions in rats with improved
hyperglycemia that underwent DJBs with a long BP limb [24], suggesting additional function of the long BP
limb.
5. Inconsistent Postoperative GIP Values after RYGB [12]
Even though there are a lot of debates regarding
the factors that may affect changing GIP values after RYGB, there appear to be
correlations between metabolic outcomes, BP limb length, and postoperative GIP
changes. Unlike GLP-1, GIP is considered a hormone targeted by the foregut
hypothesis, as it leads to hyperglycemia instead of insulin secretion. Evidence
continues to be discovered supporting the hypothesis that a sufficient length
of the BP limb enhances the effects of surgery. The mechanism underlying this
hypothesis is concomitantly associated with changes in GIP concentration and
the enteroendocrine cells responsible for GIP secretion. Ultimately,
lengthening the BP limb reduces the number of K cells in contact with
nutrients, resulting in decreased GIP secretion and improved outcomes. It can
be inferred that the postoperative decreased secretion of GIP and the BP limb
length are proportional. However, the comparison would only be accepted by
excluding other factors that impact GIP alterations, such as the incomplete
exclusion of the duodenum from nutritional exposure and weight fluctuations in
overweight subjects. Table 1 [23,25–28] demonstrates the propensity for an
inverse correlation between postoperative GIP changes and the BP limb length
ratio. Surgical patients without obesity were eligible; pylorus exclusions were
performed in every case, including RYGBs, classic BPDs, and one anastomosis
gastric bypass (OAGB).
One of the major determinants of inconsistent GIP
values after RYGB is the length of the BP limb. A shorter BP limb has been
associated with an increased GIP concentration compared with the preoperative
level, whereas a longer BP limb has been associated with a decreased
concentration. The presumed inflection point was 100 cm from Treitz’s liga ment [29].
Table 1.
Postoperative GIP alterations and length of BP limb in patients who underwent bypass surgery with total duodenal exclusions.
Table 1.
Postoperative GIP alterations and length of BP limb in patients who underwent bypass surgery with total duodenal exclusions.
| Operation |
AUC GIP
Before Operation
|
AUC GIP
After Operation
|
GIP
Alteration* (%)
|
BP
Limb (cm)
|
Reference |
| BPD |
3297.0 pmol/L |
1874.0 pmol/L |
56 |
≥ 250** |
Guidone et al. [23] |
| RYGB |
48.67 ng/L−1·min−1
|
51.56 ng/L−1·min−1
|
105 |
30 |
Laferrère et al. [25] |
| RYGB |
50.96 pmol−1·L−1·min−1
|
52.66 pmol−1·L−1·min−1
|
103 |
40 |
Laferrère et al. [26] |
| RYGB |
30.2 ng/dL·10 min |
27.0 ng/dL·10 min |
90 |
100 |
Fellici et al. [27] |
| OAGB |
184.0 pg/mL·min−1
|
98.0 pg/mL·min−1
|
53 |
200 |
Kim et al. [28] |
6. Supporting Evidence for This Hypothesis
The most effective surgical procedure at present is a classic BPD. Outcomes of pylorus-preserving BPDs with a duodenal switch (DS) could not be compared with those of classic BPDs, even though the bypassed intestinal length was the same as a classic BPD [
30]. The remaining duodenal epithelium at the anastomosis site may have been responsible for the unsatisfactory outcomes. The metabolic outcomes of revisional surgery—where the gastric pouch is reduced in size, the common-channel limb is lengthened, and the total duodenal exclusion is maintained with an undiversified BP limb length—are comparable with those of a classic BPD [
31].
When comparing the efficacy of a laparoscopic DJB(LDJB) and laparoscopic RYGB in controlling type 2 diabetes over a three-year follow-up, an LDJB group experienced a significant increase in mean weight by the third postoperative month and a considerable increase in HbA1c from the baseline at six months and two years after surgery. However, these outcomes were characterized by incomplete duodenal exclusions. The alimentary Roux limb was anastomosed with the duodenum, 3~4 cm distal to the pyloric sphincter in this study. Thus, the inadequate exclusion of the duodenum may have been responsible for the poor outcomes of the LDJB [
1].
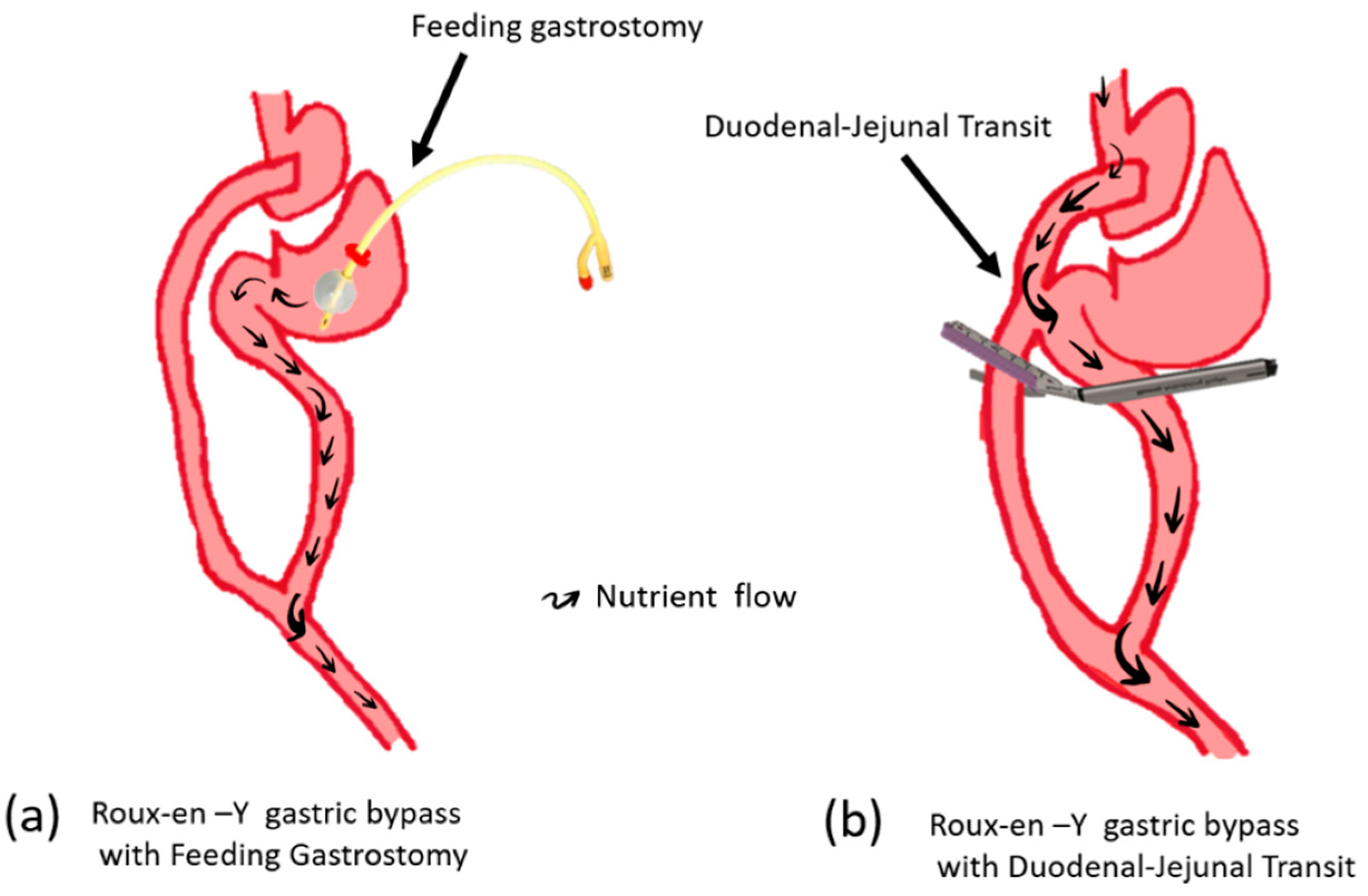
Usually, the beneficial effect of RYGB disappeared after gastrostomy feeding to the bypassed segment, including the BP limb (
Figure 1a). Surprisingly, glucose tolerance dose was not impaired with the duodenal-jejunal transit procedure following RYGB(
Figure 1b). The surgical structure of duodenal-jejunal transit with gastric bypass is as follows; concomitant RYGB with side-to-side anastomosis between the alimentary Roux limb and the anterior portion of the proximal duodenum and stapling 2 cm distal to duodenal-jejunal anastomosis without cutting for divert food passage[
32]. The preservation of the metabolic effect despite nutrients passing through the duodenum and upper jejunum has been mysterious. However, the assumption of mechanism could be illustrated by understanding the propagation of epithelial lineage to the distal intestine at the anastomosis site. The replaced intestinal identity of the duodenum and upper jejunum to that of the distal jejunum may be an acceptable consequence.
Finally, the hypothesis regarding the inconsistent outcomes of metabolic surgeries is partly based on epithelial regeneration and proliferation, which take time; noticeable discrepancies become evident a few months after surgery. The similarity between the interval and clinical recurrence after surgery—including weight gain and increased blood glucose—suggests a direct relationship between GIP alterations and recurrent hyperglycemia.
7. Simultaneous Preservation of the Pyloric Sphincter Function and Complete Exclusion of the Duodenum
The ideal surgical technique for DJBs should preserve the gastrointestinal capacity while ensuring the effectiveness of diabetes improvement. A duodenal epithelium attached to a pyloric sphincter should be completely removed from the visible border using surgical excision or cauterization, followed by interrupted stitching between the pyloric sphincter muscle and alimentary Roux limb to preserve the sphincter function.
8. Conclusions
The core mechanisms of diabetic improvements include separating the high-density K-cell area from the nutrient contact and modulating epithelial identity alteration from crosstalk with opposite mesenchymal cells and epithelium at the anastomosis site. The compensatory proliferation of the remaining intestinal epithelium intensifies the spreading of altered identity, specifically the length of the BP limb and exposure of the duodenal epithelium. These findings offer valuable insight for optimizing metabolic surgery’s effectiveness in managing type 2 diabetes.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable
Acknowledgments
The author would like to thank all the colleagues who assisted with writing the dissertation.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kim, D.J.; Paik, K.Y.; Kim, M.K.; Kim, E.; Kim, W. Three-year result of efficacy for type 2 diabetes mellitus control between laparoscopic duodenojejunal bypass compared with laparoscopic Roux-en-Y gastric bypass. Ann. Surg. Treat. Res. 2017, 93, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Rhee, N.A.; Wahlgren, C.D.; Pedersen, J.; Mortensen, B.; Langholz, E.; Wandall, E.P.; Friis, S.U.; Villman, P.; Paulsen, S.J.; Knop, F.K. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia 2015, 58, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Crosnier, C.; Stamataki, D.; Lewis, J. Organizing cell renewal in the intestine: Stem cells signals, and combinatorial control. Nat. Rev. Genet. 2006, 7, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.; DeLaForest, A.; Battle, M.A. Patterning the gastrointestinal epithelium to confer regional-specific functions. Dev. Biol. 2018, 435, 97–108. [Google Scholar] [CrossRef]
- Seiler, K.M.; Waye, S.E.; Kong, W.; Kamimoto, K.; Bajinting, A.; Goo, W.H.; Onufer, E.J.; Courtney, C.; Guo, J.; Warner, B.W.; et al. Single-cell analysis reveals regional reprogramming during adaptation to massive small bowel resection in mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 407–426. [Google Scholar] [CrossRef]
- Li, B.; Lu, Y.; Srikant, C.B.; Gao, Z.H.; Liu, J.L. Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal-jejunal bypass surgery in Zucker fatty rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G635–G645. [Google Scholar] [CrossRef]
- Larsen, H.L.; Jensen, K.B. Reprogramming cellular identity during intestinal regeneration. Curr. Opin. Genet. Dev. 2021, 70, 40–47. [Google Scholar] [CrossRef]
- Kindel, T.L.; Martins, P.J.; Yoder, S.M.; Jandacek, R.J.; Seeley, R.J.; D’Alessio, D.A.; Obici, S.; Tso, P. Bypassing the duodenum does not improve insulin resistance associated with diet-induced obesity in rodents. Obesity 2011, 19, 380–387. [Google Scholar] [CrossRef]
- Lee, H.C.; Kim, M.K.; Kwon, H.S.; Kim, E.; Song, K.H. Early changes in incretin secretion after laparoscopic duodenal-jejunal bypass surgery in type 2 diabetic patients. Obes. Surg. 2010, 20, 1530–1535. [Google Scholar] [CrossRef]
- Petry, T.Z.; Fabbrini, E.; Otoch, J.P.; Carmona, M.A.; Caravatto, P.P.; Salles, J.E.; Sarian, T.; Correa, J.L.; Schiavon, C.A.; Patterson, B.W.; et al. Effect of duodenal-jejunal bypass surgery on glycemic control in type 2 diabetes: A randomized controlled trial. Obesity 2015, 23, 1973–1979. [Google Scholar] [CrossRef]
- Klein, S.; Fabbrini, E.; Patterson, B.W.; Polonsky, K.S.; Schiavon, C.A.; Correa, J.L.; Salle, J.E.; Wajchenberg, B.L.; Cohen, R. Moderate effect of duodenal-jejunal bypass surgery on glucose homeostasis in patients with type 2 diabetes. Obesity 2012, 20, 1266–1272. [Google Scholar] [CrossRef]
- Kindel, T.L.; Yoder, S.M.; D’Alessio, D.A.; Tso, P. The effect of the duodenal-jejunal bypass on glucose-dependent insulinotropic polypeptide secretion in Wistar rats. Obes. Surg. 2010, 20, 768–775. [Google Scholar] [CrossRef]
- Heo, Y.; Ahn, J.H.; Shin, S.H.; Lee, Y.J. The effect of duodenojejunal bypass for type 2 diabetes mellitus patients below body mass index 25 kg/m2: One-year follow-up. J. Korean Surg. Soc. 2013, 85, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Grong, E.; Arbo, I.B.; Thu, O.K.; Kuhry, E.; Kulseng, B.; Marvik, R. The effect of duodenojejunostomy and sleeve gastrectomy on type 2 diabetes mellitus and gastrin secretion in Goto-Kakizaki rats. Surg. Endosc. 2015, 29, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, G.; Sun, D.; Han, H.; Hu, S. Duodenal-jejunal bypass improves glucose metabolism and adipokine expression independently of weight loss in a diabetic rat model. Obes. Surg. 2013, 23, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, K.; Yan, Z.; Zhang, G.; Liu, S.; Liu, F.; Hu, C.; Hu, S. Duodenal-jejunal bypass surgery upregulates the expression of the hepatic insulin signal in proteins and the key regulatory enzymes of intestinal gluconeogenesis in diabetic Goto-Kakizaki rats. Obes. Surg. 2013, 23, 1734–1742. [Google Scholar] [CrossRef]
- Speck, M.; Cho, Y.M.; Asadi, A.; Rubino, F.; Kieffer, T.J. Duodenal-jejunal bypass protects GK rats from β-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E923–E932. [Google Scholar] [CrossRef]
- Geloneze, B.; Geloneze, S.R.; Chaim, E.; Hirsch, F.F.; Felici, A.C.; Lambert, G.; Tambascia, M.A.; Pareja, J.C. Metabolic surgery for nonobese type 2 diabetes: Incretins, adipocytokines, and insulin secretion/resistance changes in a 1-year interventional clinical controlled study. Ann. Surg. 2012, 256, 72–78. [Google Scholar] [CrossRef]
- Geloneze, B.; Geloneze, S.R.; Fiori, C.; Stabe, C.; Tambascia, M.A.; Chaim, E.A.; Brenno, D.; Pareja, A.J. Surgery for nonobese type 2 diabetic patients: An interventional study with duodenal-jejunal exclusion. Obes. Surg. 2009, 19, 1077–1083. [Google Scholar] [CrossRef]
- Altmann, G.G.; Leblond, C.P. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. Am. J. Anat. 1970, 127, 15–36. [Google Scholar] [CrossRef]
- Jorsal, T.; Rhee, N.A.; Pedersen, J.; Wahlgren, C.D.; Mortensen, B.; Jepsen, S.L.; Dalbøge, L.; Vilmann, P.; Hassan, H.; Hendel, J.W.; et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia 2018, 61, 284–294. [Google Scholar] [CrossRef]
- Rao, R.S.; Kini, S. GIP and bariatric surgery. Obes. Surg. 2010, 21, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Guidone, C.; Manco, M.; Valera-Mora, E.; Iaconelli, A.; Gniuli, D.; Mari, A.; Nanni, G.; Castagneto, M.; Calvani, M.; Mingrone, G. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 2006, 55, 2025–2031. [Google Scholar] [CrossRef]
- Miyachi, T.; Nagao, M.; Shibata, C.; Kitahar, Y.; Tanaka, N.; Watanabe, K.; Tsuchiya, T.; Motoi, F.; Naitoh, T.; Unno, M. Biliopancreatic limb plays an important role in metabolic improvement after duodenal-jejunal bypass in a rat model of diabetes. Surgery 2016, 159, 1360–1371. [Google Scholar] [CrossRef]
- Laferrere, B.; Teixeira, J.; McGinty, J.; Tran, H.; Egger, J.R.; Colarusso, A.; Kovack, B.; Bawa, B.; Koshy, N.; Lee, H.; et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 2479–2485. [Google Scholar] [CrossRef]
- Laferrere, B.; Heshka, S.; Wang, K.; Khan, Y.; McGinty, J.; Teixeira, J.; Hart, A.B.; Olivan, B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007, 30, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Fellici, A.C.; Lambert, G.; Lima, M.M.; Pareja, J.C.; Rodovalho, S.; Chaim, E.A.; Geloneze, B. Surgical treatment of type 2 diabetes in subjects with mild obesity: Mechanisms underlying metabolic improvements. Obes. Surg. 2015, 25, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, H.K.; Byun, D.W.; Suh, K.I.; Hur, K.Y. Incretin levels 1 month after laparoscopic single anastomosis gastric bypass surgery in non-morbid obese type 2 diabetes patients. Asian J. Surg. 2014, 37, 130–137. [Google Scholar] [CrossRef]
- Whitson, B.A.; Leslie, D.B.; Kellogg, T.A.; Maddaus, M.A.; Buchwald, H.; Billington, C.J.; Ikramuddin, S. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: A preliminary study. J. Surg. Res. 2007, 141, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cossu, M.L.; Noya, G.; Tonolo, G.C.; Profili, S.; Meloni, G.B.; Ruggiu, M.; Brizzi, P.; Cossu, F.; Pilo, L.; Tilocca, P.L. Duodenal switch without gastric resection: Results and observations after 6 years. Obes. Surg. 2004, 14, 1354–1359. [Google Scholar] [CrossRef]
- Ceriani, V.; Cetta, F.; Lodi, T.; Pinna, F.; Pontiroli, A.E. Clinical and metabolic effects of biliopancreatic diversion persist after the reduction of the gastric pouch and elongation of the common alimentary tract. Preliminary report in a series of patients with a 10-year follow-up. Obes. Surg. 2017, 27, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Dolo, P.R.; Yao, L.; Li, C.; Zhu, X.; Shi, L.; Widjaja, J. Preserving duodenal-jejunal(foregut)transit does not impair glucose tolerance and diabetes remission following gastric bypass in type 2 diabetes Sparague-Dawley rat model. Obes. Surg. 2018, 28, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).