In this retrospective, observational and descriptive study were included 200 patients diagnosed with rheumatoid arthritis and treated in the Rheumatology Department, in collaboration with the Cardiology Department, of the Bihor County Clinical and Emergency Hospital, between March 2020 and March 2023. The inclusion criteria were the diagnosis of rheumatoid arthritis and the presence of cardiovascular complications. No patient was excluded from the study. At admission, it were collected data regarding the main symptoms which have led to admission in the hospital, complete medical history, history of family disease, harmful behaviors, home medication, and environment of origin, The general clinical examination was done for each patient. Moreover, we determined the blood pressure of all patients and conducted concurrent electrocardiograms in conjunction with echocardiographic examinations and laboratory tests. This data was statistically analyzed according to the intended purpose of this study. Detailed quantitative data on the amount of tobacco used and body mass index was not available.
Results
This clinical study included 200 patients, 124 women and 76 men, with a higher incidence observed in females. (
Table 1)
The highest prevalence of the disease was observed in patients aged between 50 to 69 years old. The existence of 25 cases in the 40-49 age category could be suggestive for early onset of rheumatoid arthritis and cardiovascular complications. (
Table 1)
Most of the patients were diagnosed with stage III of the disease; 13% of patients were diagnosed during stage I, 19% of patients were diagnosed with stage II, 42.5 % in stage III and quite a high percentage of 25.5% were diagnosed in stage IV. It was observed a correlation between the chronic evolution of RA , and the presence of cardiovascular complications in patients with advanced rheumatoid arthritis. (
Table 2)
The “traditional” CV risk factors were identified to nearly 50% of the total CVD risk. [
9] Smoking was more frequently observed in RA patients, since this behavior was associated with a higher incidence of RA. [
10,
11,
12]
Smoking was also significantly associated with a higher disease activity score, leading to worse clinical outcomes. [
13]
Hypertension and diabetes were also more prevalent in RA patients compared to healthy control groups. [
14]
While arterial hypertension was established as a risk factor for cardiovascular disease, there was consistent evidence that systemic inflammation, present in patients with RA, played a central role in maintaining high blood pressure values. [
15]
In addition to the traditional risk factors, patients with RA were more likely to suffer from metabolic syndrome. [
16] Metabolic syndrome was highly correlated with traditional risk factors such as hyperlipidemia, hypertension and diabetes mellitus, and resulted in a relative risk for cardiovascular disease of 1.93. [
17]
As displayed in the table above, there was a higher incidence in men regarding traditional and non-traditional CV risk factors. (
Table 3)
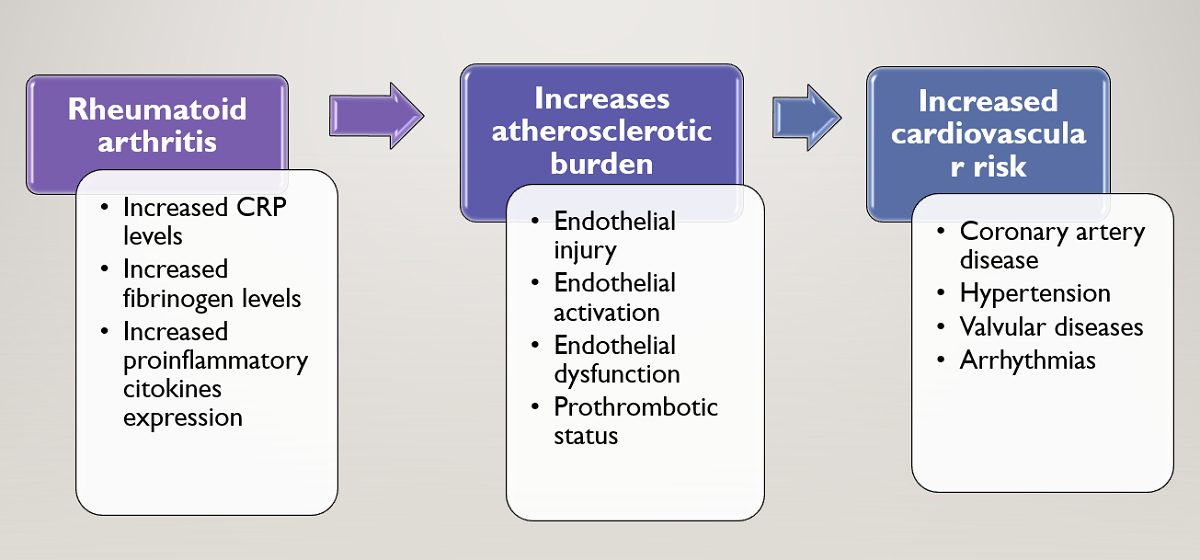
In addition to traditional risk factors, novel “non-traditional” risk factors involved in acceleration of atherosclerosis were emphasized in recent years. In this study, non-traditional risk factors were identified and distributed by gender.
The inflammatory mechanisms involved in the pathophysiology of RA enhance atherogenesis in several ways. C-reactive protein, a useful marker of disease activity, is elevated in RA and has significant prognostic value. [
18] It also causes endothelial injury directly through the activation of endothelial cells due to T-cell mediated cytotoxicity. [
19] Circulating cytokines in RA, such as TNF-α, result in endothelial activation and up-regulation of adhesion molecules. [
20] Endothelial dysfunction is frequently present in RA patients, even in the absence of identifiable CV risk factors, and improves with anti-TNF-α therapy. [
21,
22]
In recent years, new cardiovascular risk factors, suggested to be useful in cardiovascular risk stratification, were described: C-reactive protein, interleukin 6, TNF- α, endothelial dysfunction,homocysteine, prothrombotic status, rheumatoid factor. The presence of “traditional” and “non-traditional” risk factors in rheumatoid arthritis increases the risk of atherosclerosis and cardiovascular comorbidities.
Based on collected data, we determined the atherogenic coefficient, men scoring an average value of 4,52 +/- 0,27 (median = 4.60, IQR = 4-5) and women scoring 3,13 +/- 0,20 (median = 2.75, IQR = 2.37-4.10), the
Mann-Whitney U Test showing higher values in men versus women (
p<0.001). In conclusion, both groups scored an atherogenic coefficient over 2, indicating a higher risk of atherogenesis, more frequently in men (100%) than women (93.5%) (
Fisher’s Exact Test – p=0.025). (
Figure 1)
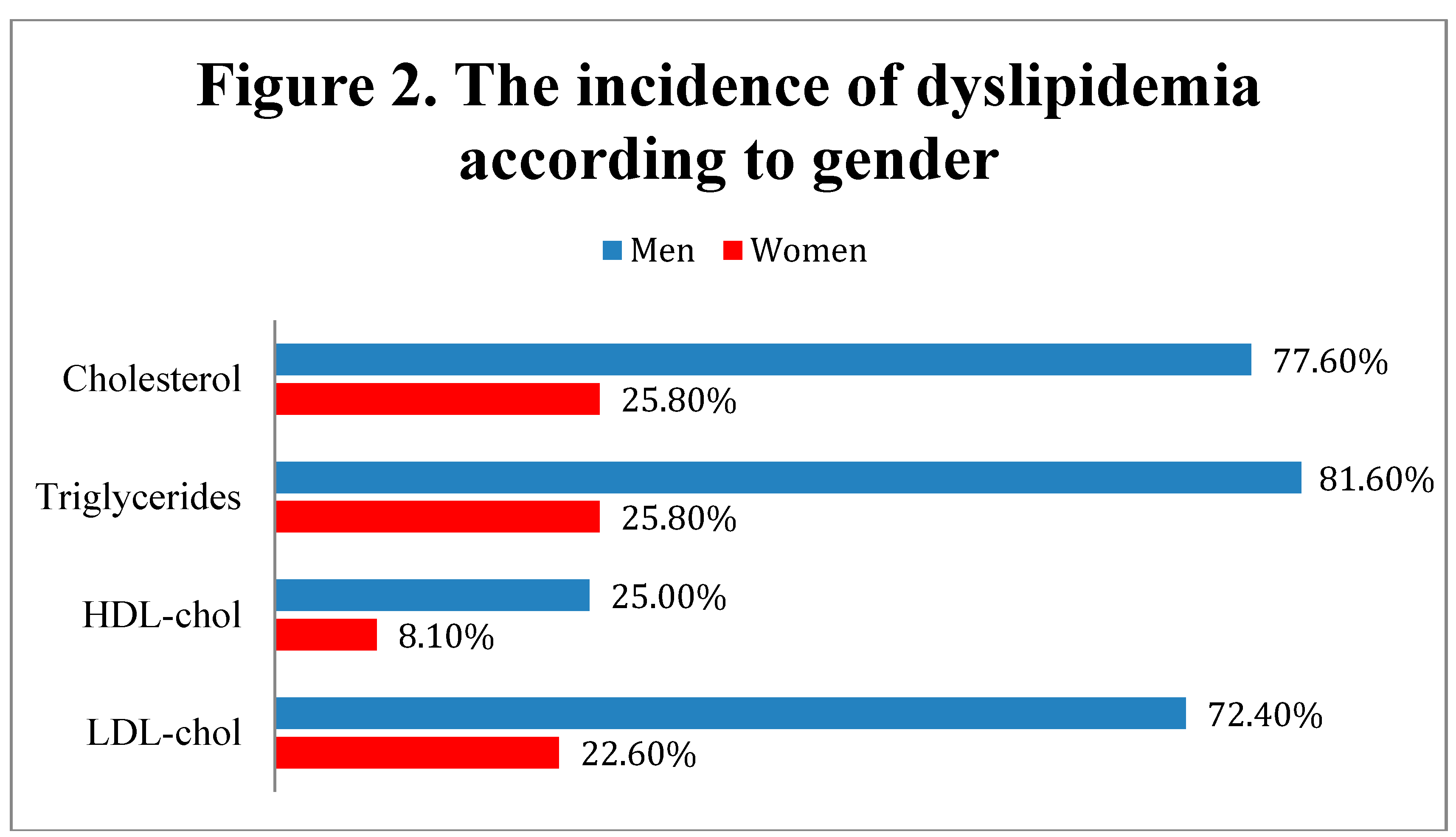
According to the data presented in Figure 2, men were significantly more associated with dyslipidemia criteria, having more frequently elevated levels of total cholesterol (≥ 200 mg/dL) (77.6% - men vs. 25.8% - women, Fisher’s Exact Test – p<0.001), LDL-cholesterol (≥ 140 mg/dL) (72.4% - men vs. 22.6% - women, Fisher’s Exact Test – p<0.001), triglycerides (≥ 150 mg/dL) (81.6% - men vs. 25.8% - women, Fisher’s Exact Test – p<0.001) and lower concentrations of HDL-cholesterol (< 40 mg/dL) (25% - men vs. 8.1% - women, Fisher’s Exact Test – p=0.002),
As displayed in the table above, men presented a higher incidence of coronary disease compared to women (
Fisher’s Exact Test with Bonferroni corrected Z-tests – p<0.001). 42.1% of men presented monovascular coronary artery disease, 19.7% bivascular coronary artery disease, and 11.8% trivascular coronary artery disease. Women exhibited monovascular coronary artery disease in 24.1% of cases, bivascularcoronary artery disease in 9.6% of cases, and trivascular coronary artery disease in 4% of cases. (
Table 4)
Electrocardiogram examinations was recorded for every patient enrolled in the study. Left ventricular hypertrophy was significantly more frequent in men (51.3%) than women (27.4%),
p=0.001. Secondary ST-T appeared in 39.5% of men and 26.6% of women (showing a tendency towards statistical significance in the direction of higher frequencies in men than women, p=0.062), while atrial fibrillation was more frequently in women (32.3%) than men (17.1%) (
p=0.021). Thus, a higher incidence of ECG abnormalities could be observed in men. The examination of the ECGs highlighted the presence of arrhythmias and conduction disorders (supraventricular extrasystoles, left anterior fascicular block, left bundle branch block, premature ventricular contractions, atrial fibrillation). (
Table 5)
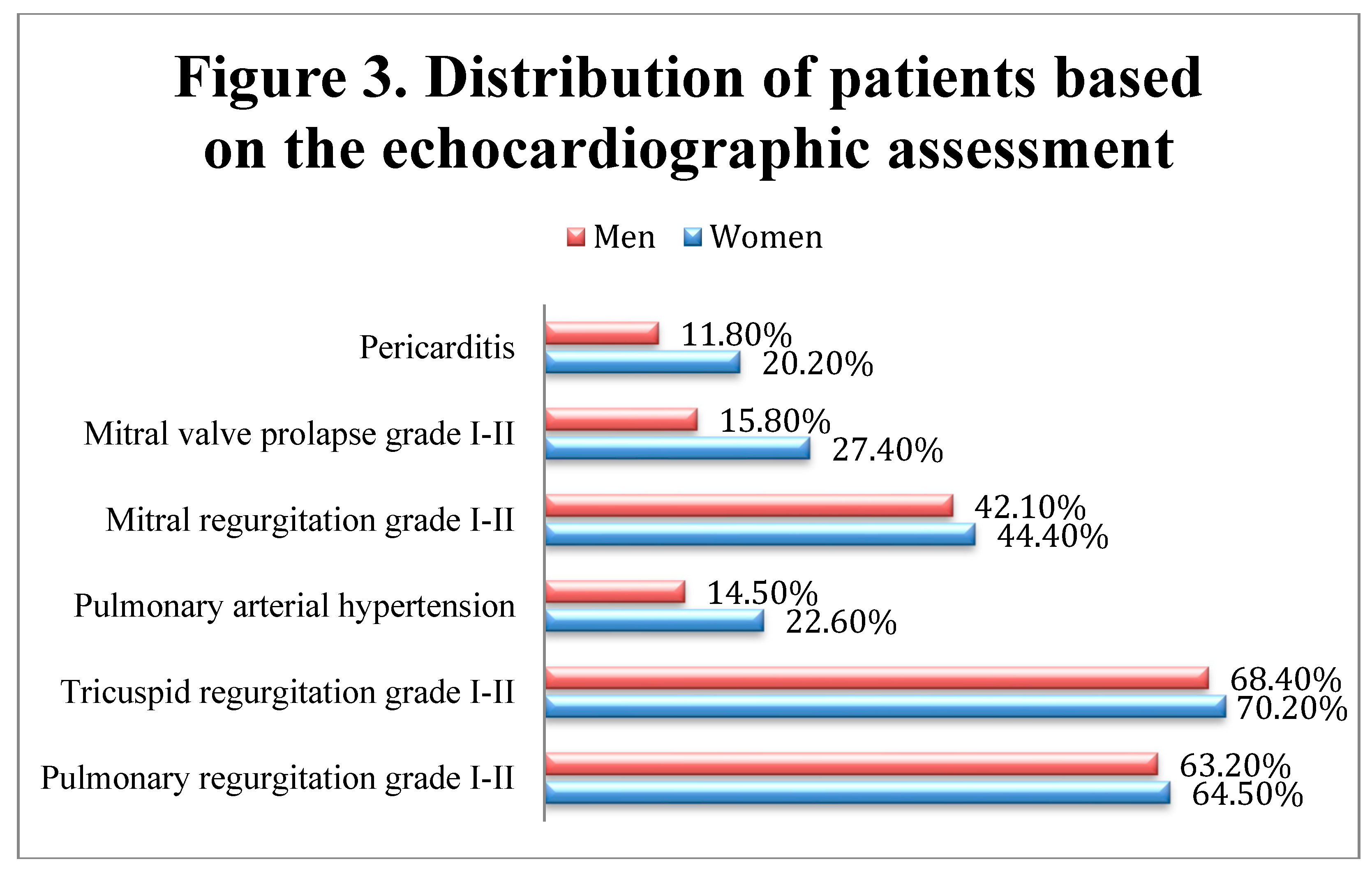
The echocardiography confirmed the presence of ventricular hypertrophy, previously reported on the ECGs. Moreover, pericarditis, pulmonary arterial hypertension, and mitral valve prolapse appeared to have a higher incidence in women compared to men, although significant differences between genders were not observed (Fisher’s Exact Tests – pericarditis (p=0.174), mitral Valve Prolapse (p=0.083), mitral Regurgitation (p=0.771), pulmonary arterial hypertension (p=0.199), tricuspid regurgitation (p=0.874), pulmonary regurgitation (p=0.880)), while wall motion abnormalities (hypokinesia and akinesia) were present in 31 patients (17 men and 14 women), possibly due to past myocardial infarctions. (Figure 3)
The etiology of congestive heart failure is complex and varies according to geographical and socio-economic factors. During the initial clinical evaluation, numerous patients complained about heart failure symptomes, which were correlated with echocardiography findings. As result, 19 patients (9.5%) were diagnosed with congestive heart failure.
Coronary artery disease and high blood pressure were the most common causes of congestive heart failure. The main cause for heart failure was arterial hypertension (44.5%) , while coronary artery disease was responsible for 17.5%, valvular heart disease for 14% of cases, and arrhythmias and conduction disorders for 7.5% of cases. (
Table 6)
The comparision of analyzed parameters between patients according to the existence of cardiac complications showed that in patients with pericarditis, only HDL-cholesterol and atrial fibrillation were significantly different between groups. Patients with pericarditis had significantly higher values of HDL-cholesterol (median = 42, IQR = 40-45) in comparison to patients without pericarditis (median = 40, IQR = 40-43) (p=0.039). Also,atrial fibrillation were sig nificantly more frequently associated with pericarditis (41.2% vs. 23.5%, (p=0.033).
Data from Table no 7 show the logistic regression models used for the prediction of cardiac complications In patients with mitral valve prolapse, only frequency of LVH was significantly different between groups, patients with LVH were significantly less associated with mitral valve prolapse (40.9% vs. 21.7%) than patients without LVH (78.3% vs. 59.1%) (p=0.023). CRP, tryglicerides, HDL-cholesterol and secondary ST-T changes were significantly different between groups in patients with mitral regurgitation. This patients had a higher CRP values (median = 121, IQR = 4-150 vs. median = 105, IQR = 2-132.5, p=0.012), lower values of HDL-cholesterol (median = 40, IQR = 40-42 vs. median = 42, IQR = 40-44, p=0.018), higher frequencies of triglycerides dyslipidemia (55.2% vs. 40.7%, p=0.047) and lower frequencies of secondary ST-T changes (38.1% vs. 23%, p=0.031) than patients without mitral regurgitation.
In case of pulmonary arterial hypertension, only rheumatoid factor and HDL-cholesterol were significantly different between groups, patients with pulmonary arterial hypertension had significantly higher values of rheumatoid factor (median = 206, IQR = 137-216 vs. median = 202, IQR = 123-211, p=0.036) and higher values of HDL-cholesterol (median = 42, IQR = 40-45 vs. median = 40, IQR = 40-43, p=0.045) in comparison to patients without pulmonary arterial hypertension. In case of tricuspid regurgitation, none of the parameters were significantly different between groups (p>0.05).
LVH was a significant predictor for mitral valve prolapse (p=0.020). Patients without LVH have increased odds of having mitral valve prolapse by 2.493 times (95% C.I.: 1.153-5.376); In case of mitral regurgitation, in univariate models, HDL-cholesterol was not a significant predictor (p=0.055), while CRP (p=0.019), triglycerides dyslipidemia (p=0.043) and secondary ST-T changes (p=0.024) were significant predictors. Each increase of 1 unit of CRP was associated with increased odds of having mitral regurgitation by 1.005 times (95% C.I.:1.001-1.009); Patients with dyslipidemia had increased odds of having mitral regurgitation by 1.793 times (95% C.I.:1.019-3.154);. Patients without secondary ST-T changes had increased odds of having mitral regurgitation by 2.057 times (95% C.I.:1.098-3.861);
In case of pulmonary arterial hypertension, none of the variables were significant predictors (p>0.05). For pulmonary regurgitation, left bundle branch block was a significant predictor (p=0.009), patients with this condition had increased odds of having pulmonary regurgitation by 7.264 times (95% C.I.: 1.656-31.869).
Table 8.
Comparison of analyzed parameters between patients according to the existence of atrial fibrillation (*Fisher’s Exact Test, **Mann-Whitney U Test, ***Pearson Chi-Square Test, ****Fisher’s Exact Test with Bonferroni corrected Z-tests).
Table 8.
Comparison of analyzed parameters between patients according to the existence of atrial fibrillation (*Fisher’s Exact Test, **Mann-Whitney U Test, ***Pearson Chi-Square Test, ****Fisher’s Exact Test with Bonferroni corrected Z-tests).
| Parameter / Group |
Atrial fibrillation |
p |
| Absent (N=147) |
Present (N=53) |
| Gender (Male) (Nr., %) |
63 (42.9%) |
13 (24.5%) |
0.021* |
| Age (Median (IQR)) |
63 (55-73) |
61 (55-70.5) |
0.642** |
| Stages of RA disease (Nr., %) |
|
|
0.890* |
| Stage I |
20 (13.6%) |
6 (11.3%) |
| Stage II |
28 (19%) |
10 (18.9%) |
| Stage III |
60 (40.8%) |
25 (47.2%) |
| Stage IV |
39 (26.5%) |
12 (22.6%) |
| Smoking (Nr., %) |
59 (40.1%) |
11 (20.8%) |
0.012* |
| Alcohol consumption (Nr., %) |
37 (25.2%) |
10 (18.9%) |
0.450* |
| Hypertension (Nr., %) |
85 (57.8%) |
21 (39.6%) |
0.025* |
| Hypercholesterolemia (Nr., %) |
72 (49%) |
19 (35.8%) |
0.110* |
| Diabetes mellitus (Nr., %) |
24 (16.3%) |
4 (7.5%) |
0.165* |
| CRP (Median (IQR)) |
110 (2-135) |
114 (3-147) |
0.505** |
| Fibrinogen (Median (IQR)) |
4.7 (4.5-5.1) |
4.6 (4.5-4.85) |
0.022** |
| ESR (Median (IQR)) |
98 (14-106) |
98 (15.5-110.5) |
0.535** |
| Rheumatoid factor (Median (IQR)) |
201 (121-213) |
206 (168.5-213.5) |
0.047** |
| Atherogenic coefficient (Median (IQR)) |
3.375 (2.75-4.75) |
2.90 (2.29-4.67) |
0.016** |
| Total cholesterol (Median (IQR)) |
180 (157-230) |
160 (145-222.5) |
0.003** |
| Dyslipidemia – Total cholesterol (Nr., %) |
72 (49%) |
19 (35.8%) |
0.110* |
| Triglycerides (Median (IQR)) |
150 (100-170) |
110 (85-157.5) |
0.028** |
| Dyslipidemia – Triglycerides (Nr., %) |
74 (50.3%) |
20 (37.7%) |
0.148* |
| HDL-cholesterol (Median (IQR)) |
40 (40-44) |
40 (40-44.5) |
0.460** |
| Dyslipidemia – HDL-cholesterol (Nr., %) |
24 (16.3%) |
5 (9.4%) |
0.262* |
| LDL-cholesterol (Median (IQR)) |
116 (95-154) |
100 (83.5-149) |
0.008** |
| Dyslipidemia – LDL-cholesterol (Nr., %) |
65 (44.2%) |
18 (34%) |
0.255* |
| Coronary artery disease (Nr., %) |
|
|
0.030**** |
| Absent |
64 (43.5%) |
33 (62.3%) |
| Monovascular artery disease |
46 (31.3%) |
16 (30.2%) |
| Bivascular artery disease |
24 (16.3%) |
3 (5.7%) |
| Trivascular artery disease |
13 (8.8%) |
1 (1.9%) |
| Pericarditis (Nr., %) |
20 (13.6%) |
14 (26.4%) |
0.033*** |
| Mitral valve prolapse (Nr., %) |
33 (22.4%) |
13 (24.5%) |
0.849* |
| Mitral regurgitation (Nr., %) |
63 (42.9%) |
24 (45.3%) |
0.872* |
| Pulmonary arterial hypertension (Nr., %) |
24 (16.3%) |
15 (28.3%) |
0.070* |
| Tricuspid regurgitation (Nr., %) |
102 (69.4%) |
37 (69.8%) |
1.000* |
| Pulmonary regurgitation (Nr., %) |
90 (61.2%) |
38 (71.7%) |
0.186* |
| CHF etiology |
| Hypertension (Nr., %) |
69 (46.9%) |
20 (37.7%) |
0.264* |
| Coronary artery disease (Nr., %) |
30 (20.4%) |
5 (9.4%) |
0.091* |
| Valvular heart disease (Nr., %) |
18 (12.2%) |
10 (18.9%) |
0.252* |
| Arrhythmias (Nr., %) |
1 (0.7%) |
14 (26.4%) |
<0.001* |
Data from Table no.8 show the comparison of analyzed parameters between patients according to the existence of atrial fibrillation. Men were significantly less associated with atrial fibrillation (42.9% vs. 24.5%) than women (75.5% vs. 57.1%) (p=0.021) Patients with arterial hypertension were significantly less associated with atrial fibrillation (57.8% vs. 39.6%) than patients without arterial hypertension (60.4% vs. 42.2%) (p=0.025). Fibrinogen levels were significantly lower in patients with atrial fibrillation (median = 4.6, IQR = 4.5-4.85) than in patients without atrial fibrillation (median = 4.7, IQR = 4.5-5.1) (p=0.022). Rheumatoid factor levels were significantly higher in patients with atrial fibrillation (median = 206, IQR = 168.5-213.5) than in patients without atrial fibrillation (median = 201, IQR = 121-213) (p=0.047);
Patients without coronary artery disease were significantly more associated with atrial fibrillation (62.3% vs. 43.5%) (p=0.030);
Data from
Table 9 show the logistic regression models used for the prediction of atrial fibrillation. In univariate models, each of the analyzed parameters were significant predictors (p<0.05). Female patients had increased odds of atrial fibrillation by 2.308 times (95% C.I.:1.139-4.674) (p=0.020), Non-smokers had increased odds of atrial fibrillation by 2.557 times (95% C.I.:1.219-5.376) (p=0.013) and non-hypertensive patients had increased odds of atrial fibrillation by 2.087 times (95% C.I.:1.101-3.968) (p=0.024). Each decrease of 1 unit of fibrinogen had increased odds of atrial fibrillation by 3.322 times (95% C.I.:1.183-9.345) (p=0.023), each increase of 1 unit of rheumatoid factor had increased odds of atrial fibrillation by 1.009 times (95% C.I.: 1.001-1.017) (p=0.020). Also, patients without coronary artery disease had increased odds of atrial fibrillation by 1.972 times (95% C.I.:1.008-3.861) (p=0.047); Each decrease of 1 unit of atherogenic coefficient had increased odds of atrial fibrillation by 1.388 times (95% C.I.:1.055-1.828) (p=0.019) and each decrease of 1 unit of total cholesterol had increased odds of atrial fibrillation by 1.010 times (95% C.I.:1.002-1.018) (p=0.011);
The multivariable model was selected using the forward step-wise selection, as such only total cholesterol, coronary artery disease and pericarditis were the selected variables in the prediction, all of them being independent significant predictors
The progression of rheumatoid arthritis is characterized by flare episodes, alternating with periods of remission. Assessing the active stage of the disease relies on evaluating inflammatory joints signs, functional status, and various immunological activity tests, including erythrocyte sedimentation rate (ESR) and C-reactive protein. While ESR alone cannot diagnose a specific disease, it plays a crucial role in monitoring the activity of inflammatory conditions like rheumatoid arthritis and gauging their response to therapy. Additionally, ESR is essential for tracking the progression and establishing prognostics in congestive heart failure.
In certain diseases, such as rheumatoid arthritis, serial measurements of C-reactive protein hold prognostic value. [
23]
As displayed in
Table 10, 70% of patients (140 individuals) presented an ESR exceeding the upper limits, while the remaining cases presented values within the normal range. Additionally, C-reactive protein levels were within the normal range for 34.5% of patients (69 cases), while 65.5% (132 patients) displaying elevated C-reactive protein values.
These results provide new evidence suggesting that inflammatory factors present in rheumatoid arthritis patients may contribute to both the onset and prognosis of congestive heart failure. ESR, along with other specific inflammatory markers, may play a potential role in assessing congestive heart failure in patients with rheumatoid arthritis.
Rheumatoid arthritis (RA) is asociated with an increased mortality, primarily due to accelerated coronary artery and cerebrovascular atherosclerosis, which occurs in both early and established RA. The QUEST-RA study demonstrates that the prolonged use of medications such as methotrexate, sulfasalazine, glucocorticoids, leflunomide, and TNF-α blockers reduces the risk of cardiovascular events. [
24]
All patients received DMARDs, with 40 (20%) of them also taking NSAIDs. Remissive treatment was administered as follows: triple therapy (methotrexate, hydroxychloroquine, and sulfasalazine) to 50 patients (25%), DMARDs inmonotherapy to 93 patients (46.5%), and DMARDs in dual therapy to 57 patients (28.5%). Corticosteroids were included in the remissive treatment of 86 patients (43%). (
Table 11)
Ninety-seven patients in the study were on statin treatment (Atorvastatin), consisting of 51 men (67.10%) and 46 women (37%), men being more frequently associated with statin treatment than women ( p<0.001). Among these patients, improvements were observed in lipid profiles, along with visible clinical improvements and reductions in inflammatory markers such as ESR and CRP.
Statins, by reducing LDL-C levels, significantly lower the risk of major cardiovascular events in both high and low-risk patients for CVD. [
25,
26] In addition to their lipid-lowering effects, statins possess properties like stabilizing and regressing atherosclerotic plaques and limiting LDL-C oxidation, theoretically contributing to the prevention of primary atherosclerosis in RA. [
27,
28] These pleiotropic effects consist in anti-inflammatory, antiproliferative, antithrombotic, antioxidative, and immunomodulatory properties. [
29]