Submitted:
15 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
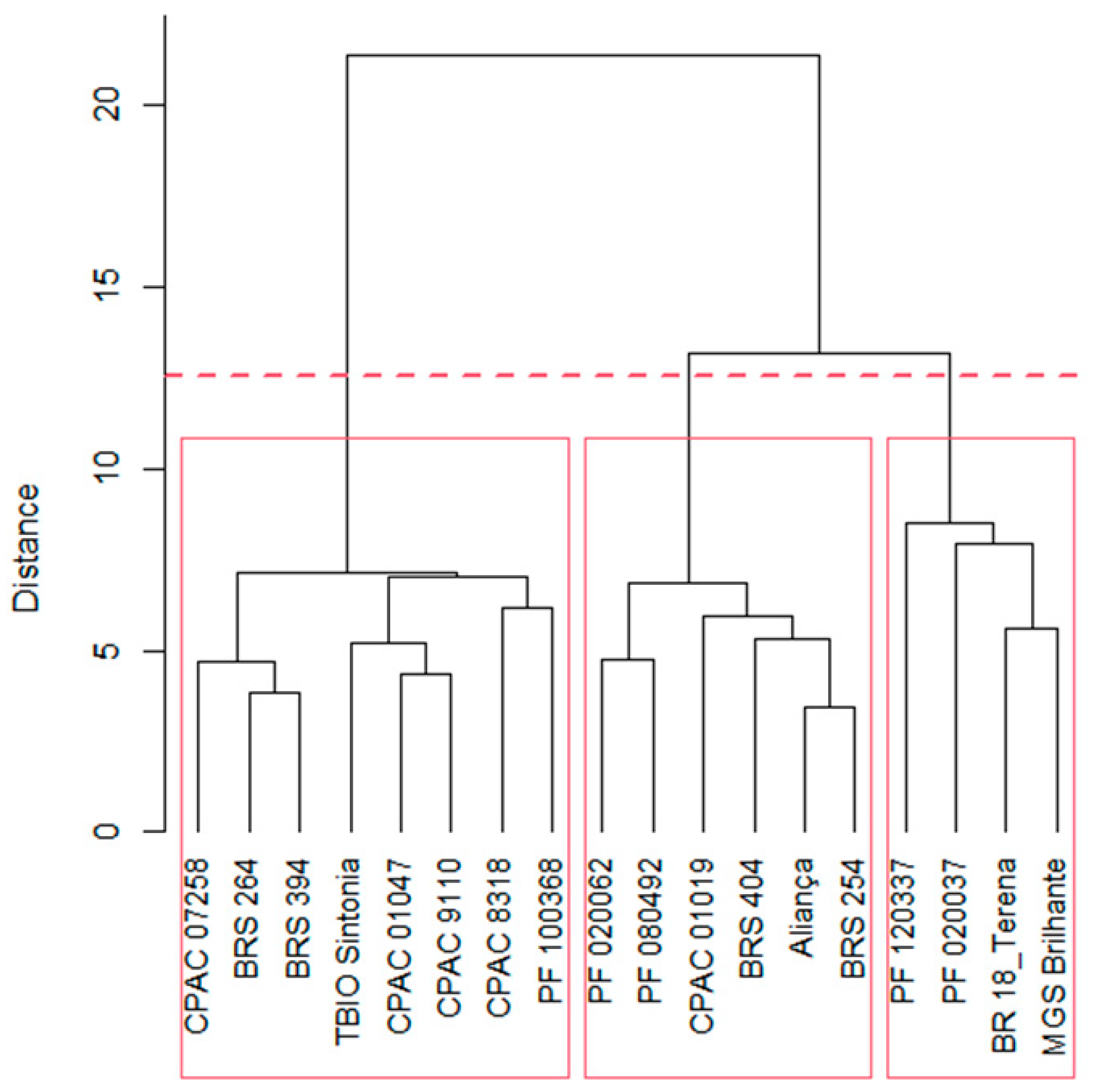
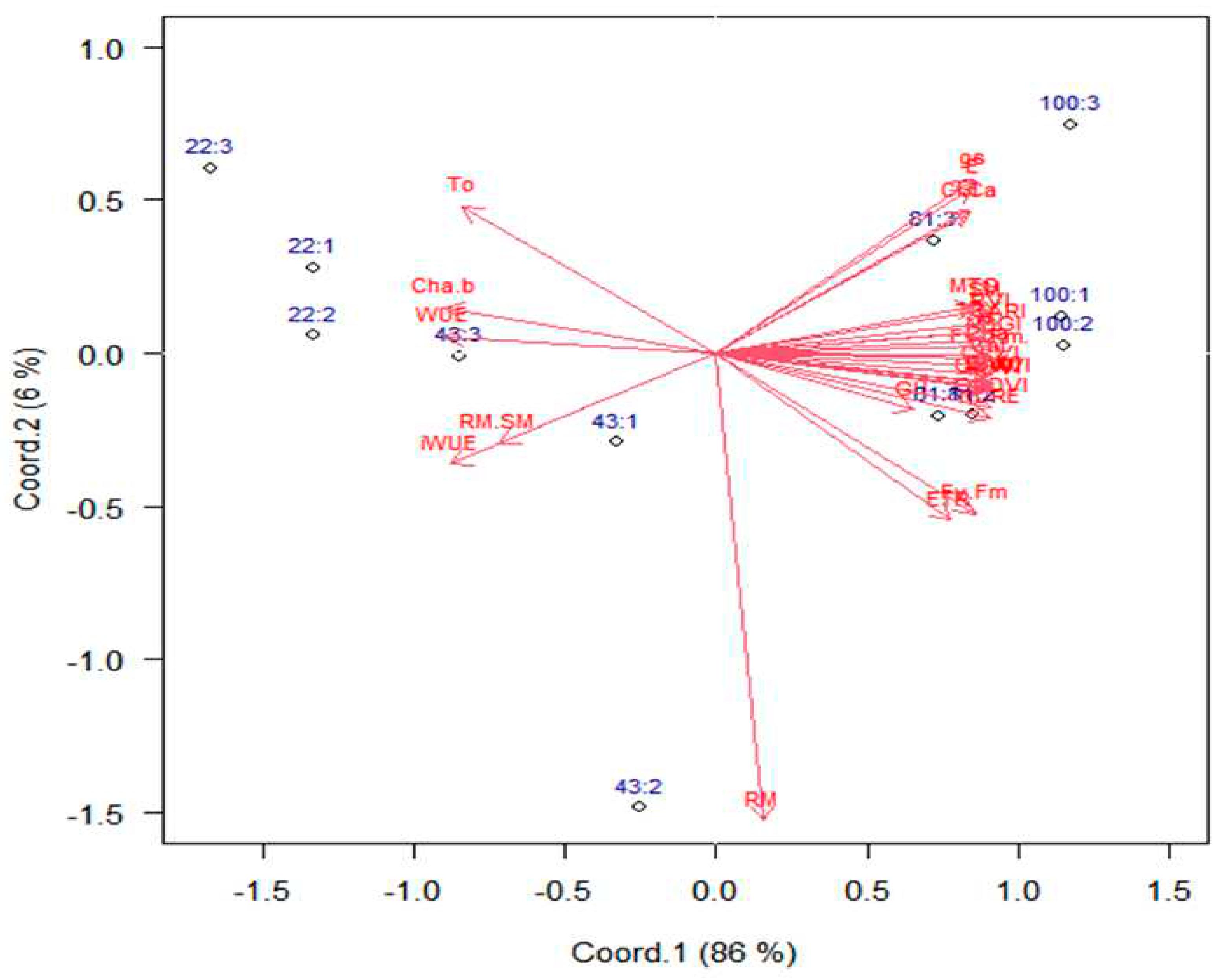
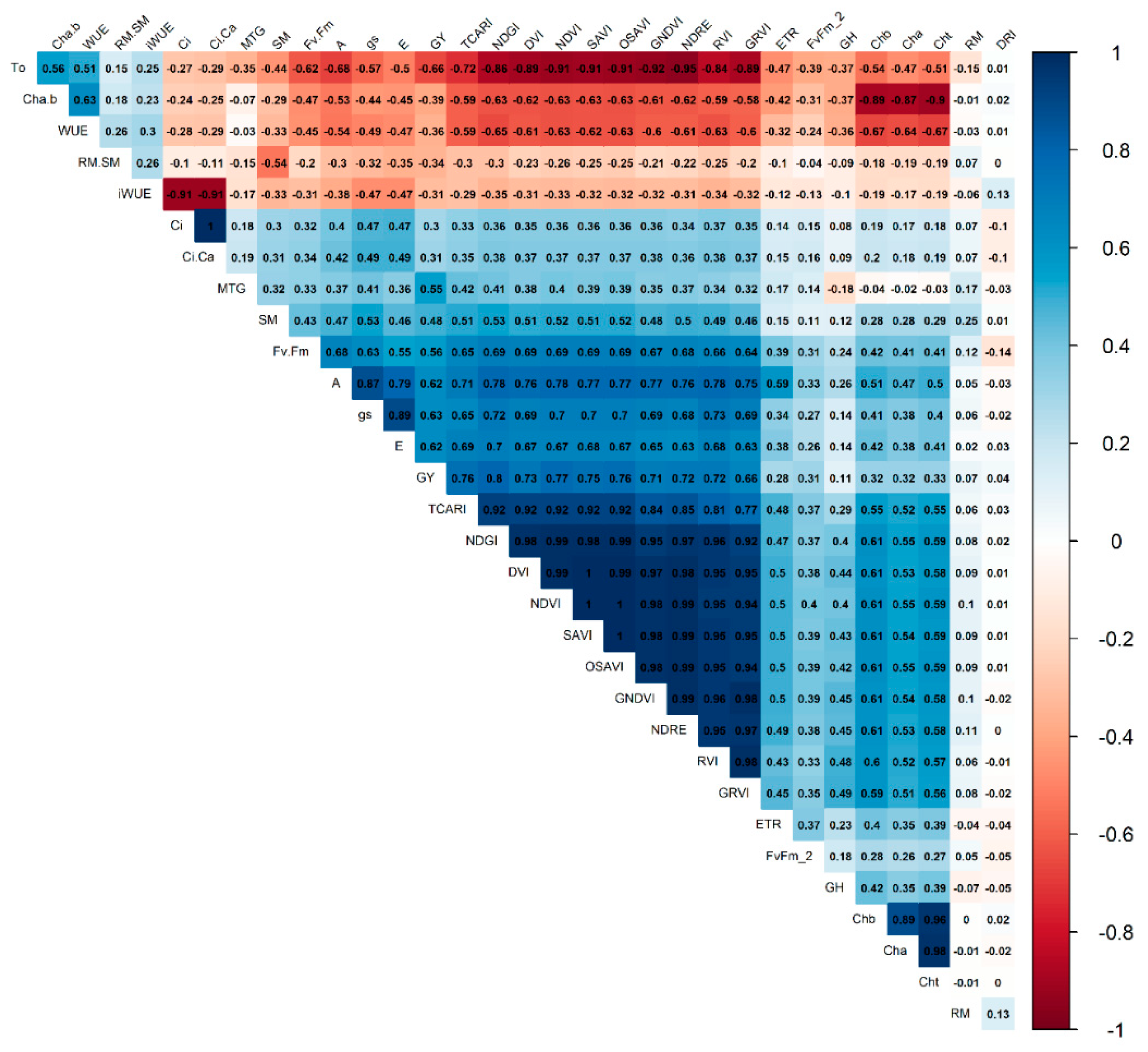
2.1. Variable contributions in the multivariate response
3. Discussion
4. Materials and Methods
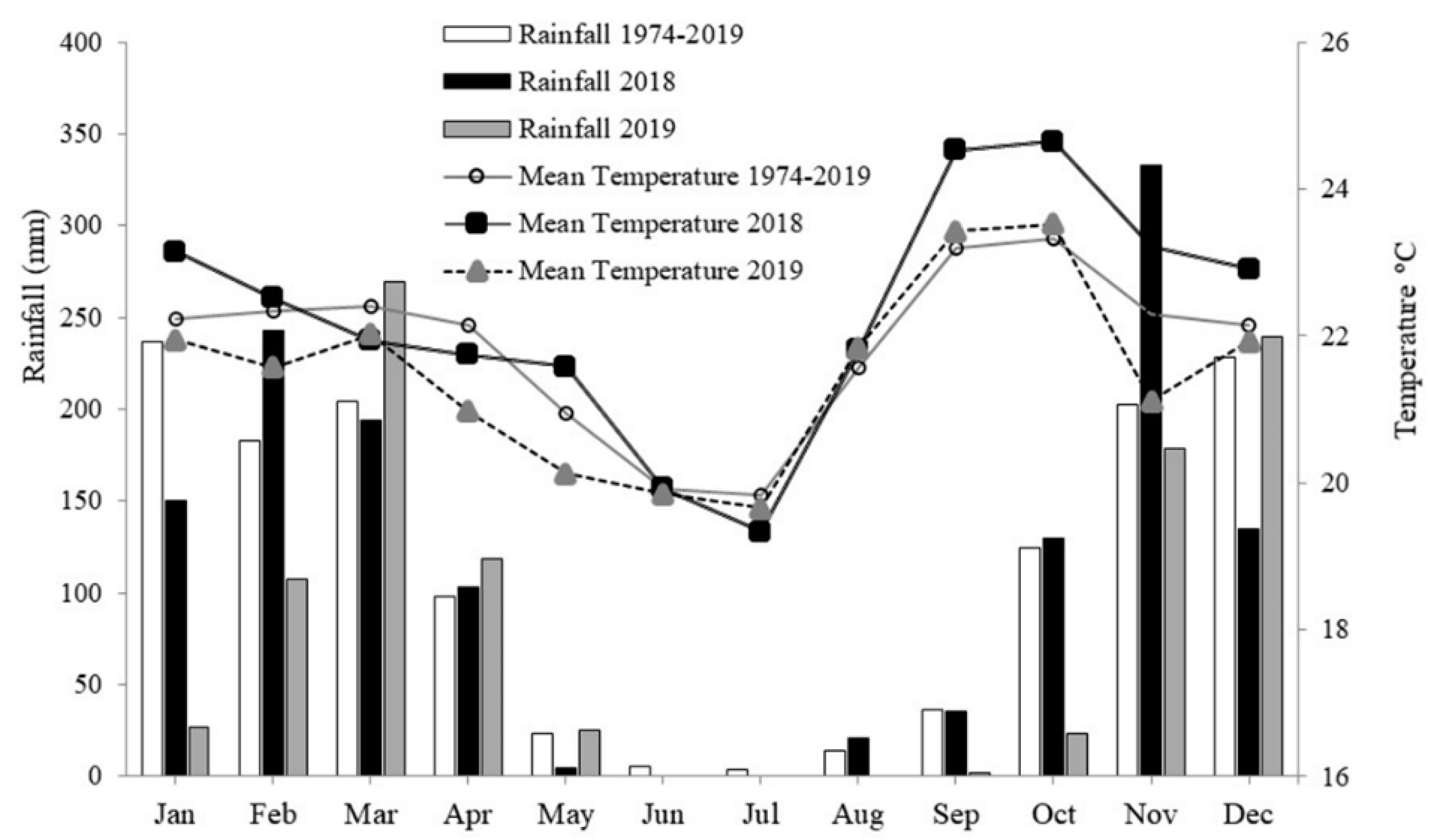
4.1. Experimental design and conducting the experiment
4.2. Analyzed variables
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef]
- Liu, W.; Wang, J.; Wang, C.; Ma, G.; Wei, Q.; Lu, H.; Xie, Y.; Ma, D.; Kang, G. Root growth, water and nitrogen use efficiencies in winter wheat under different irrigation and nitrogen regimes in North China Plain. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Soares, D.C.; Bonfim-Silva, E.M.; Silva, T.J.A.; Anicésio, E.C.A.; Duarte, T.F.; Oliveira, J.F. Growth and production of wheat cultivars under water tensions in Cerrado soil. Rev. Bras. Eng. Agríc. Ambiental. 2023, 27, 279–286. [Google Scholar] [CrossRef]
- Soares, G.F.; Ribeiro Júnior, W.Q.; Pereira, L.F.; Lima, C.A.; Soares, D.S.; Muller, O.; Rascher, U.; Ramos, M.L.G. Characterization of wheat genotypes for drought tolerance and water use efficiency. Scient. Agricola. 2021, 78, e20190304. [Google Scholar] [CrossRef]
- Pereira, J.F.; Cunha, G.R.; Moresco, E.R. Improved drought tolerance in wheat is required to unlock the production potential of the Brazilian Cerrado. Crop. Breed. Appl. Biotechnol. 2019, 19, 217–225. [Google Scholar] [CrossRef]
- Cattelan, A.J.; Dall’agnol, A. The rapid soybean growth in Brazil. OCL. 2018, 25, 1–12. [Google Scholar] [CrossRef]
- Schauberger, B.; Rolinski, S.; Schaphoff, S.; Muller, C. Global historical soybean and wheat yield loss estimates from ozone pollution considering water and temperature as modifying effects. Agric. For. Meteorol. 2019, 265, 1–15. [Google Scholar] [CrossRef]
- Peña-Gallardo, M.; Vicente-Serrano, S.M.; Quiringb, S.; Svoboda, M.; Hannafordd, M.; Hannafordd, J.; Tomas-Burguera, M.; Martín-Hernándeza, N.; Domínguez-Castro, F.; Kenawy, E.A. Response of crop yield to different timescales of drought in the United States: Spatio-temporal patterns and climatic and environmental driver. Agric. For. Meteorol. 2019, 264, 40–55. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.-D.; Sajjad, M.; Li, M.; Azmat, M.A.; Rizwan, M.; Maqsood, R.H.; Khan, S.H. Selection Criteria for Drought-Tolerant Bread Wheat Genotypes at Seedling Stage. Sustainability 2019, 11, 2584. [Google Scholar] [CrossRef]
- Conab. (2023). Companhia Nacional de Abastecimento. Acompanhamento da safra brasileira de grãos. Safra 2022/2023, n. 1 – Boletim de monitoramento agrícola – cultivos de verão e inverno, p. 1-20, 2023. Available online: https://www.conab.gov.br/info-agro/safras/graos/monitoramento-agricola (accessed on 20 June 2023).
- Hatfield, J.L. Increased temperatures have dramatic effects on growth and grain yield of three maize hybrids. Agric. Environ. Lett. 2016, 1. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Chen, W.; Li, H.; Deng, X. Physiological mechanisms contributing to increased water-use efficiency in winter wheat under organic fertilization. PLoS ONE. 2017, 12, 1–21. [Google Scholar] [CrossRef]
- Li, P.; Ma, B.; Palta, J.A.; Ding, T.; Cheng, Z.; Lv, G.; Xiong, X. Wheat breeding highlights drought tolerance while ignores the advantages of drought avoidance: A meta-analysis. Eur. J. Agron. 2021, 122, 126196. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.-D.; Zeng, Y.; Yang, X.; Anwaar, H.A.; Mansha, M.Z.; Hanif, C.M.S.; Ikram, K.; Ullah, A.; Alghanem, S.M.S. Conferring drought-tolerant wheat genotypes through morpho-physiological and chlorophyll indices at seedling stage. Saudi J. Biol. Sci. 2020, 27, 2116–2123. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Mohammadi, R.; Etminan, A.; Shooshtari, L.; Maleki-Tabrizi, N.; Poczai, P. Effects of drought stress on some agronomic and morpho-physiological traits in Durum wheat genotypes. Sustainability 2020, 12, 5610. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water. 2020, 12, 1–19. [Google Scholar] [CrossRef]
- Anwaar, H.A.; Perveen, R.; Mansha, M.Z.; Abid, M.; Sarwar, M.; Aatif, H.M.; Umar, U.U.D.; Sajid, M.; Aslam, H.M.U.; Alam, M.M.; Rizwan, M.; Ikram, R.M.; Alghanem, S.M.S.; Rashid, A.; Khan, K.A. Assessment of grain yield indices in response to drought stress in wheat (Triticum aestivum L.). Saudi J. Biol. Sci. 2020, 27, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef]
- Anderegg, J.; Yu, K.; Aasen, H.; Walter, A.; Liebisch, F.; Hund, A. Spectral vegetation indices to track senescence dynamics in diverse wheat germplasm. Front. Plant Sci. 2020, 10, 1749. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Hassan, M.A.; Xu, K.; Zheng, C.; Rasheed, A.; Zhang, Y.; Jin, X.; Xia, X.; Xiao, Y.; He, Z. Assessment of water and nitrogen use efficiencies through uav-based multispectral phenotyping in winter wheat. Front. Plant Sci. 2020, 11, 927. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.E.; Al-Suhaibani, N.A.; Elsayed, S.; Hassan, W.M.; Dewir, Y.H.; Refay, Y.; Abdella, K.A. Potential of the existing and novel spectral reflectance indices for estimating the leaf water status and grain yield of spring wheat exposed to different irrigation rates. Agri. Water Manag. 2019, 217, 356–37. [Google Scholar] [CrossRef]
- Qiu, R.C.; Wei, S.; Zhang, M.; Sun, H.; Li, H.; Liu, G.; Li, M. Sensors for measuring plant phenotyping: A review. Int. J. Agric. Biol. Eng. 2018, 11, 1–17. [Google Scholar] [CrossRef]
- Sobejano-Paz, V.; Mikkelsen, T.N.; Baum, A.; Mo, X.; Liu, S.; Köppl, C.J.; Johnson, M.S.; Gulyas, L.; García, M. Hyperspectral and Thermal Sensing of Stomatal Conductance, Transpiration, and Photosynthesis for Soybean and Maize under Drought. Remote Sens. 2020, 12, 3182. [Google Scholar] [CrossRef]
- Andrade-Sanchez, P.; Gore, M.A.; Heun, J.T.; Thorp, K.R.; Carmo-Silva, A.E.; French, A.N.; SalvuccI, M.E.; White, J.W. Development and evaluation of a field-based high-throughput phenotyping platform. Funct. Plant Biol. 2014, 41, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Haghighattalab, A.; Gonzalez, L.; Mondal, S.; Singh, D.; Schinstock, D.; Rutkoski, J.; Ortiz-Monasterio, I.; Singh, R.; Goodin, D.; Poland, J. Application of unmanned aerial systems for high throughput phenotyping of large wheat breeding nurseries. Plant methods. 2016, 35. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.; Vass, I.; Matsubara, S.; Paul, K.; Jedmowski, C.; Pieruschka, R.; Nedbal, L.; Rascher, U.; Muller, O. Maximum fluorescence and electron transport kinetics determined by light-induced fluorescence transients (LIFT) for photosynthesis phenotyping. Photosynth. Res. 2018, 140, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A Physio-Morphological Trait-Based Approach for Breeding Drought Tolerant Wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Knapp, S.; Schmidhalter, U. Advancing High-Throughput Phenotyping of Wheat in Early Selection Cycles. Remote Sens. 2020, 12, 574. [Google Scholar] [CrossRef]
- Yang, M.; Hassan, M.A.; Xu, K.; Zheng, C.; Rasheed, A.; Zhang, Y.; Jin, X.; Xia, X.; Xiao, Y.; He, Z. Assessment of water and nitrogen use efficiencies through uav-based multispectral phenotyping in winter wheat. Front. Plant Sci. 2020, 11, 927. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.N.; Ramos, M.L.G.; Ribeiro Junior, W.Q.; Alencar, E.R.; Silva, P.C.; Lima, C.A.; Vinson, C.C.; Silva, M.A.V. Water stress alters physical and Chemical quality ingrains of common bean, triticale and wheat. Agric. Water Manag. 2020, 231, 16023. [Google Scholar] [CrossRef]
- Tavares, C.J.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Pereira, L.F.; Casari, R.A.C.N.; Pereira, A.F.; de Sousa, C.A.F.; Silva, A.R.; Silva Neto, S.P.; Mertz-Henning, L.M. Water Stress Alters Morphophysiological, Grain Quality and Vegetation Indices of Soybean Cultivars. Plants. 2022, 11, 559. [Google Scholar] [CrossRef]
- Casari, R.A.C.N.; Paiva, D.S.; Silva, V.N.B.; Ferreira, T.M.M.; Souza, M.T.; Oliveira, N.G.; Kobayashi, A.; Molinari, H.B.C.; Santos, T.T.; Gomide, R.L.; Magalhães, P.C.; Sousa, C.A.F. Using thermography to confirm genotypic variation for drought response in maize. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef]
- Vitek, P.; Veselá, V.; Klem, K. Spatial and temporal variability of plant leaf responses cascade after PSII inhibition: raman, chlorophyll fluorescence and infrared thermal imaging. Sensors. 2020, 20, 1015. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.N.; Ramos, M.L.G.; Ribeiro Junior, W.Q.; da Silva, P.C.; Soares, G.F.; Casari, R.A.D.C.N.; Vinson, C.C. Use of Thermography to Evaluate Alternative Crops for Off-Season in the Cerrado Region. Plants. 2023, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Bowman, B.; Chen, J.; Zhang, J.; Wheeler, J.; Wang, Y.; Zhao, W.; Nayak, S.; Heslot, N.; Bockelman, H.; Bonman, J. Evaluating grain yield in spring wheat with canopy spectral reflectance. Crop Sci. 2015, 55, 1881–1890. [Google Scholar] [CrossRef]
- Prey, L.; Schmidhalter, U. Simulation of satellite reflectance data using high-frequency ground based hyperspectral canopy measurements for in-season estimation of grain yield and grain nitrogen status in winter wheat. ISPRS J. Photogramm. Remot. 2019, 149, 176–187. [Google Scholar] [CrossRef]
- Frels, K.; Guttieri, M.; Joyce, B.; Leavitt, B.; Baenziger, P.S. Evaluating canopy spectral reflectance vegetation indices to estimate nitrogen use traits in hard winter wheat. Field Crops Res. 2018, 217, 82–92. [Google Scholar] [CrossRef]
- Damm, A.; Paul-Limoges, E.; Haghighi, E.; Simmer, C.; Morsdorf, F.; Schneider, F.D.; Van Der Tol, C.; Migliavacca, M.; Rascher, U. Remote sensing of plant-water relations, An overview and future perspectives. J. Plant Physiol. 2018, 227, 3–19. [Google Scholar] [CrossRef]
- Cao, Z.; Yao, X.; Liu, H.; Liu, B.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Comparison of the abilities of vegetation indices and photosynthetic parameters to detect heat stress in wheat. Agric. For. Meteorol. 2019, 265, 121–136. [Google Scholar] [CrossRef]
- Ballester, C.; Zarco-Tejada, P.J.; Nicolás, E.; Alarcón, J.J.; Fereres, E.; Intrigliolo, D.S.; Gonzalez-Dugo, V. Evaluating the performance of xanthophyll, chlorophyll and structure-sensitive spectral indices to detect water stress in five fruit tree species. Precis. Agric. 2018, 19, 178–193. [Google Scholar] [CrossRef]
- Marček, T.; Hamow, K.; Végh, B.; Janda, T.; Darko, E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE. 2019, 14, 1–23. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Zhan, A.; Lynch, J. P. Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J. Exp. Bot. 2015, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Young, I.M.; Hallett, P.D.; White, P.J. Root hairs improve root penetration, root – soil contact, and phosphorus acquisition in soils of different strength. J. Exp. Bot. 2013, 64, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, Q.; Blłaszkiewicz, Z.; Sun, J.; Sun, Q.; He, R.; Li, Y. Phenotyping for the dynamics of field wheat root system architecture. Sci. Rep. 2017, 7, 37649. [Google Scholar] [CrossRef]
- Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.M.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Tavera, V.C.; Mancilla, C.L.A.; Gallegos, J.A.C.; Pimentel, J.G.R.; Arriaga, A.I.M.; Ruiz Nietto, J.E. Mechanisms for water use efficiency between bean cultivars tolerant to drought are different. Acta Sci. Agron. 2018, 40, e39378. [Google Scholar] [CrossRef]
- Soil Survey Staff. 2014. ‘Keys to Soil Taxonomy’. In: Burt, R., Soil Survey Staff (Eds.), Soil Survey Field and Laboratory Methods Manual. Soil Survey Investigations Report No. 51, Version 2.0. U.S. Department of Agriculture, Natural Resources Conservation Service. Washington, DC.
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Embrapa. 2011. Programa de Monitoramento da Irrigação. EMBRAPA, Brasília.
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration: guidelines for computing crop water requirements. FAO Irrigation and Drainage Paper, 1998, 56, Rome, p. 300.
- Jayme-Oliveira, A.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Ziviani, A.C.; Jakelaitis, A. Amaranth, quinoa, and millet growth and development under different water regimes in the Brazilian Cerrado. Pesqui. Agropecu. Bras. 2017, 52, 561–571. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Rigon, J.P.G.; Capuani, S.; Beltrão, N.M.; Brito Neto, J.F.; Sofiatti, V.; França, V.F. Non-destructive determination of photosynthetic pigments in the leaves of castor oil plants. Acta Sci. Agron. 2012, 34, 325–329. [Google Scholar] [CrossRef]
- Ratke, R.F.; Pereira, H.S.; Santos Junior, D.G.; Frazão, J.J.; Barbosa, J.M.; Dias, B.O. Root growth, nutrition and yield of maize with applied different limestone particle size in the cerrado soil. Am. J. Plant Sci. 2014, 5, 463–472. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Sauer, T.J.; Prueger, J.H. Managing soils to achieve greater water use efficiency: a review. Agronomy. 2001, 93, 271–280. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Mojena, R. Hierarchical grouping methods and stopping rules: an evaluation. J. Comput. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant Breed. 1981, 41, 237–245. [Google Scholar]
- Gabriel, K.R. The biplot graphical display of matrices with application to principal component analysis. Biometrika. 1971, 58, 453–467. [Google Scholar] [CrossRef]
| Source | Df | Pillai | Approx F | Num Df | Den Df | Probabilty |
|---|---|---|---|---|---|---|
| Year | 1 | 0.882 | 44.831 | 31 | 186 | 2.2e-16 ** |
| Genotypes | 17 | 6.5224 | 4.056 | 527 | 3434 | 2.2e-16 ** |
| Water regime | 3 | 2.6453 | 45.234 | 93 | 564 | 2.2e-16 ** |
| Year:Block | 4 | 2.2584 | 7.906 | 124 | 756 | 2.2e-16 ** |
| Year:Genotypes | 17 | 1.6662 | 0.708 | 527 | 3434 | 1ns |
| Year:Water regime | 3 | 0.9002 | 2.600 | 93 | 564 | 7.026e-12 ** |
| Genotypes:Water regime | 51 | 9.6958 | 1.928 | 1581 | 564 | 2.2e-16 ** |
| Year:Block:Genotypes | 68 | 9.9413 | 1.500 | 2108 | 6699 | 2.2e-16 ** |
| Year:Genotypes:Water regime | 51 | 1.8329 | 0.266 | 1581 | 6699 | 1ns |
| Residuals | 216 |
| G1/ | WR | Variables | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDVI | SAVI | PRI | DVI | GRVI | GNDVI | NDRE | TCARI | OSAVI | TO | GH | MTG | GY | |||
| 1 | 22 | 0.27 | 0.18 | 0.1 | 1.81 | 3.09 | 0.5 | 0.12 | 0.12 | 0.22 | 0.54 | 10.5 | 32.97 | 2337 | |
| 43 | 0.45 | 0.28 | 0.16 | 3.17 | 3.89 | 0.58 | 0.25 | 0.14 | 0.36 | 0.4 | 10.8 | 35.19 | 3691 | ||
| 81 | 0.62 | 0.38 | 0.21 | 5.58 | 4.7 | 0.63 | 0.34 | 0.18 | 0.49 | 0.38 | 11.3 | 37.56 | 5076 | ||
| 100 | 0.65 | 0.39 | 0.22 | 6.27 | 5.06 | 0.65 | 0.35 | 0.18 | 0.51 | 0.37 | 11.4 | 38.74 | 5604 | ||
| 2 | 22 | 0.29 | 0.19 | 0.12 | 1.93 | 3.15 | 0.51 | 0.14 | 0.12 | 0.24 | 0.54 | 11.2 | 33.68 | 1983 | |
| 43 | 0.47 | 0.3 | 0.17 | 3.38 | 3.97 | 0.58 | 0.26 | 0.15 | 0.38 | 0.41 | 10.9 | 36.2 | 3913 | ||
| 81 | 0.63 | 0.39 | 0.23 | 5.91 | 4.82 | 0.63 | 0.35 | 0.18 | 0.5 | 0.37 | 11.6 | 36.99 | 5095 | ||
| 100 | 0.65 | 0.41 | 0.23 | 6.6 | 5.2 | 0.66 | 0.37 | 0.18 | 0.52 | 0.36 | 11.8 | 38.43 | 5439 | ||
| 3 | 22 | 0.24 | 0.16 | 0.09 | 1.67 | 2.93 | 0.49 | 0.11 | 0.11 | 0.2 | 0.55 | 10.3 | 32.43 | 2366 | |
| 43 | 0.4 | 0.24 | 0.14 | 2.56 | 3.51 | 0.55 | 0.21 | 0.13 | 0.31 | 0.43 | 10.3 | 36.31 | 4013 | ||
| 81 | 0.6 | 0.37 | 0.21 | 5.04 | 4.38 | 0.61 | 0.31 | 0.19 | 0.47 | 0.4 | 10.6 | 39.97 | 5653 | ||
| 100 | 0.64 | 0.39 | 0.22 | 6.04 | 4.86 | 0.64 | 0.33 | 0.19 | 0.51 | 0.39 | 10.8 | 40.43 | 6052 | ||
| Mean | 0.49 | 0.31 | 0.17 | 4.16 | 4.13 | 0.59 | 0.26 | 0.16 | 0.39 | 0.43 | 10.9 | 36.57 | 4269 | ||
| SE | 0.05 | 0.03 | 0.01 | 0.55 | 0.24 | 0.02 | 0.03 | 0.01 | 0.04 | 0.02 | 0.15 | 0.76 | 414.5 | ||
| CV | 3.28 | 3.1 | 2.97 | 4.62 | 1.98 | 1.03 | 3.67 | 2.02 | 3.19 | 1.66 | 0.46 | 0.72 | 3.36 | ||
| G1/ | WR | Variables | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WUE | Cha | Chb | RM | SM | A | gs | iWUE | Ci | E | Fv’/Fm’ | ETR | Fv/Fm | DRI | |||
| 22 | 22.7 | 30.9 | 7.2 | 4.9 | 10.4 | 8.7 | 0.09 | 121.9 | 188 | 2.5 | 0.46 | 122 | 0.8 | |||
| 1 | 43 | 19.5 | 35.5 | 10.5 | 5 | 12.2 | 15.8 | 0.13 | 96.8 | 204 | 3.2 | 0.5 | 144 | 0.82 | 1.01 | |
| 81 | 14.3 | 39.4 | 14.1 | 5.8 | 15.7 | 22.3 | 0.33 | 67.8 | 248 | 6.3 | 0.57 | 148 | 0.82 | |||
| 100 | 12.5 | 41.0 | 16.0 | 5.7 | 15.6 | 22.7 | 0.48 | 47.2 | 275 | 8.7 | 0.59 | 151 | 0.83 | |||
| 22 | 20.3 | 32.0 | 8.2 | 5.4 | 11.1 | 9.7 | 0.09 | 125.6 | 193 | 2.5 | 0.47 | 133 | 0.81 | |||
| 2 | 43 | 19.1 | 35.3 | 11.4 | 9.8 | 12.9 | 15.1 | 0.12 | 108.4 | 175 | 3.2 | 0.51 | 146 | 0.83 | 0.95 | |
| 81 | 14.1 | 40.2 | 16.1 | 6.1 | 13.6 | 21.6 | 0.39 | 55.5 | 263 | 7.8 | 0.6 | 150 | 0.83 | |||
| 100 | 12.2 | 41.6 | 16.7 | 5.6 | 15.2 | 22.1 | 0.53 | 41.8 | 273 | 9.9 | 0.6 | 164 | 0.83 | |||
| 22 | 22.0 | 27.4 | 5.4 | 4.8 | 9.9 | 8.1 | 0.09 | 127.1 | 168 | 2.6 | 0.4 | 99 | 0.8 | |||
| 3 | 43 | 20.3 | 32.6 | 8.2 | 5.1 | 11.6 | 16.0 | 0.1 | 112.0 | 144 | 2.9 | 0.5 | 116 | 0.81 | 0.93 | |
| 81 | 16.0 | 38.6 | 13 | 5.2 | 15.7 | 23.1 | 0.41 | 56.4 | 259.9 | 7.68 | 0.6 | 159 | 0.82 | |||
| 100 | 13.6 | 40.2 | 14.2 | 5.5 | 17.1 | 23.9 | 0.61 | 49.3 | 294.9 | 11.55 | 0.6 | 142 | 0.83 | |||
| Mean SE CV |
17.2 | 36.1 | 11.7 | 5.7 | 13.4 | 17.4 | 0.28 | 84.1 | 224.2 | 5.75 | 0.5 | 140 | 0.82 | 0.96 | ||
| 1.1 | 1.34 | 1.1 | 0.4 | 0.7 | 1.7 | 0.06 | 4.87 | 14.56 | 0.96 | 0.02 | 5.49 | 0.09 | 0.01 | |||
| 2.2 | 1.28 | 3.3 | 2.3 | 1.8 | 3.4 | 7.05 | 1.12 | 2.25 | 5.77 | 1.29 | 1.36 | 0.13 | 0.11 | |||
| Variables | Percentage (%) |
|---|---|
| SAVI | 33 |
| OSAVI | 33 |
| DVI | 11 |
| NDRE | 11 |
| GNDVI | 3 |
| NDGI | 2 |
| RVI | 2 |
| NDVI | 1 |
| GRVI | 1 |
| GY | 1 |
| Other | < 2 |
| Variable | Group of genotype | Equation | R squared |
|---|---|---|---|
| Main Coordinate 1 (latent variable) | 1 | 0.98 | |
| 2 | 0.98 | ||
| 3 | 0.98 | ||
| Grain yield (kg ha-1) | 1 | 0.99 | |
| 2 | 0.99 | ||
| 3 | 0.99 | ||
| Net CO2 assimilation (A, µmol CO2 m-2 s-1) | 1 | 0.99 | |
| 2 | 0.99 | ||
| 3 | 0.99 | ||
| Normalized Difference Vegetation Index (NDVI) | 1 | 0.99 | |
| 2 | 0.99 | ||
| 3 | 0.99 | ||
| Water use efficiency (WUE) | 1 | 0.98 | |
| 2 | 0.99 | ||
| 3 | 0.99 | ||
| Intrinsic water use efficiency (iWUE) | 1 | 0.96 | |
| 2 | 0.98 | ||
| 3 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





