Submitted:
17 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Design
2.3. Statistical Analysis
3. Results
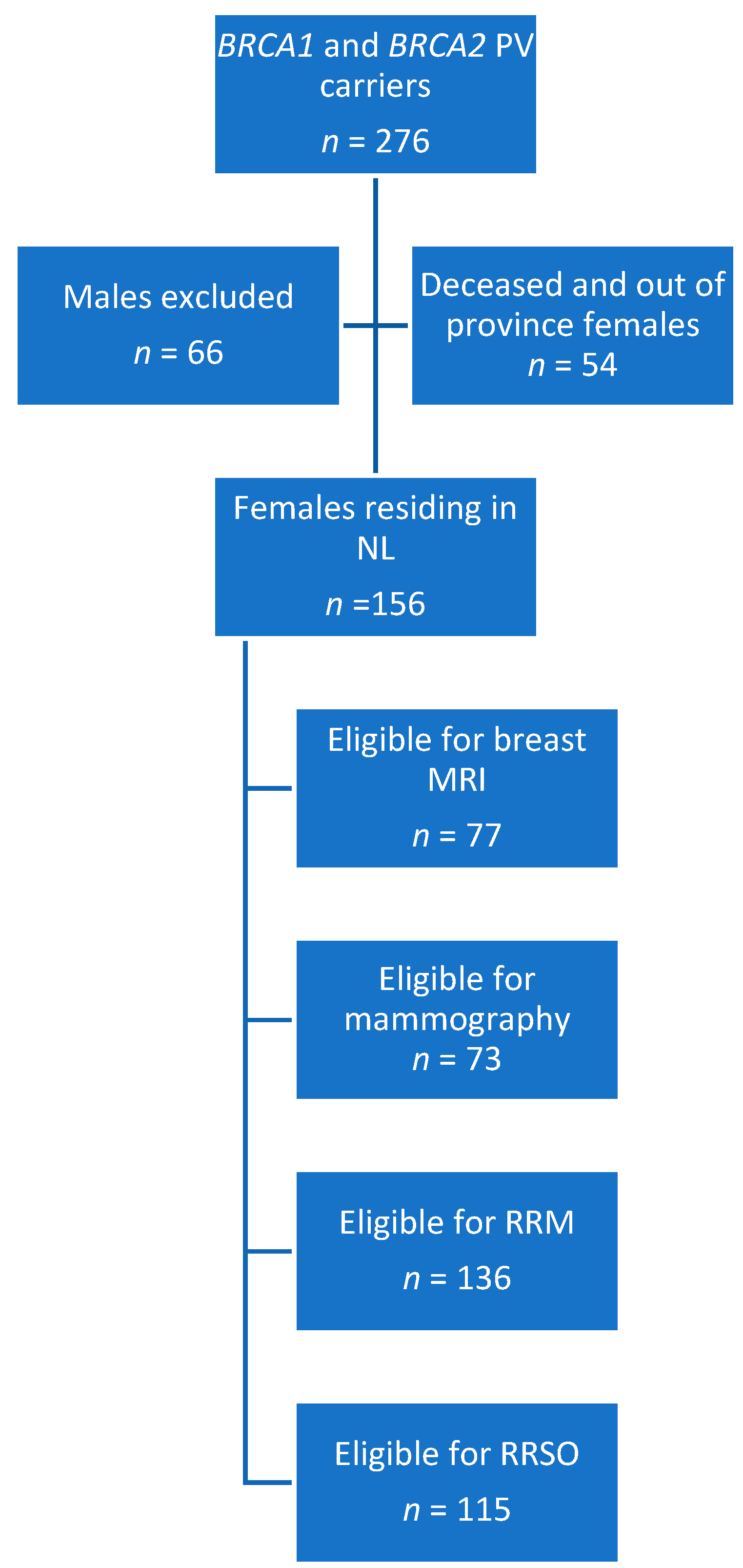
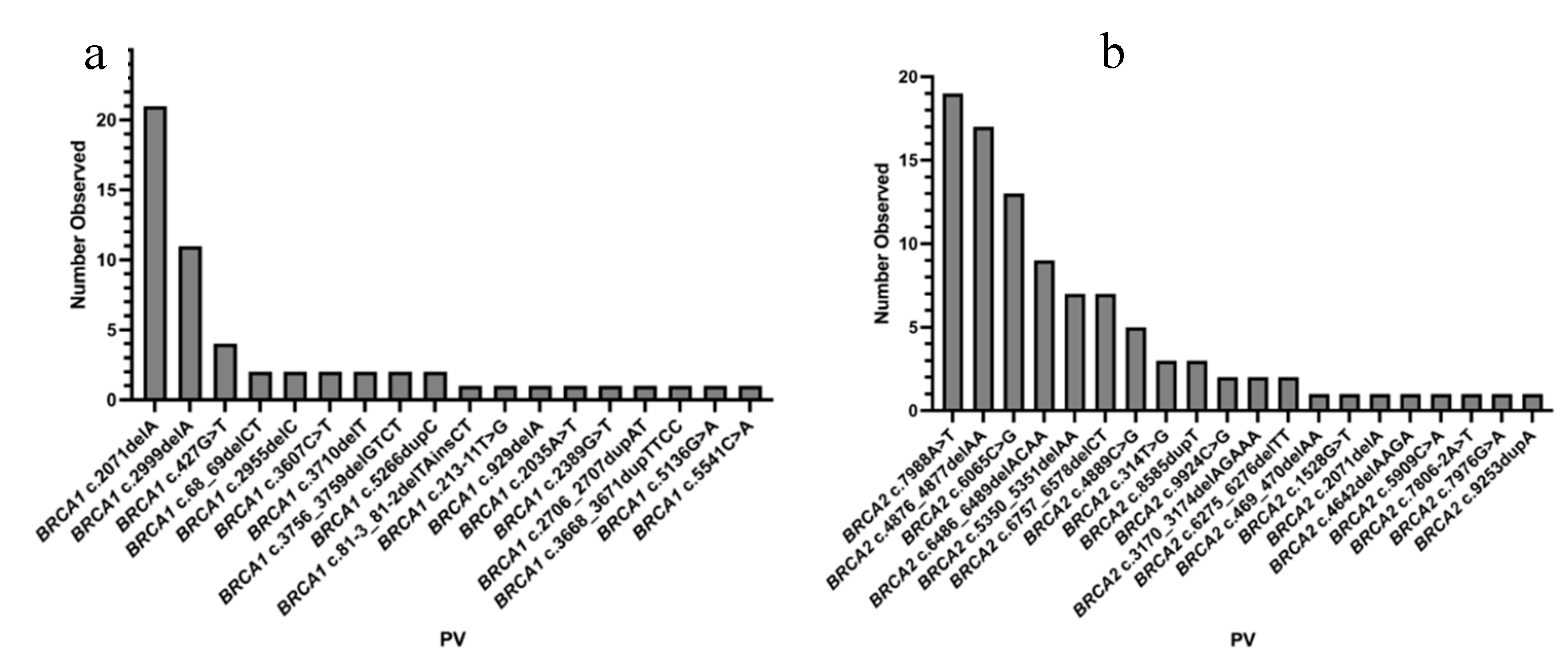
3.1. Study Population
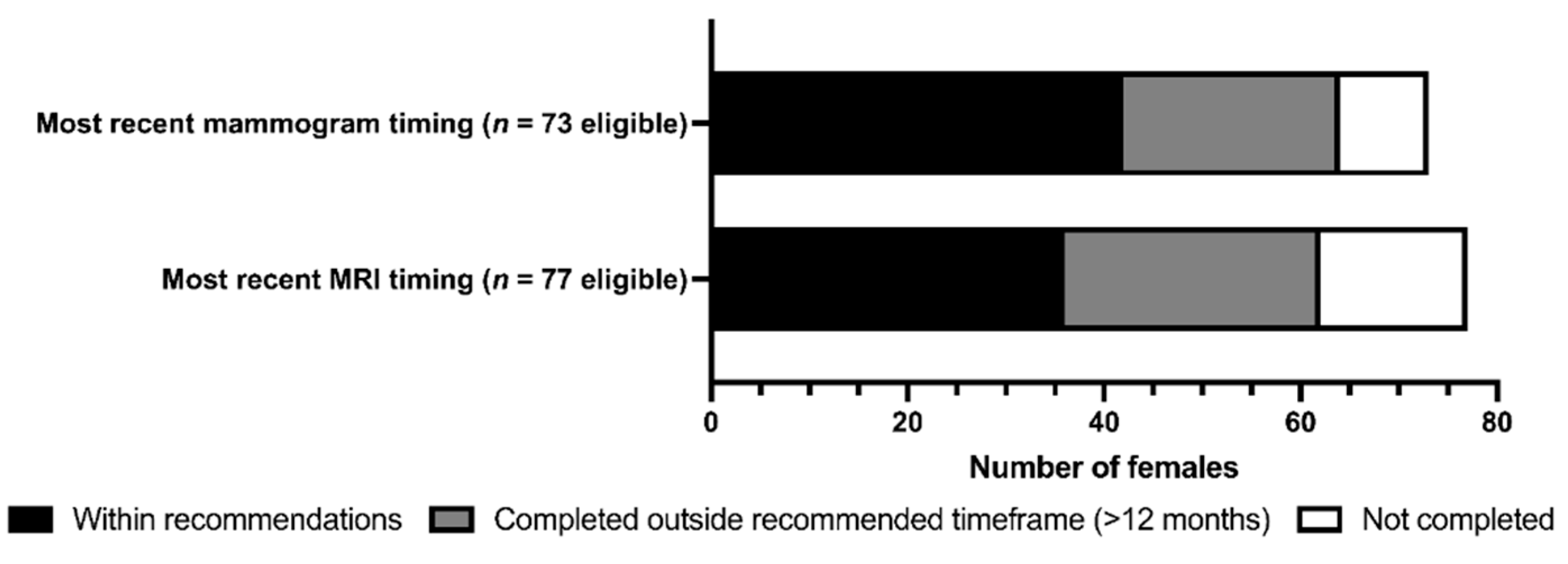
3.2. Screening and Preventative Interventions
4. Discussion
4.1. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.C.; Nathanson, K.L.; et al. Association of Type and Location of BRCA1 and BRCA2 Mutations with Risk of Breast and Ovarian Cancer. JAMA - Journal of the American Medical Association 2015, 313. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; Van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA - Journal of the American Medical Association 2017, 317. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Cancer 2008. [Google Scholar]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA: A Cancer Journal for Clinicians 2018, 68. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y.; Swensen, J.; Shattuck-Eidens, D.; Futreal, P.A.; Harshman, K.; Tavtigian, S.; Liu, Q.; Cochran, C.; Bennett, L.M.; Ding, W.; et al. A Strong Candidate for the Breast and Ovarian Cancer Susceptibility Gene BRCA1. Science 1994, 266. [Google Scholar] [CrossRef]
- Wooster, R.; Bignell, G.; Lancaster, J.; Swift, S.; Seal, S.; Mangion, J.; Collins, N.; Gregory, S.; Gumbs, C.; Micklem, G.; et al. Identification of the Breast Cancer Susceptibility Gene BRCA2. Nature 1995, 378. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M. Meta-Analysis of Risk Reduction Estimates Associated with Risk-Reducing Salpingo-Oophorectomy in BRCA1 or BRCA2 Mutation Carriers. Journal of the National Cancer Institute 2009, 101. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Gareth Evans, D.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of Risk-Reducing Surgery in BRCA1 or BRCA2 Mutation Carriers with Cancer Risk and Mortality. JAMA 2010, 304. [Google Scholar] [CrossRef]
- Godet, I.; M. Gilkes, D. BRCA1 and BRCA2 Mutations and Treatment Strategies for Breast Cancer. Integrative Cancer Science and Therapeutics 2017, 4. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; AlHilli, Z.; Arun, B.; Buys, B.B.; Cheng, H.; Churpek, J.; Domchek, S.M.; Friedman, S.; Giri, V.; et al. Genetic/Familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2024. National Comprehensive Cancer Network; 2023.
- Klarenbach, S.; Sims-Jones, N.; Lewin, G.; Singh, H.; Thériault, G.; Tonelli, M.; Doull, M.; Courage, S.; Garcia, A.J.; Thombs, B.D.; et al. Recommendations on Screening for Breast Cancer in Women Aged 40-74 Years Who Are Not at Increased Risk for Breast Cancer. CMAJ. Canadian Medical Association Journal 2018, 190. [Google Scholar] [CrossRef]
- Warner, E.; Hill, K.; Causer, P.; Plewes, D.; Jong, R.; Yaffe, M.; Foulkes, W.D.; Ghadirian, P.; Lynch, H.; Couch, F.; et al. Prospective Study of Breast Cancer Incidence in Women with a BRCA1 or BRCA2 Mutation under Surveillance with and without Magnetic Resonance Imaging. Journal of Clinical Oncology 2011, 29. [Google Scholar] [CrossRef] [PubMed]
- Passaperuma, K.; Warner, E.; Causer, P.A.; Hill, K.A.; Messner, S.; Wong, J.W.; Jong, R.A.; Wright, F.C.; Yaffe, M.J.; Ramsay, E.A.; et al. Long-Term Results of Screening with Magnetic Resonance Imaging in Women with BRCA Mutations. British Journal of Cancer 2012, 107. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Kesavan, N.; Lim, Y.; Gadde, S.; Hurley, E.; Massat, N.J.; Maxwell, A.J.; Ingham, S.; Eeles, R.; Leach, M.O.; et al. MRI Breast Screening in High-Risk Women: Cancer Detection and Survival Analysis. Breast Cancer Res Treat 2014, 145, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, S.; Obdeijn, I.M.; Rutgers, E.J.; Oosterwijk, J.C.; Tollenaar, R.A.; Woldringh, G.H.; Bergers, E.; Verhoef, C.; Heijnsdijk, E.A.; Hooning, M.J.; et al. Survival Benefit in Women with BRCA1 Mutation or Familial Risk in the MRI Screening Study (MRISC). International Journal of Cancer 2015, 137. [Google Scholar] [CrossRef]
- Warner, E. Screening BRCA1 and BRCA2 Mutation Carriers for Breast Cancer. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; You, R.; Wang, X.; Liu, C.; Xu, Z.; Zhou, J.; Yu, B.; Xu, T.; Cai, H.; Zou, Q. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-Analysis and Systematic Review. Clinical Cancer Research 2016, 22. [Google Scholar] [CrossRef]
- Kenkhuis, M.J.A.; de Bock, G.H.; Oude Elferink, P.; Arts, H.J.G.; Oosterwijk, J.C.; Jansen, L.; Mourits, M.J.E. Short-Term Surgical Outcome and Safety of Risk Reducing Salpingo-Oophorectomy in BRCA1/2 Mutation Carriers. Maturitas 2010, 66. [Google Scholar] [CrossRef]
- Johansen, N.; Liavaag, A.H.; Iversen, O.E.; Dørum, A.; Braaten, T.; Michelsen, T.M. Use of Hormone Replacement Therapy after Risk-Reducing Salpingo-Oophorectomy. Acta Obstetricia et Gynecologica Scandinavica 2017, 96. [Google Scholar] [CrossRef]
- Sahni, S.; Lobo-Romero, A.; Smith, T. Contemporary Non-Hormonal Therapies for the Management of Vasomotor Symptoms Associated with Menopause: A Literature Review. European Endocrinology 2021, 17. [Google Scholar] [CrossRef] [PubMed]
- Kauff, N.D.; Domchek, S.M.; Friebel, T.M.; Robson, M.E.; Lee, J.; Garber, J.E.; Isaacs, C.; Evans, D.G.; Lynch, H.; Eeles, R.A.; et al. Risk-Reducing Salpingo-Oophorectomy for the Prevention of BRCA1- and BRCA2-Associated Breast and Gynecologic Cancer: A Multicenter, Prospective Study. Journal of Clinical Oncology 2008, 26. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.P.M.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of Oophorectomy on Cancer Incidence and Mortality in Women with a BRCA1 or BRCA2 Mutation. Journal of Clinical Oncology 2014, 32. [Google Scholar] [CrossRef] [PubMed]
- Gaba, F.; Blyuss, O.; Tan, A.; Munblit, D.; Oxley, S.; Khan, K.; Legood, R.; Manchanda, R. Breast Cancer Risk and Breast-Cancer-Specific Mortality Following Risk-Reducing Salpingo-Oophorectomy in BRCA Carriers: A Systematic Review and Meta-Analysis. Cancers 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Plevritis, S.K.; Kurian, A.W.; Sigal, B.M.; Daniel, B.L.; Ikeda, D.M.; Stockdale, F.E.; Garber, A.M. Cost-Effectiveness of Screening BRCA1/2 Mutation Carriers with Breast Magnetic Resonance Imaging. JAMA 2006, 295. [Google Scholar] [CrossRef] [PubMed]
- Petelin, L.; Trainer, A.H.; Mitchell, G.; Liew, D.; James, P.A. Cost-Effectiveness and Comparative Effectiveness of Cancer Risk Management Strategies in BRCA1/2 Mutation Carriers: A Systematic Review. Genetics in Medicine 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.; Eccleston, A.; Hurry, M.; Dyer, M. Targeted Surgical Prevention of Epithelial Ovarian Cancer Is Cost Effective and Saves Money in BRCA Mutation Carrying Family Members of Women with Epithelial Ovarian Cancer. A Canadian Model. Gynecologic Oncology 2019, 153. [Google Scholar] [CrossRef]
- Statistics Canada. Population and dwelling count highlight tables, 2021 census. Ottawa, ON: Statistics Canada; 2022.
- Zhai, G.; Zhou, J.; Woods, M.O.; Green, J.S.; Parfrey, P.; Rahman, P.; Green, R.C. Genetic Structure of the Newfoundland and Labrador Population: Founder Effects Modulate Variability. European Journal of Human Genetics 2016, 24. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. ; on behalf of the ESMO Guidelines Committee Prevention and Screening in BRCA Mutation Carriers and Other Breast/Ovarian Hereditary Cancer Syndromes: ESMO Clinical Practice Guidelines for Cancer Prevention and Screening. Annals of Oncology 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstetrics & Gynecology 2017, 130. [CrossRef]
- Jacobson, M.; Bernardini, M.; Sobel, M.L.; Kim, R.H.; McCuaig, J.; Allen, L. No. 366-Gynaecologic Management of Hereditary Breast and Ovarian Cancer. Journal of Obstetrics and Gynaecology Canada 2018, 40. [Google Scholar] [CrossRef]
- Metcalfe, K.; Eisen, A.; Senter, L.; Armel, S.; Bordeleau, L.; Meschino, W.S.; Pal, T.; Lynch, H.T.; Tung, N.M.; Kwong, A.; et al. International Trends in the Uptake of Cancer Risk Reduction Strategies in Women with a BRCA1 or BRCA2 Mutation. British Journal of Cancer 2019, 121. [Google Scholar] [CrossRef]
- Buchanan, A.H.; Voils, C.I.; Schildkraut, J.M.; Fine, C.; Horick, N.K.; Marcom, P.K.; Wiggins, K.; Skinner, C.S. Adherence to Recommended Risk Management among Unaffected Women with a BRCA Mutation. J Genet Couns 2017, 26, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Hanley, G.E.; McAlpine, J.N.; Cheifetz, R.; Schrader, K.A.; McCullum, M.; Huntsman, D. Selected Medical Interventions in Women with a Deleterious BRCA Mutation: A Population-Based Study in British Columbia. Current Oncology 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Isaacs, C.; Graves, K.D.; Poggi, E.; Peshkin, B.N.; Gell, C.; Finch, C.; Kelly, S.; Taylor, K.L.; Perley, L. Long-Term Outcomes of BRCA1/BRCA2 Testing: Risk Reduction and Surveillance. Cancer 2012, 118. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Wendt, J.; Lyon, L.; Jones, J.; Littell, R.D.; Armstrong, M.A.; Raine-Bennett, T.; Powell, C.B. Risk Management Options Elected by Women after Testing Positive for a BRCA Mutation. Gynecologic Oncology 2014, 132. [Google Scholar] [CrossRef] [PubMed]
- Dicks, E.; Roome, R.; Chafe, J.; Powell, E.; McCrate, F.; Simmonds, C.; Etchegary, H. Factors Influencing Surgical Treatment Decisions for Breast Cancer: A Qualitative Exploration of Surgeon and Patient Perspectives. Current Oncology 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.G.; Arts-de Jong, M.; Horstik, K.; Manders, P.; Massuger, L.F.A.G.; Hermens, R.P.M.G.; Hoogerbrugge, N.; Woldringh, G.H.; de Hullu, J.A. Very High Uptake of Risk-Reducing Salpingo-Oophorectomy in BRCA1/2 Mutation Carriers: A Single-Center Experience. Gynecologic Oncology 2016, 143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Lim, M.C.; Lee, D.O.; Kong, S.Y.; Seo, S.S.; Kang, S.; Lee, E.S.; Park, S.Y. Uptake of Risk-Reducing Salpingo-Oophorectomy among Female BRCA Mutation Carriers: Experience at the National Cancer Center of Korea. Journal of Cancer Research and Clinical Oncology 2016, 142. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.; Dawson, L.M.; MacMillan, A.; Warden, G.; Green, J. Analysis of BRCA Mutations in Newfoundland and Labrador: Is There Evidence of a Founder Effect? Journal of Obstetrics and Gynaecology Canada 2013, 35. [Google Scholar]
- Petty, E.M.; Green, J.S.; Marx, S.J.; Taggart, R.T.; Farid, N.; Bale, A.E. Mapping the Gene for Hereditary Hyperparathyroidism and Prolactinoma (MEN1Burin) to Chromosome 11q: Evidence for a Founder Effect in Patients from Newfoundland. Am J Hum Genet 1994, 54, 1060–1066. [Google Scholar]
- Green, R.C.; Narod, S.A.; Morasse, J.; Young, T.L.; Cox, J.; Fitzgerald, G.W.; Tonin, P.; Ginsburg, O.; Miller, S.; Jothy, S. Hereditary Nonpolyposis Colon Cancer: Analysis of Linkage to 2p15-16 Places the COCA1 Locus Telomeric to D2S123 and Reveals Genetic Heterogeneity in Seven Canadian Families. Am J Hum Genet 1994, 54, 1067–1077. [Google Scholar]
- Dawson, L.M.; Smith, K.N.; Werdyani, S.; Ndikumana, R.; Penney, C.; Wiede, L.L.; Smith, K.L.; Pater, J.A.; MacMillan, A.; Green, J.; et al. A Dominant RAD51C Pathogenic Splicing Variant Predisposes to Breast and Ovarian Cancer in the Newfoundland Population Due to Founder Effect. Mol Genet Genomic Med 2020, 8, e1070. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics 2021. Available online: cancer.ca/Canadian-Cancer-Statistics-2021-EN (accessed on 1 September 2023).
- Metcalfe, K.A.; Ghadirian, P.; Rosen, B.; Foulkes, W.; Kim-Sing, C.; Eisen, A.; Ainsworth, P.; Horsman, D.; Maugard, C.; Provencher, D.; et al. Variation in Rates of Uptake of Preventive Options by Canadian Women Carrying the BRCA1 or BRCA2 Genetic Mutation. Open medicine : a peer-reviewed, independent, open-access journal 2007, 1. [Google Scholar]
- Simard, J.; Dumont, M.; Moisan, A.M.; Gaborieau, V.; Vézina, H.; Durocher, F.; Chiquette, J.; Plante, M.; Avard, D.; Bessette, P.; et al. Evaluation of BRCA1 and BRCA2 Mutation Prevalence, Risk Prediction Models and a Multistep Testing Approach in French-Canadian Families with High Risk of Breast and Ovarian Cancer. Journal of Medical Genetics 2007, 44. [Google Scholar] [CrossRef]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.C.; Rosen, B.; Bradley, L.; Fan, I.; Tang, J.; Li, S.; Zhang, S.; Shaw, P.A.; et al. Population BRCA1 and BRCA2 Mutation Frequencies and Cancer Penetrances: A Kin-Cohort Study in Ontario, Canada. Journal of the National Cancer Institute 2006, 98. [Google Scholar] [CrossRef] [PubMed]
- Rowley, S.M.; Mascarenhas, L.; Devereux, L.; Li, N.; Amarasinghe, K.C.; Zethoven, M.; Lee, J.E.A.; Lewis, A.; Morgan, J.A.; Limb, S.; et al. Population-Based Genetic Testing of Asymptomatic Women for Breast and Ovarian Cancer Susceptibility. Genetics in Medicine 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Abul-Husn, N.S.; Soper, E.R.; Odgis, J.A.; et al. Exome sequencing reveals a high prevalence of BRCA1 and BRCA2 founder variants in a diverse population-based biobank. Genome Med 2020, 12, 2. [Google Scholar] [CrossRef]
- Saletta, F.; Dalla Pozza, L.; Byrne, J.A. Genetic Causes of Cancer Predisposition in Children and Adolescents. Translational pediatrics 2015, 4. [Google Scholar] [CrossRef]
- Vetter, L.; Keller, M.; Bruckner, T.; Golatta, M.; Eismann, S.; Evers, C.; Dikow, N.; Sohn, C.; Heil, J.; Schott, S. Adherence to the Breast Cancer Surveillance Program for Women at Risk for Familial Breast and Ovarian Cancer versus Overscreening: A Monocenter Study in Germany. Breast Cancer Research and Treatment 2016, 156. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.Y.; Jie, Y.E.; Cheng, S.W.; Ling, G.L.; Ming, H.V.Y. PARP Inhibitors in Breast and Ovarian Cancer. Cancers 2023, 15. [Google Scholar] [CrossRef]
| Characteristic | Overall | BRCA1 PV | BRCA2 PV | p-value* |
| Total (n) | 156 | 36.5% (57) | 63.5% (99) | < 0.001 |
| Age at time of study (y) | ||||
| Mean (± SD) | 53.3 ± 14.2 | 52.1 ± 13.6 | 54.0 ± 14.5 | 0.412 |
| Median | 54.0 | 54.0 | 53.0 | |
| Range | 24-89 | 24-79 | 28-89 | |
| Age at genetic testing (y) | ||||
| Mean (± SD) | 47.1 ± 14.0 | 43.7 ± 12.7 | 49.1 ± 14.4 | 0.020 |
| Median | 45.5 | 42.0 | 50.0 | |
| Range | 18-83 | 18-67 | 24-83 | |
| Genetic testing after BC/OC diagnosis | 31.4% (49) | 79.2% (19) | 96.8% (30) | 0.038 |
| BC and/or OC (n) | 36.5% (57) | 45.6% (26) | 31.3% (31) | 0.074 |
| BC (n) | 32.7% (51) | 40.4% (23) | 28.3% (28) | 0.122 |
| Age at BC diagnosis (y) | ||||
| Mean (± SD) | 46.5 y ± 9.7 | 43.8 ± 9.5 y | 48.5 ± 9.5 y | 0.091 |
| Median | 44.0 | 42.0 | 49.0 | |
| Range | 31-65 | 31-64 | 32-65 | |
| OC (n) | 5.1% (8) | 7.0% (4) | 4.0% (4) | 0.417 |
| Age at OC diagnosis (y) | ||||
| Mean (± SD) | 51.6 ± 11.4 | 47.5 ± 13.0 | 55.8 ± 9.5 y | 0.344 |
| Median | 52.0 | 47.5 | 56.5 | |
| Range | 32-66 | 32-63 | 44-66 | |
| Assessed by medical oncology (n) | 56.0% (84) | 45.5% (25) | 62.1% (59) | 0.048 |
| Assessed by ICPC (n) | 56.7% (85) | 49.1% (27) | 61.1% (58) | 0.154 |
| Received specialty care† (n) | 77.3% (116) | 67.3% (37) | 83.2% (79) | 0.025 |
| Primary care provider on record (n) | 95.7% (135) | 96.3% (52) | 95.4% (83) | 0.798 |
| Rural residence‡ (n) | 39.1% (61) | 35.1% (20) | 41.4% (41) | 0.436 |
| Patient* | Tumour type | Age of onset | Tumour histology | Stage | Grade | Receptor status† |
| 1 | Breast | |||||
| 2 | Breast | 49 | IDC | II | ER+PR+Her2- | |
| 3 | Breast | 33 | IDC | II | ER-PR-Her2- | |
| 4 | Breast | 42 | DCIS | II | ER-PR- | |
| 5 | Breast | 37 | IDC | II | 3 | ER-PR-Her2- |
| 6 | Breast | 34 | IDC | 3 | ER+PR-Her2- | |
| 7 | Breast | 40 | IDC | 3 | ||
| 8 | Breast | 34 | II | ER-PR- | ||
| 9 | Ovary | 63 | Serous | II | 3 | n/a |
| 10 | Breast | 50 | ER-PR-Her2- | |||
| 11 | Breast | 48 | IDC | II | 3 | ER-PR-Her2- |
| 12 | Breast | 31 | IDC | |||
| 13 | Breast | 50 | IDC | I | 3 | ER-PR- |
| 14 | Breast | 42 | IDC | II | 3 | ER+PR+Her2- |
| 15 | Breast | 34 | IDC | II | 3 | ER+PR+Her2+ |
| 16 | Breast | 37 | IDC | I | 3 | ER-PR-Her2- |
| 17 | Breast | 41 | MDC | I | ER-PR+ | |
| 18 | Breast | 65 | IDC | |||
| 19 | Breast | 64 | IDC | II | 3 | ER-PR-Her2- |
| 20 | Breast | 41 | DCIS | I | ||
| 21 | Breast | 33 | IDC | |||
| 22 | Breast | 42 | DCIS | |||
| 23 | Breast | 41 | IDC | II | 3 | ER-PR- |
| 24 | Breast | 37 | IDC | |||
| 25 | Breast | 64 | IDC | I | 3 | ER-PR-Her2- |
| 26 | Breast | 51 | IDC | II | 3 | ER+PR-Her2- |
| 27 | Breast | 56 | IDC | I | 1 | ER+PR+Her2- |
| 28 | Breast | IDC | ||||
| 29 | Breast | 44 | IDC | II | 3 | ER-PR- |
| 30 | Breast | 40 | IDC | I | ER-PR- | |
| Ovary | 60 | n/a | ||||
| 31 | Breast | 61 | IDC | 2 | ER+PR+Her2- | |
| 32 | Breast | 57 | IDC | III | 3 | ER-PR-Her2- |
| 33 | Breast | 53 | DCIS | 3 | ||
| 34 | Breast | 51 | IDC | II | 3 | ER-PR-Her2+ |
| 35 | Breast | 36 | IDC | II | 3 | ER-PR- |
| 36 | Breast | 43 | IDC | II | 3 | ER-PR-Her2- |
| 37 | Breast | 44 | IDC | I | 3 | ER-PR-Her2- |
| 38 | Breast | 45 | IDC | II | 2 | ER+PR+Her2- |
| 39 | Breast | 42 | DCIS | 0 | 2 | ER+PR+Her2+ |
| 40 | Ovary | 53 | Serous | n/a | ||
| 41 | Breast | 60 | IDC | II | 3 | ER+PR+Her2- |
| Ovary | 51 | n/a | ||||
| 42 | Breast | 55 | IDC | I | 3 | ER-PR-Her2- |
| 43 | Breast | 41 | IDC | |||
| 44 | Breast | 59 | DCIS | |||
| 45 | Breast | 43 | IDC | II | 3 | ER+PR+Her2+ |
| 46 | Breast | 32 | IDC | 3 | ||
| 47 | Breast | 53 | IDC | I | 3 | ER-PR-Her2- |
| 48 | Breast | 41 | DCIS | |||
| 49 | Breast | 63 | IDC | I | 2 | ER+PR-Her2- |
| 50 | Ovary | 66 | Serous | III | 3 | n/a |
| 51 | Ovary | 44 | Serous | III | 2 | n/a |
| 52 | Breast | 57 | IDC | 3 | ER+PR+ | |
| 53 | Breast | 49 | DCIS | 2 | ||
| 54 | Breast | 56 | IDC | III | 3 | ER+PR-Her2- |
| 55 | Breast | 56 | DCIS | I | 3 | |
| 56 | Ovary | 32 | Serous | IV | n/a | |
| 57 | Ovary | 44 | Serous | II | n/a |
| Risk-Reducing Intervention* | |||||||||
| MRI | Mammogram | RRM | RRSO | ||||||
| Eligible | 49.4% (77) | 46.8% (73) | 88.3% (136) | 67.3% (115) | |||||
| Uptake | 61.0% (47) | 61.6% (45) | 39.0% (53) | 75.7% (87) | |||||
| p-value† | < 0.001 | < 0.001 | 0.025 | < 0.001 | |||||
| Factor | % (n) | p-value | % (n) | p-value | % (n) | p-value | % (n) | p-value | |
| BRCA1 PV | 50.0% (12) | 0.181 | 52.2% (12) | 0.259 | 41.7% (20) | 0.634 | 75.6% (34) | 0.985 | |
| BRCA2 PV | 66.0% (35) | 66.0% (33) | 37.5% (33) | 75.7% (53) | |||||
| Proband | 55.6% (15) | 0.510 | 59.3% (16) | 0.803 | 32.5% (13) | 0.276 | 79.4% (27) | 0.612 | |
| Personal history of BC | 54.5% (6) | 0.633 | 45.5% (5) | 0.231 | 64.7% (22) | < 0.001 | 81.4% (35) | 0.267 | |
| 1st degree relative(s) with BC | 54.5% (18) | 0.264 | 51.6% (16) | 0.159 | 47.7% (31) | 0.082 | 78.9% (45) | 0.887 | |
| 1st degree relative(s) with OC | 56.3% (9) | 0.628 | 68.8% (11) | 0.444 | 32.0% (8) | 0.331 | 71.4% (15) | 0.381 | |
| Assessed by medical oncology | 71.4% (30) | 0.041 | 56.1% (23) | 0.270 | 41.9% (31) | 0.590 | 86.4% (57) | 0.003 | |
| Assessed by ICPC | 63.3% (31) | 0.596 | 63.0% (29) | 0.748 | 38.8% (31) | 0.750 | 83.9% (52) | 0.038 | |
| Received specialty care‡ | 66.1% (39) | 0.099 | 60.7% (34) | 0.767 | 42.3% (44) | 0.273 | 84.1% (74) | < 0.001 | |
| Primary care provider on record | 65.2% (43) | 0.539 | 59.4% (38) | 0.105 | 42.4% (50) | 0.662 | 79.4% (81) | 0.092 | |
| Rural residence§ | 55.9% (19) | 0.409 | 65.6% (21) | 0.537 | 35.7% (20) | 0.515 | 72.9% (35) | 0.563 | |
| Distance to MRI > 2 h | 50.0% (4) | 0.499 | 62.5% (5) | 0.958 | 57.9% (11) | 0.068 | 75.0% (12) | 0.948 | |
| Distance to surgical centre > 2 h | 37.5% (3) | 0.149 | 62.5% (5) | 0.958 | 55.6% (10) | 0.121 | 75.0% (12) | 0.948 | |
| Age ≥ 50 y | 65.0% (26) | 0.459 | 62.5% (25) | 0.868 | 45.1% (37) | 0.070 | 75.9% (60) | 0.912 | |
| Factor | Veryadherent* | Adherent | OR (CI)† | p-value†‡ | Non-adherent | OR (CI)¶ | p-value¶ |
| BRCA1 PV | 66.7% (36) | 7.4% (4) | 1.581(0.685-3.651) | 0.122 | 25.9% (14) | 0.399(0.124-1.278) | 0.283 |
| BRCA2 PV | 65.6% (61) | 18.3% (17) | 16.1% (15) | ||||
| Personal history of BC and/or OC | 74.0% (37) | 12.0% (6) | 0.649(0.231-1.820) | 0.411 | 14.0% (7) | 0.516(0.201-1.326) | 0.170 |
| Personal history of other cancer(s) | 60.0% (6) | 30.0% (3) | 2.528(0.578-11.052) | 0.218 | 10.0% (1) | 0.542(0.063-4.692) | 0.578 |
| 1st degree relative(s) with BC | 68.1% (47) | 14.5% (10) | 1.307(0.457-3.738) | 0.617 | 17.4% (12) | 0.998(0.399-2.496) | 0.997 |
| 1st degree relative(s) with OC | 56.0% (14) | 28.0% (7) | 3.800(1.238-11.664) | 0.020 | 16.0% (4) | 1.143(0.338-3.870) | 0.830 |
| Assessed by medical oncology | 76.3% (61) | 12.5% (10) | 0.537(0.207-1.388) | 0.199 | 11.3% (9) | 0.312(0.126-0.774) | 0.012 |
| Assessed by ICPC | 69.9% (58) | 15.7% (13) | 1.093(0.414-2.882) | 0.858 | 14.5% (12) | 0.576(0.241-1.378) | 0.215 |
| Received specialty care§ | 73.2% (82) | 13.4% (15) | 0.457(0.153-1.367) | 0.161 | 13.4% (15) | 0.249(0.096-0.647) | 0.004 |
| Primary care provider on record | 72.1% (93) | 11.6% (15) | 0.108(0.017-0.698) | 0.019 | 16.3% (21) | 0.452(0.039-5.216) | 0.524 |
| Rural residence | 56.9% (33) | 22.4% (13) | 3.152(1.188-8.362) | 0.021 | 20.7% (12) | 1.369(0.585-3.203) | 0.469 |
| Distance to MRI > 2 h | 68.4% (13) | 15.8% (3) | 1.077(0.278-4.174) | 0.915 | 15.8% (3) | 0.746(0.197-2.820) | 0.665 |
| Distance to surgical centre > 2 h | 57.9% (11) | 21.1% (4) | 1.840(0.523-6.466) | 0.342 | 21.1% (4) | 1.2551(0.366-4.271) | 0.721 |
| Age ≥ 50 y | 66.7% (60) | 21.1% (19) | 5.858(1.290-26.613) | 0.022 | 12.2% (11) | 0.377(0.160-0.866) | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





