Submitted:
18 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
3. Results
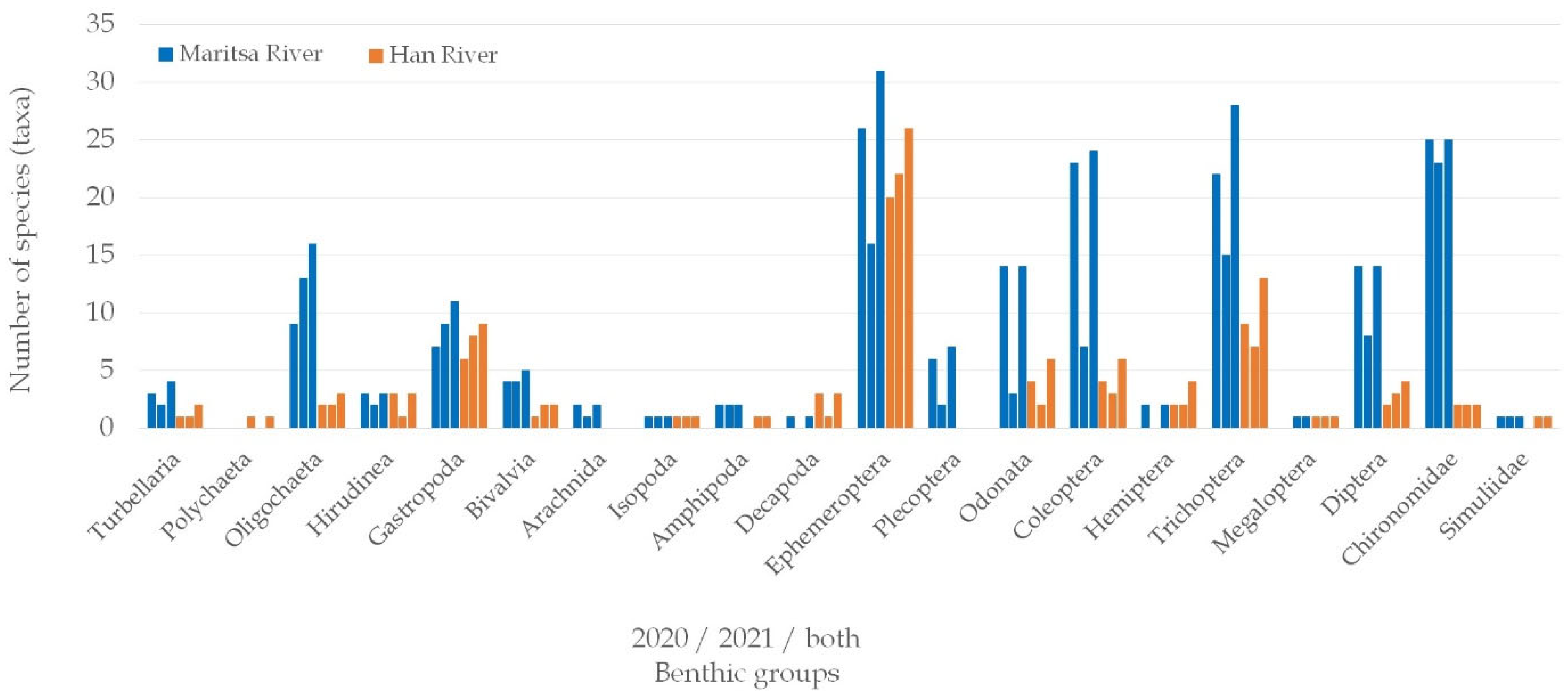
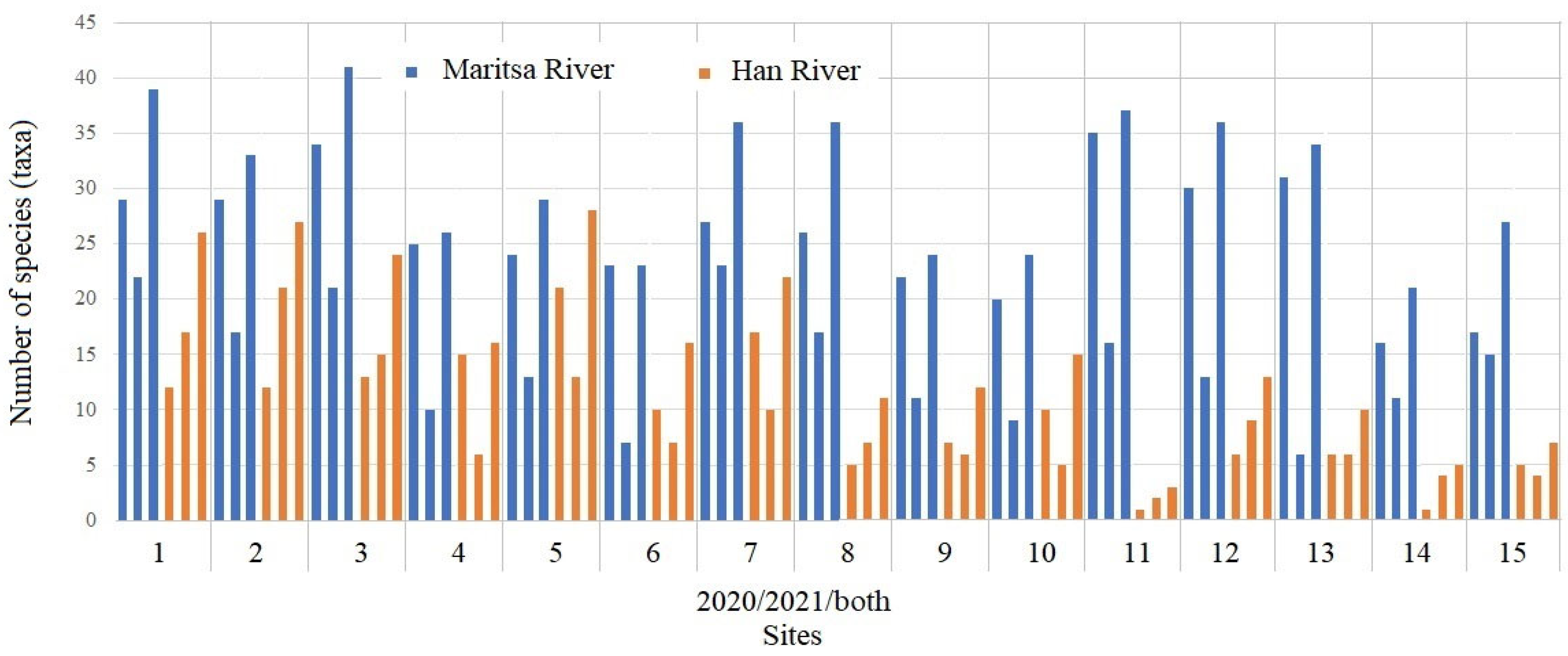
3.1. Taxonomic Composition and Number of Macrozoobenthic Taxa
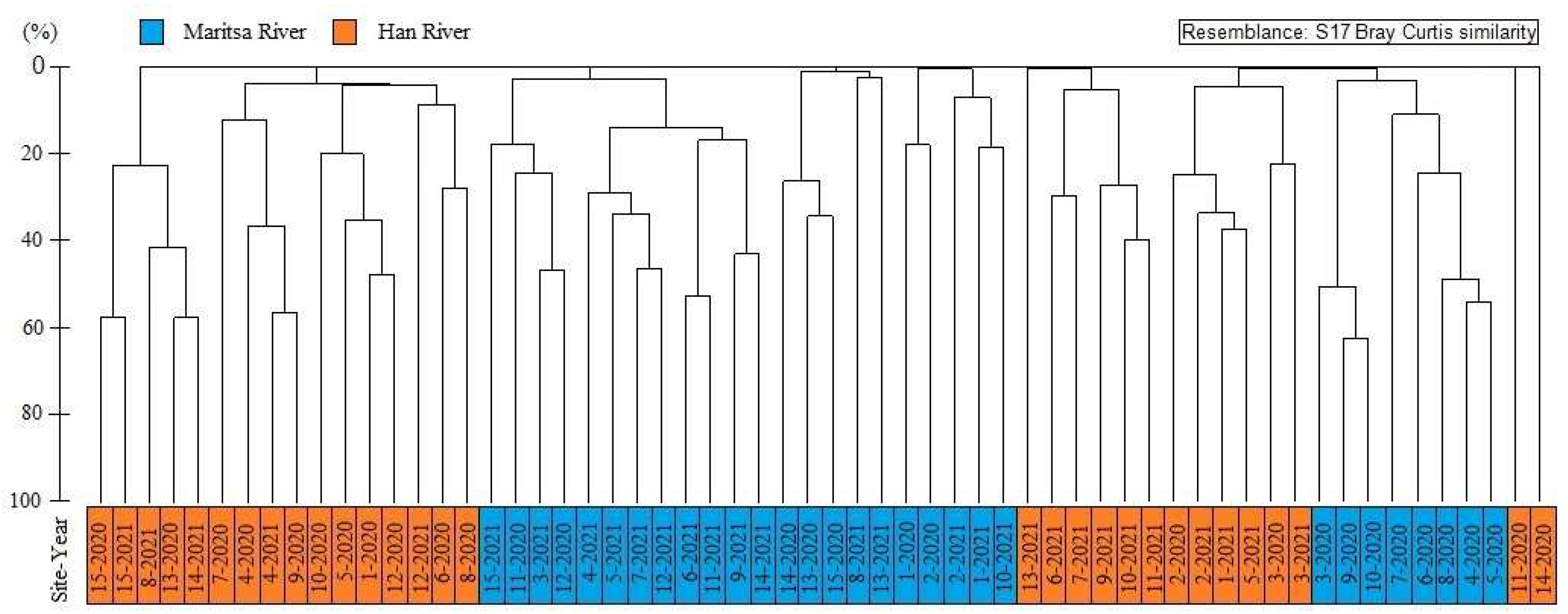
3.2. Similarity Analysis
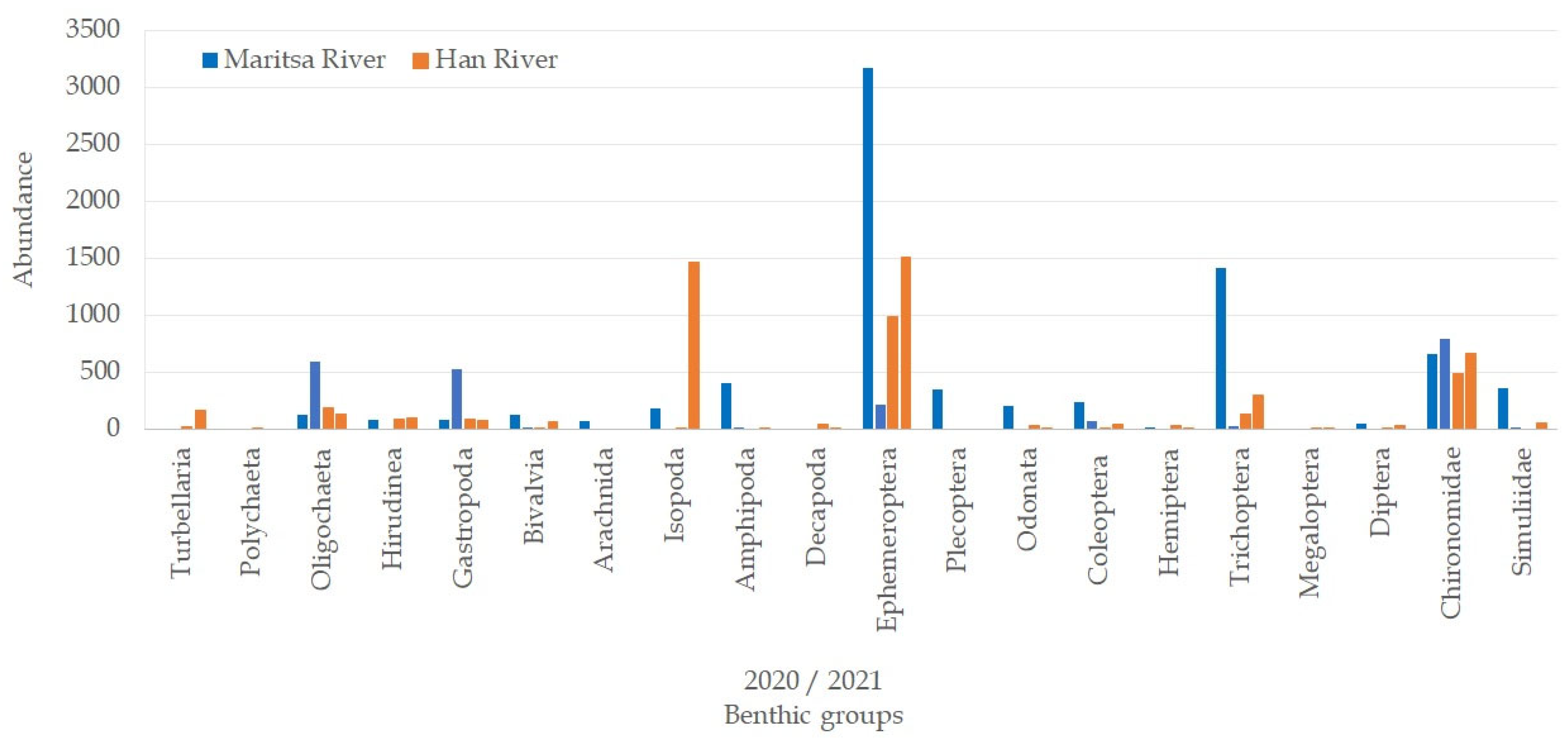
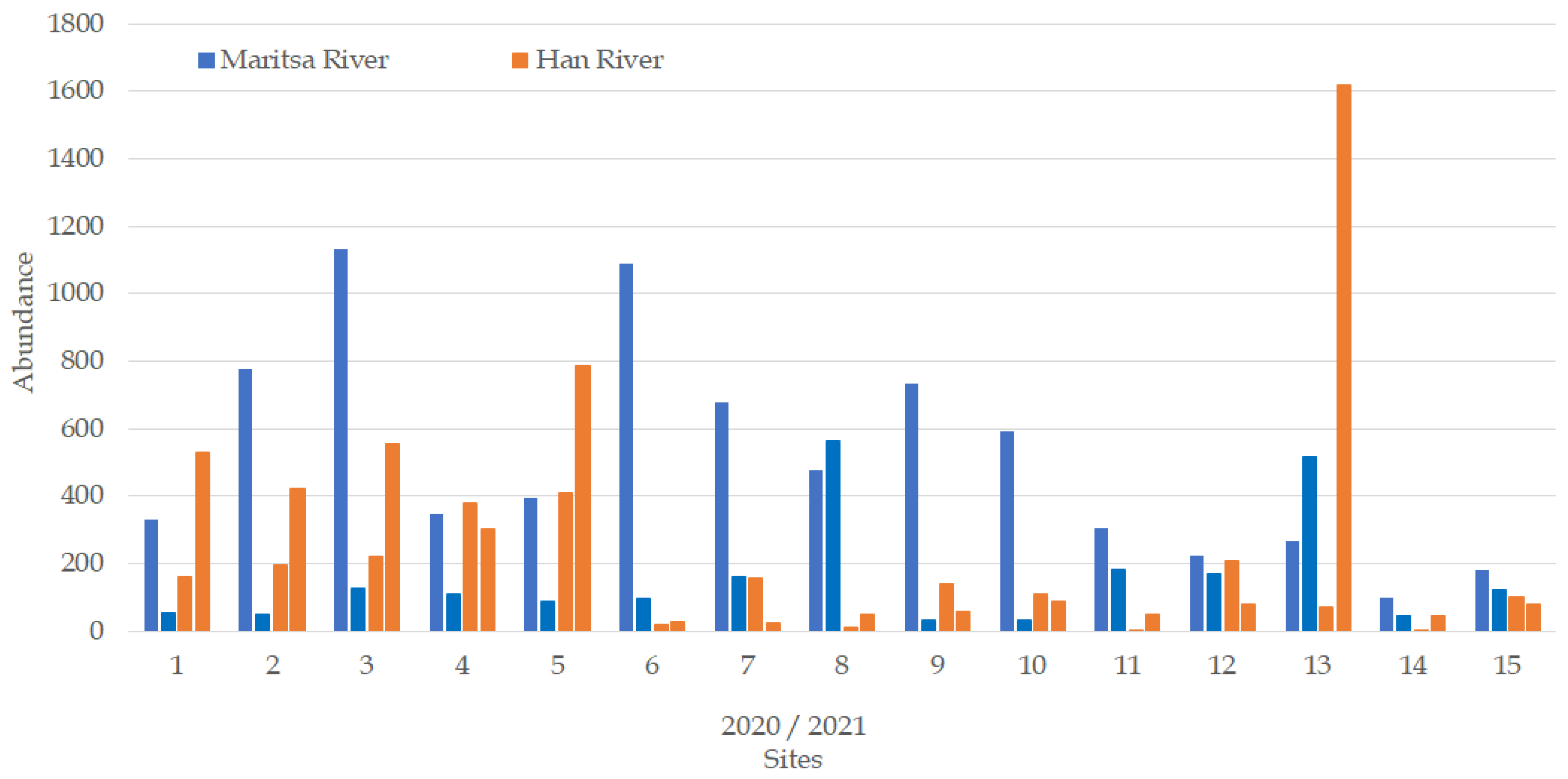
3.3. Abundance of the Macrozoobenthos
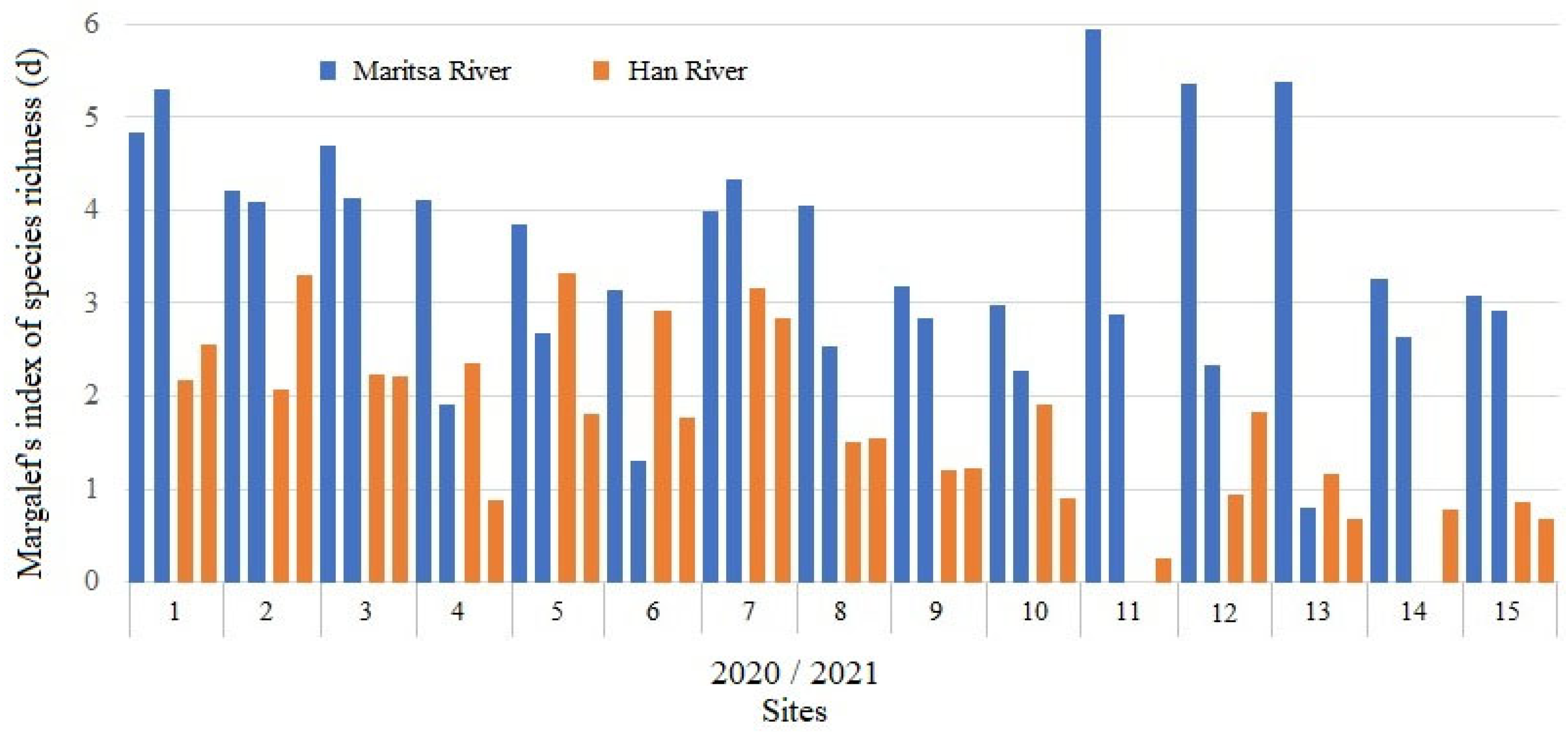
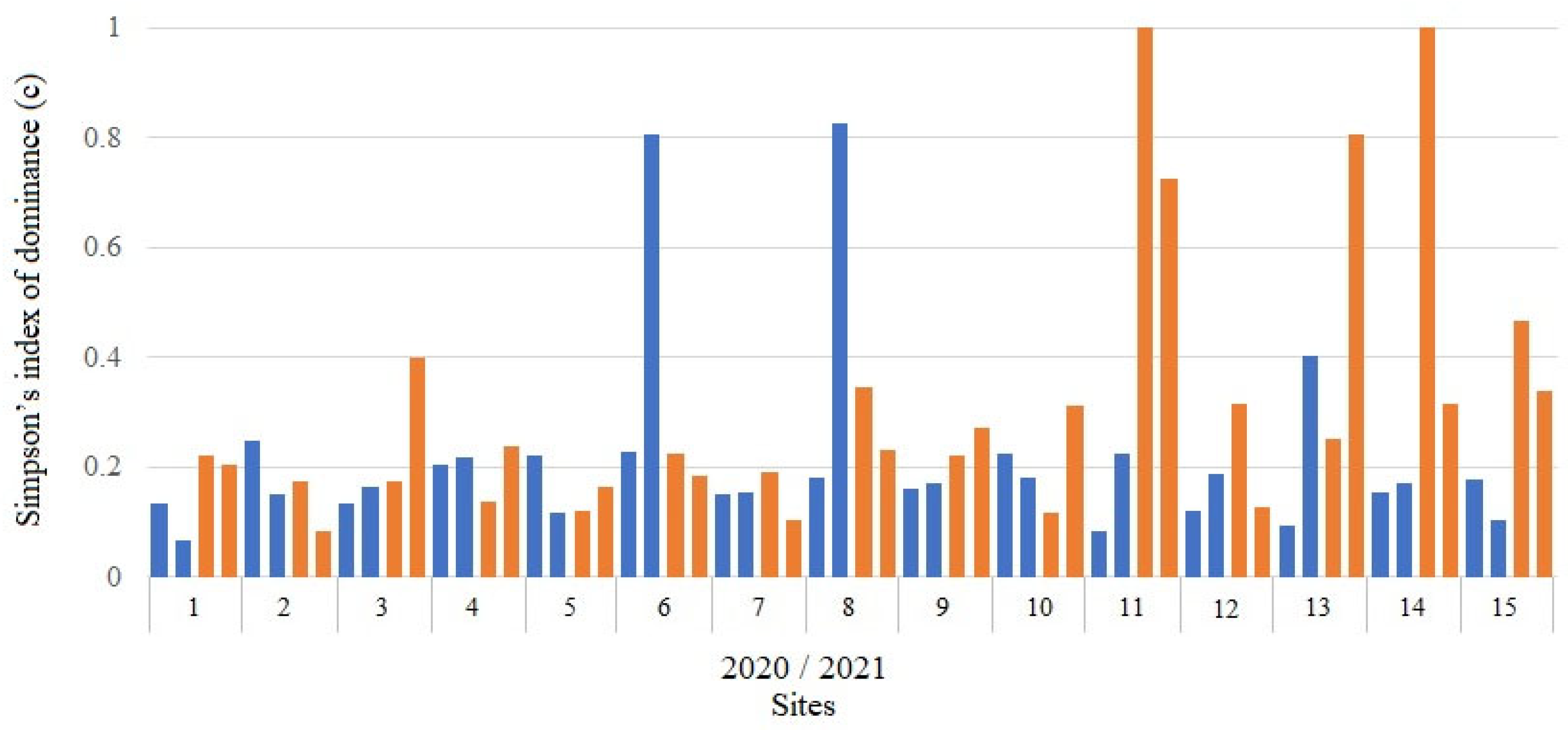
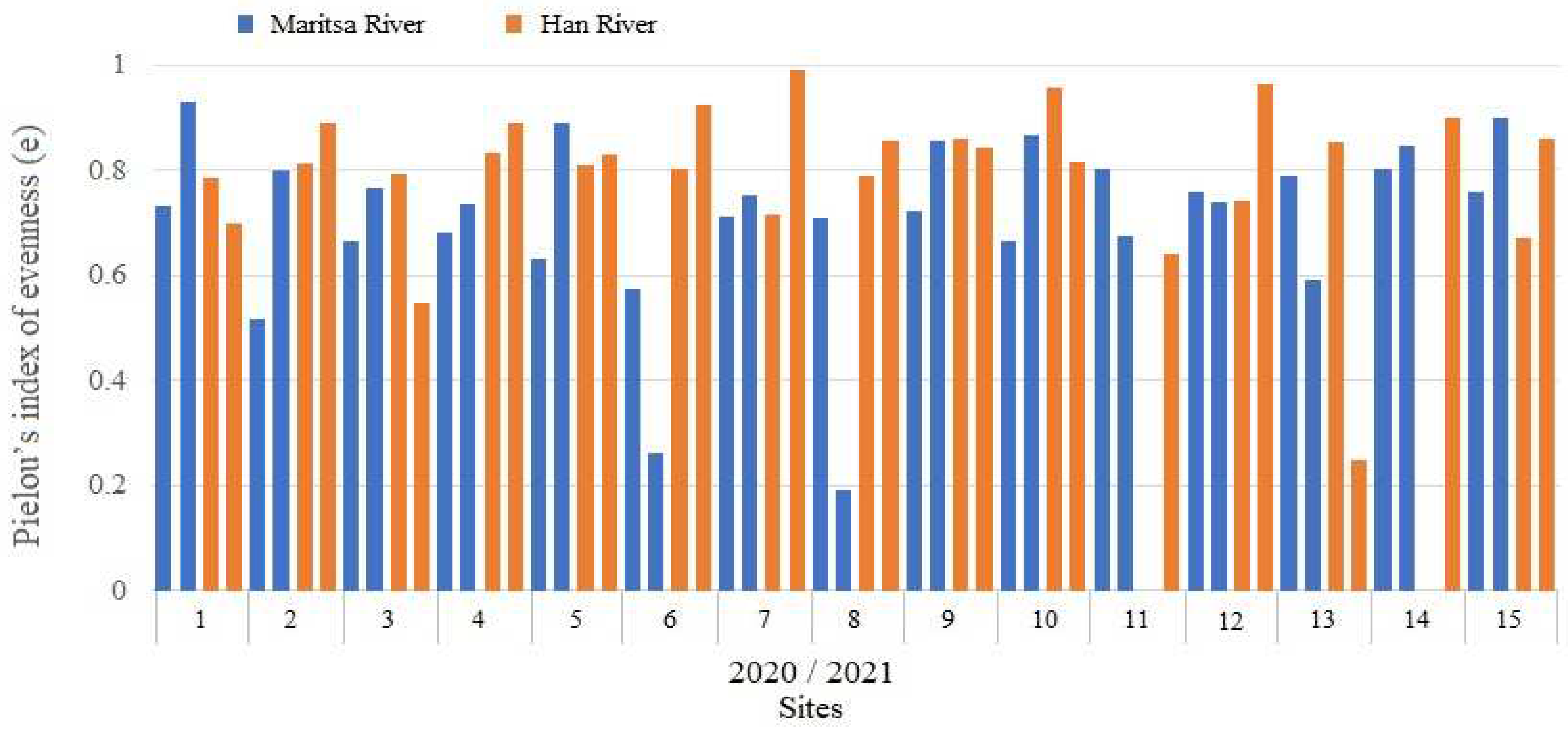
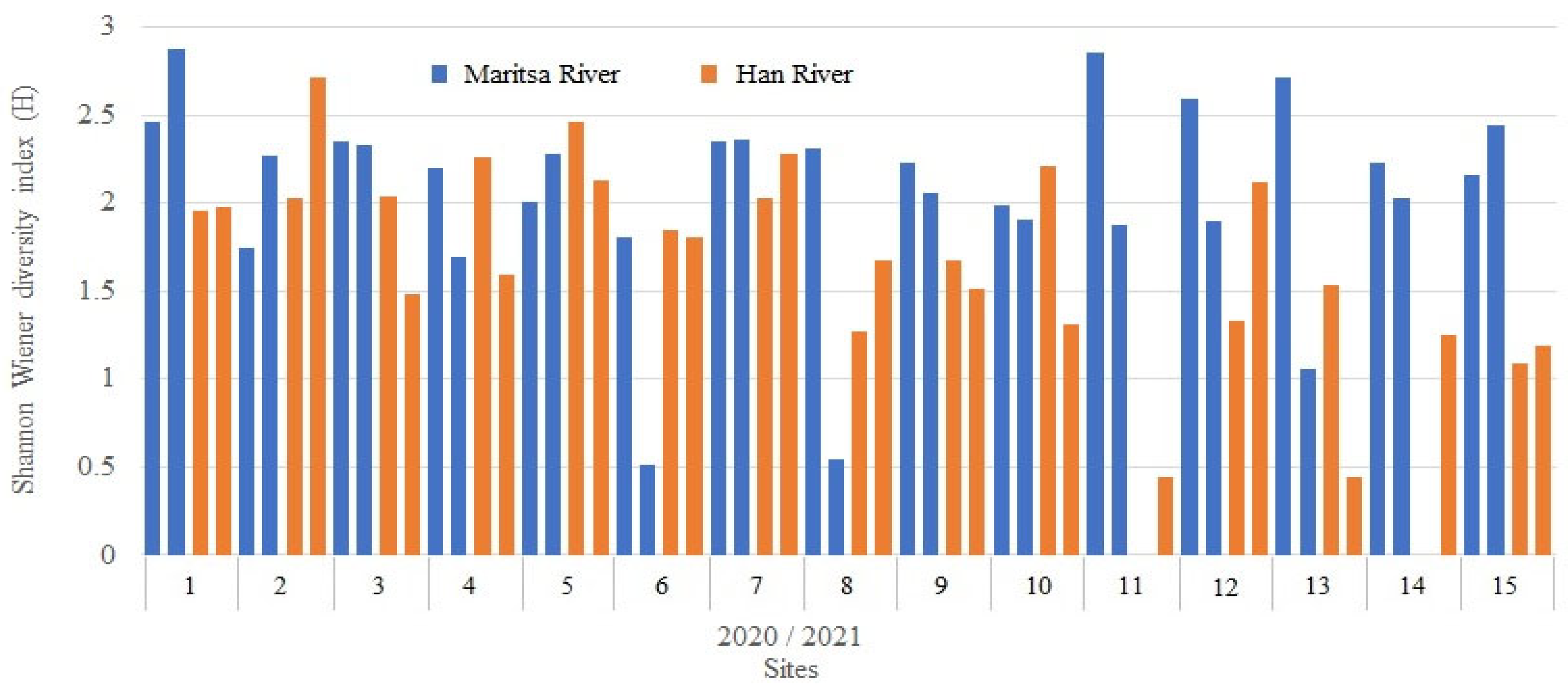
3.4. Species Diversity
4. Discussion
5. Conclusions
Funding
Acknowledgments
References
- Bae, K.S. , Kim, H. J. & Kim, J.Y. Biological estimation of water quality by benthic macroinvertebrates in the Han River Water System. Seoul institute of health and environment 1994, 30, 268–282. (In Korean) [Google Scholar]
- Bae, K.S. , Gil, H.K., Yoo, D.G., Kim, R.R. & Shin, J. Long-term Variation of Benthic Macroinvertebrate Fauna and Diversity in the Lower Han River, Korean Society of Environmental Biology, Spring Joint Conference and Symposium, 2002, , 50. (In Korean). 1 May.
- Evtimova, V. , Tyufekchieva, V. , Varadinova, E., Vidinova, Y., Ihtimanska, M., Georgieva, G., Todorov, M. & Soufi, R. Macroinvertebrate Communities of sub-Mediterranean Intermittent Rivers in Bulgaria: Association with Environmental Parameters and Ecological Status, Ecologia Balkanica, Special Edition 2021, 4, 49–64. [Google Scholar]
- Ihtimanska, M. , Varadinova, E. , Kazakov, S., Hristova, R., Naumova, S. & Pehlivanov, L. Preliminary Results about the Distribution of Macrozoobenthos along the Bulgarian Stretch of the Danube River with Respect to Loading of Nutrients, Heavy Metals and Arsenic, Acta Zoologica Bulgaria, Supplement 2014, 7, 165–171. [Google Scholar]
- Janeva, I. , Pehlivanov, L., Vidinova, Y., Stoichev, S., Tyufekchieva, V. & Kumanski, K. A comparative ecological characterization of lotic benthal zoocoenoses from two streams under different anthropogenic influence. – In: Ecomonitoring in Rozhen and Srednogorie – Bulgaria. Ministry of Environment, SDC – Swiss Agency for Development and Cooperation, Sofia 1997, 101-112.
- Kim, M.C. , Lee, E.J., Heo, J.W., Lee, Y.K., Ryu, S.M., Kim, J.B., Jeon, N.S., Lee, J.G., Kim, J.W., Lee, T.G., Park, S.H., Jeong, S.Y., Lee, J.W., Lee, G.H., Lee, M.D., Noh, S.E., Bae, D.Y., Lee, Y.Y., Kim, E.Y. & Park. J.S. Survey of aquatic organisms in major streams, Ecological Conservation Research Institute, Ministry of Environment. 2017; (In Korean). [Google Scholar] [CrossRef]
- Koperski, P. Diversity of freshwater macrobenthos and its use in biological assessment: a critical review of current applications. Environ. Rev. 2011, 19, 16–31. [Google Scholar] [CrossRef]
- Kwak, I.-S.; 전남대학교환경해양학전공; Lee, D. -S.; Hong, C.; Park, Y.-S. Distribution Patterns of Benthic Macroinvertebrates in Streams of Korea. Korean J. Ecol. Environ. 2018, 51, 60–70. [Google Scholar] [CrossRef]
- Guidelines for Aquatic Ecosystem Status survey and health assessment method (stream edition). No. 2019-52. Article 9 of the Water Environment Conservation Act. National Institute of Environmental Research (NIER). 2019. (In Korean).
- Lee, S.D. , Kim, J.G., Lee, J.C., Heo, S.W., Kim, H.J., Noh, Y.M. and Gi, N.E. Conservation and management plan of aquatic ecosystem in the mouth of the Han River, Seoul Green Environment Support Center, Final report 2011, SGEC2011-0727076.
- Lock, K.; Asenova, M.; Goethals, P.L. Benthic macroinvertebrates as indicators of the water quality in Bulgaria: A case-study in the Iskar river basin. Limnologica 2011, 41, 334–338. [Google Scholar] [CrossRef]
- Margalef, R. Information Theory in Ecology, General Systems. 1958, 3, 36–71. [Google Scholar]
- Min, J.-K.; Kim, Y.-J.; Kong, D.-S. Spatial distribution patterns of benthic macroinvertebrate functional feeding groups by stream size and gradient in Republic of Korea. J. Freshw. Ecol. 2019, 34, 715–738. [Google Scholar] [CrossRef]
- Min, J.-K.; Kong, D.-S. Distribution patterns of benthic macroinvertebrate communities based on multispatial-scale environmental variables in the river systems of Republic of Korea. J. Freshw. Ecol. 2020, 35, 323–347. [Google Scholar] [CrossRef]
- Min, J.-K.; Lee, H.; Kong, D. Development of a benthic macroinvertebrate predictive model based on the physical and chemical variables of rivers in the Republic of Korea. J. Freshw. Ecol. 2022, 37, 425–453. [Google Scholar] [CrossRef]
- Ministry of Environment and National Institute of Environmental Research (ME & NIER). Survey and Assessment of Estuary Ecosystem (2021): final report. 2021, Publication No. 11-1480523-004744-01, NIER No. SP2021-329. (In Korean).
- Ministry of Environment and National Institute of Environmental Research (ME & NIER). Water Environment Information System, Biometric Network, Benthic Macroinvertebrates 2023, Retrieved from http://water.nier.go.kr.
- Moskova, G., Uzunov, Y., Yaneva, I., Stoichev, St., Vidinova, Y., Tyufekchieva, V. & Kenderov, L. Species Content and Distribution of the Macrozoobenthos along Rilska River, South-West Bulgaria. Bioautomation 2009, 13, 231–238.
- Park, J. , Sakelarieva, L. & Varadinova, E. Comparative Analysis of the Water Policies and the Systems for Ecological Status Assessment of the Running Waters in Bulgaria and South Korea: Case study on Maritsa River and Han River, Ecologia Balkanica 2022, 13(2), 171-189.
- Park, J. , Sakelarieva, L., Varadinova, E., Evtimova, V., Vidinova, Y., Tyufekchieva, V., Georgieva, G., Ihtimanska, M. & Todorov, M. Taxonomic Composition and Dominant Structure of the Macrozoobenthos in Maritsa River and Some Tributaries, South Bulgaria, Acta Zoologica Bulgaria, Supplement 2022, 16(06). (In press).
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Russev, B.K. Hydrobiological research of Maritsa River. І. Sofia, Bulgaria: Bulgarian Academy of Sciences. Zoological institute with museum 1966. (In Bulgarian).
- Russev, B.K. Hydrobiological research of Maritsa River. Ⅱ. Saprobiological assessment for 1965 and 1966. Sofia, Bulgaria: Bulgarian Academy of Sciences. Zoological institute with museum 1967. (In Bulgarian).
- Russev, B.K. & Janeva, I. J. The significance of mayflies (Ephemeroptera, Insecta) as structural constituents of benthic zoocenoses of the Maritsa River. Bulgarian Academy of Sciences. Hydrobiology (Sofia) 1983, 19, 14–23. [Google Scholar]
- Sakelarieva, L. , Janeva, I., Michailov, M. & Hristov, H. Dependences between the river flow and some parameters of macrozoobenthic communities in the Blagoevgradska Bistritsa River, South-West Bulgaria, 12th International Conference on Wetland Systems for Water Pollution Control, -8, 2010, Venice, Italy, Conference Paper 2: 801-1616. 4 October.
- Sakelarieva, L. , Yaneva, I., Uzunov, Y., Kumanski, K., Stoichev, S., Vidinova, Y. & Tyufekchieva, V. Taxonomic Composition and Dominant Structure of Macrozoobenthos in the Blagoevgradska Bistritsa River, Acta Zoologica Bulgaria 2008, Supplement 2: 219-23.
- Shannon, C.E. & Weaver, W.W. The Mathematical Theory of Communication. Urbana, USA: University of Illinois Press 1964.
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 668. [Google Scholar] [CrossRef]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Kongelige Danske Videnskabernes Selskab 1948, 5, 1–34. [Google Scholar]
- Stoykov, S.; Uzunova, S. Dynamics of macrozoobenthos in the Southern Bulgarian Black Sea coastal and open-sea areas. Mediterr. Mar. Sci. 2001, 2, 27. [Google Scholar] [CrossRef]
- Sueb, S.; Damayanti, J.; Rohman, F. Macrozoobenthos diversity as bioindicator water quality of Metro River, Malang City. 2353, 0300; 50. [Google Scholar] [CrossRef]
- Superada, J.L.; Tampus, A.D. Macroinvertebrates as Indicators of Water Quality in Three Estuary Sites in Iligan City, Philippines. J. Multidiscip. Stud. 2015, 4. [Google Scholar] [CrossRef]
- Uzunov, J.I. & Kovachev, S. G. The effect of the substrate on the structure of the macrozoobenthic communities in the Maritsa River. Bulgarian Academy of Sciences. Hydrobiology (Sofia) 1981, 14, 65–74. (In Bulgarian) [Google Scholar]
- Uzunov, J.I. & Kovachev, S. G. Macroinvertebrate communities structures in the Maritsa River under human activity impact. Bulgarian Academy of Sciences. Hydrobiology (Sofia) 1985, 24, 33–47. [Google Scholar]
- Uzunov, J.I. , Russev, B. K., Kovachev, S.G. & Janeva, I.J. Species composition and distribution of the macrozoobenthos of the Maritsa River. Bulgarian Academy of Sciences. Hydrobiology (Sofia) 1981, 14, 3–15. (In Bulgarian) [Google Scholar]
- Varadinova, E.; Sakelarieva, L.; Park, J.; Ivanov, M.; Tyufekchieva, V. Characterisation of Macroinvertebrate Communities in Maritsa River (South Bulgaria)—Relation to Different Environmental Factors and Ecological Status Assessment. Diversity 2022, 14, 833. [Google Scholar] [CrossRef]
- Vidinova, Y.N. , Evtimova, V. V. & Tyufekchieva, V.G. Ephemeroptera, Plecoptera and Trichoptera (Insecta) from Water Bodies in the Region of Plovdiv City. Faunistic diversity of the city of Plovdiv (Bulgaria), Vol.1 – Invertebrates. Bulletin of the Natural History Museum – Plovdiv, Supplement 2018, 1, 69–79. [Google Scholar]
- Zhelev, Z. Ecological status of the river Sazliyka and its tributaries (Southern Bulgaria) as indicated by developmental stability of Pelophylax ridibundus (Amphibia: Ranidae). Acta Zoologica Bulgarica 2013, 65(3), 371–380. [Google Scholar]
- Zhelev, Z. , Arnaudov, A. , Popgeorgiev, G. and Dimitrov, H. Assessment of Ecological Status of Two Rivers with Different Types of Anthropogenic Pollution in Southern Bulgaria Based on the Level of Fluctuating Asymmetry in the Populations of Marsh Frog Rana ridibunda (Amphibia: Ranidae). Acta Zoologica Bulgarica 2012, 64, 229–235. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




