Submitted:
01 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Solvents, solutions and reagents
2.2. Preparation of the biosorbent
2.3. Equipments
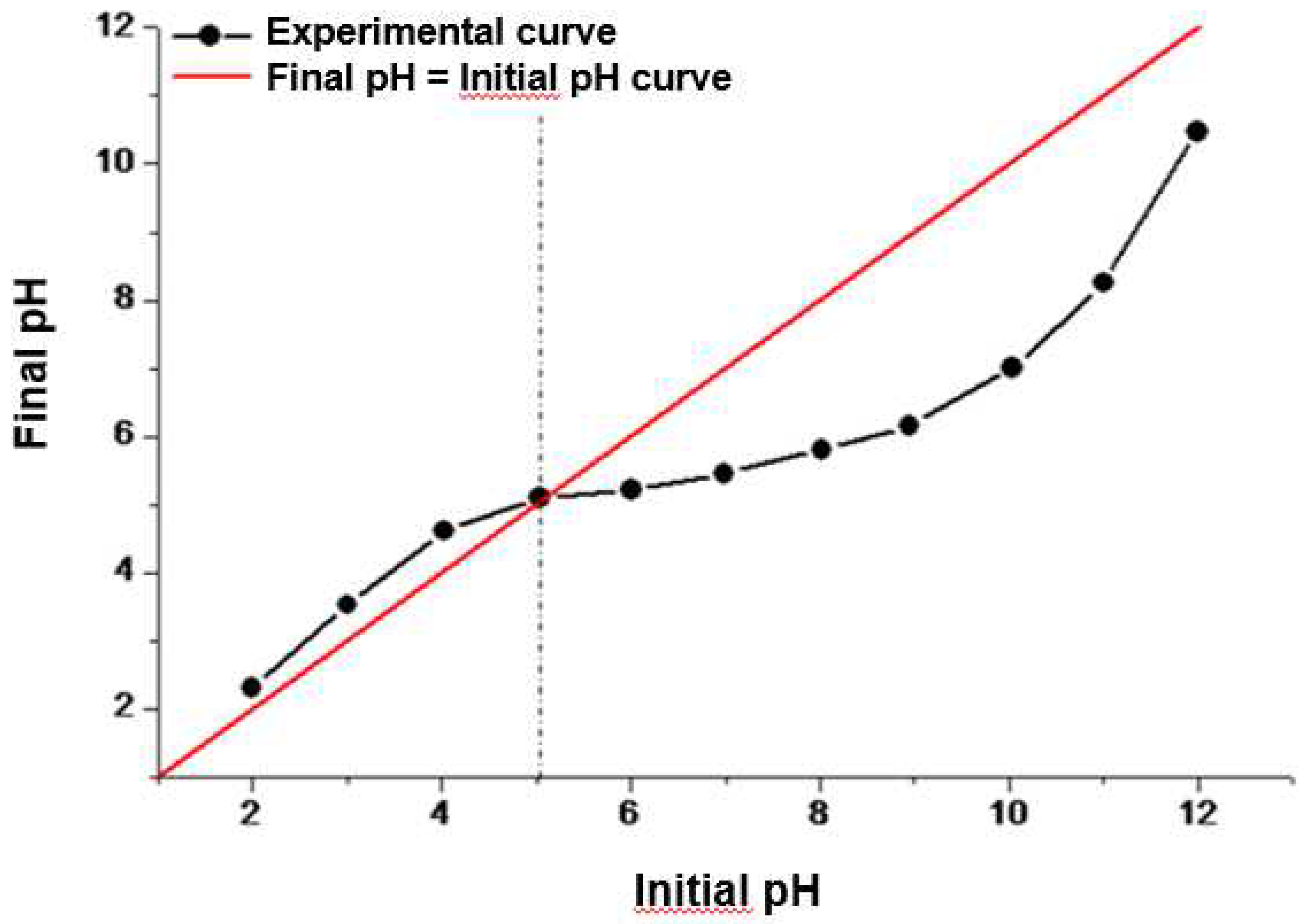
2.4. Point of zero charge (pHPZC)
2.5. Batch adsorption experiments
3. Results and Discussion
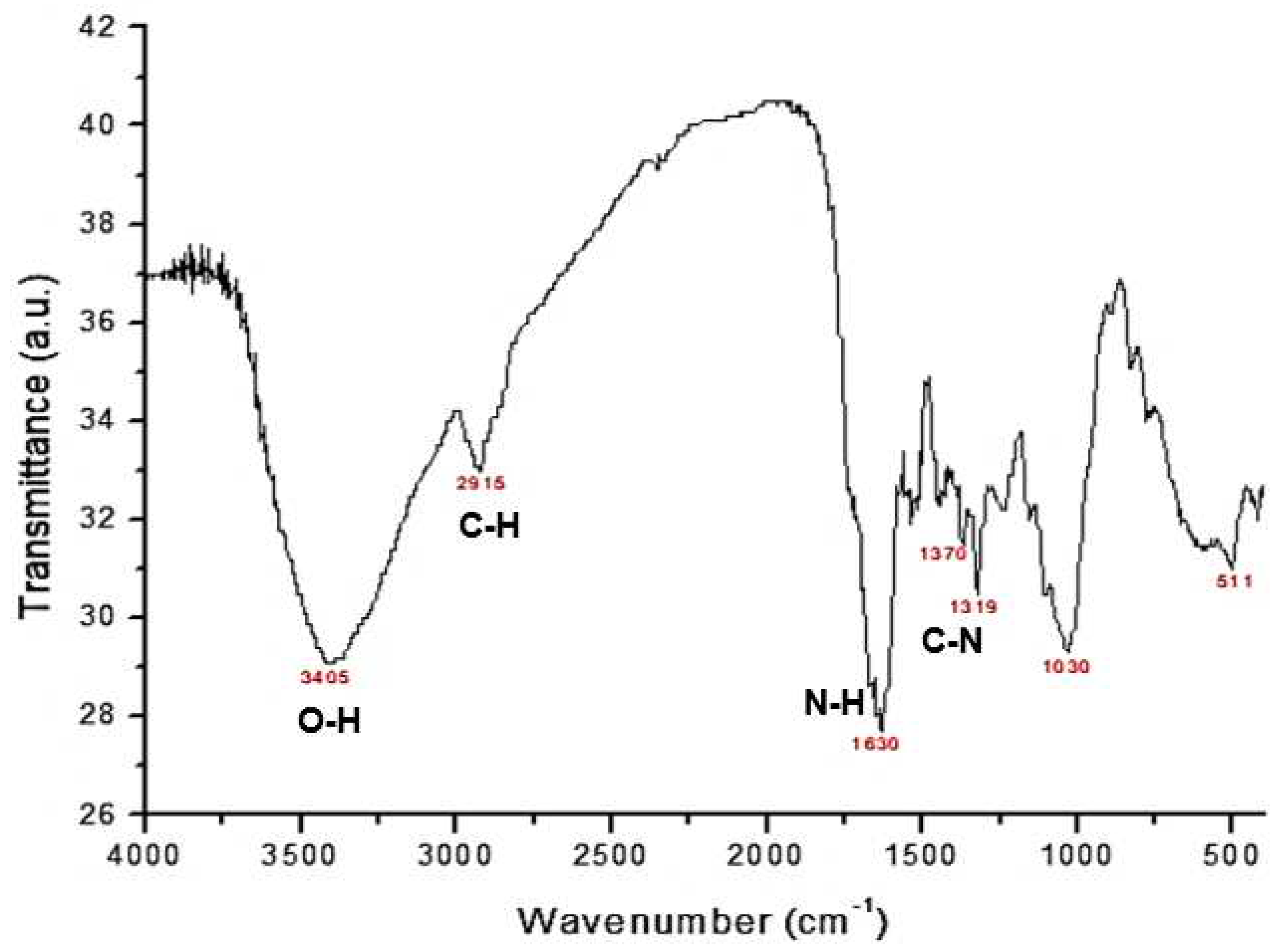
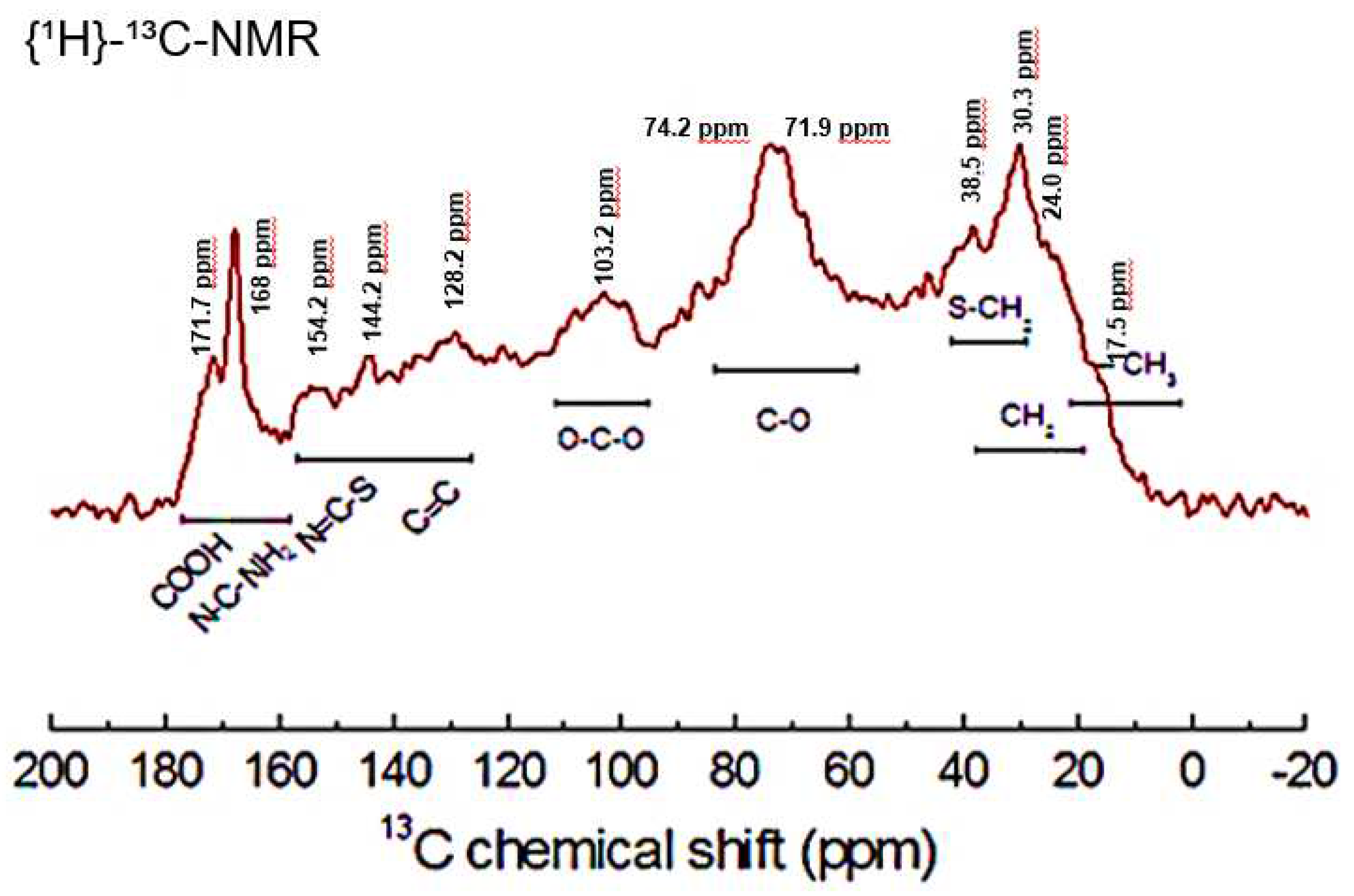
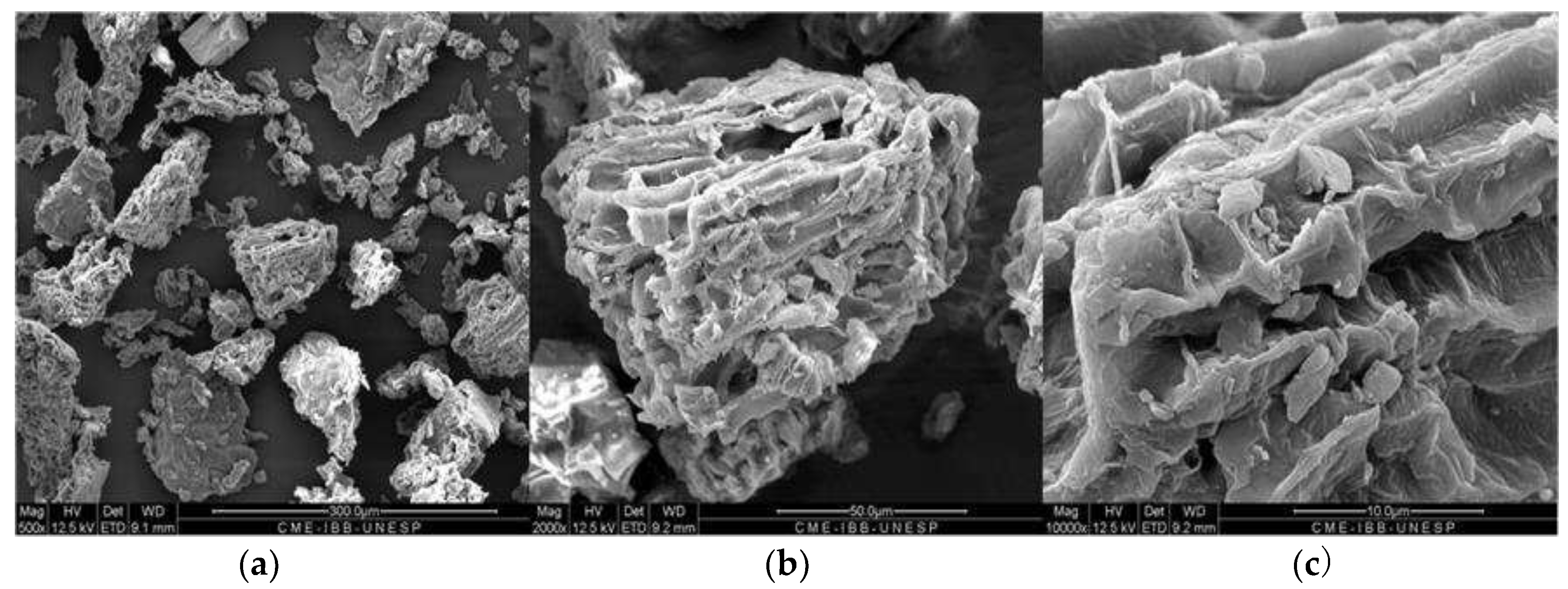
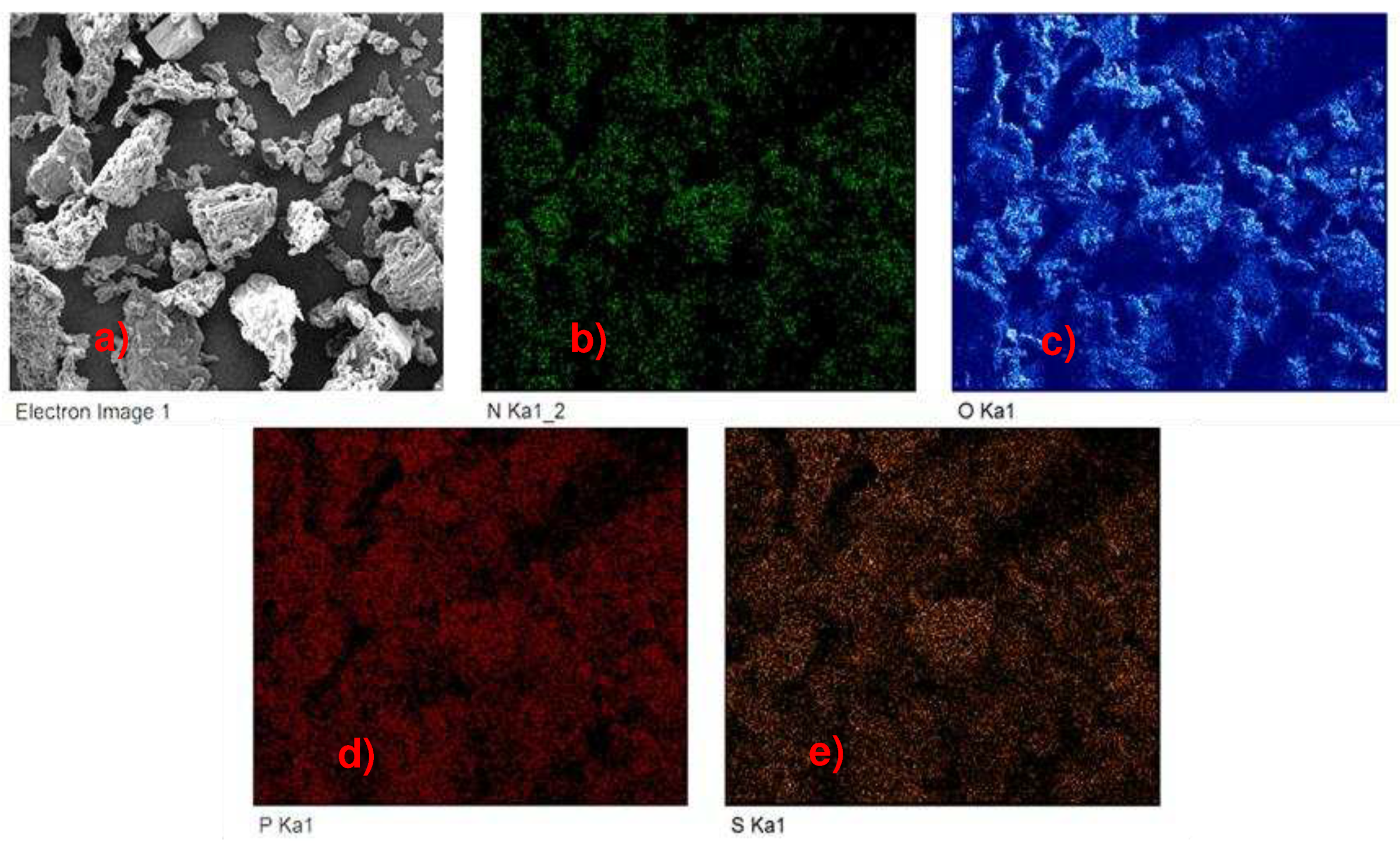
3.1. Characterization of the material
3.2. Batch adsorption experiments
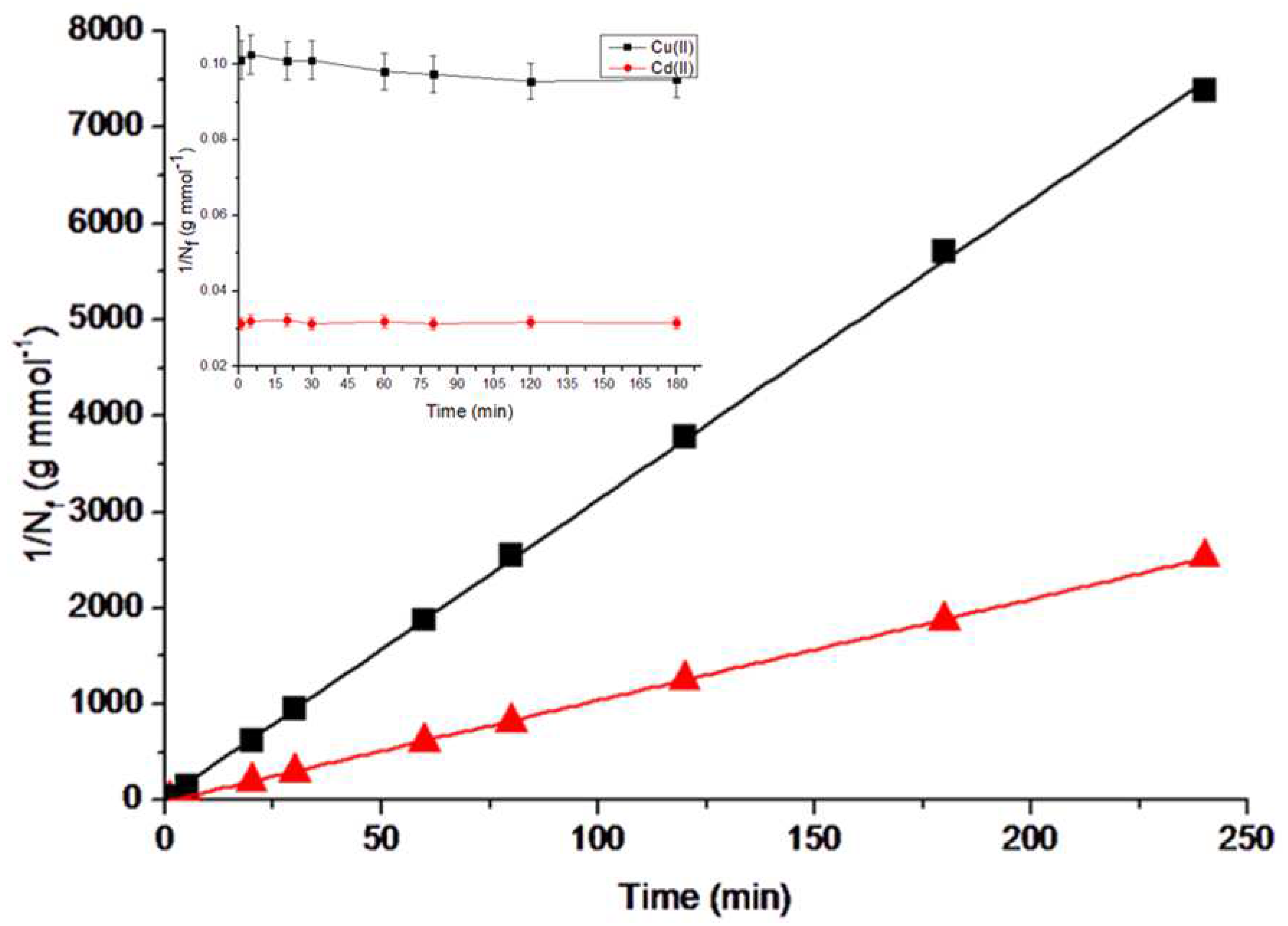
3.2.1. Adsorption kinetics
3.2.2. Effect of the pH of the medium
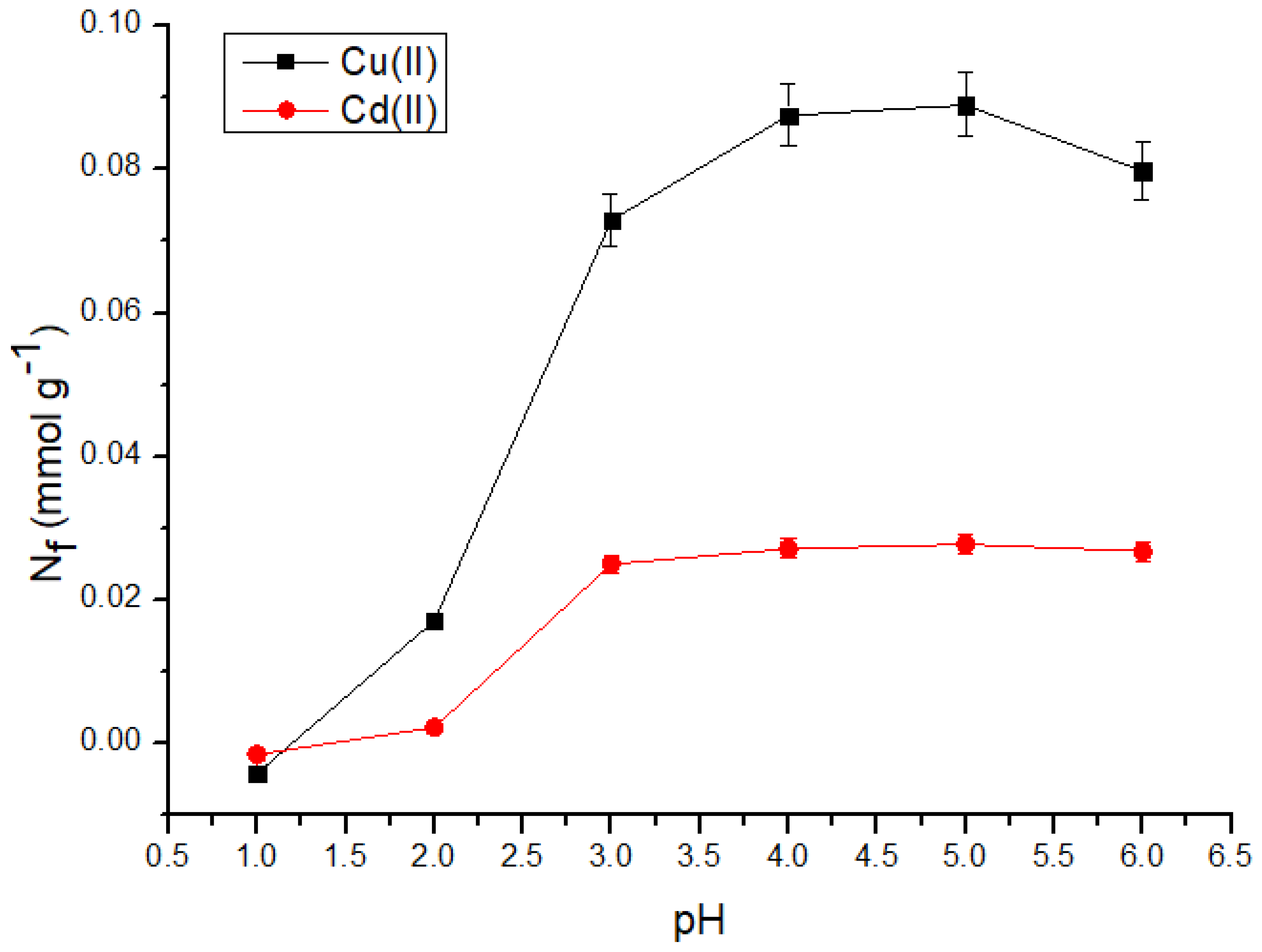
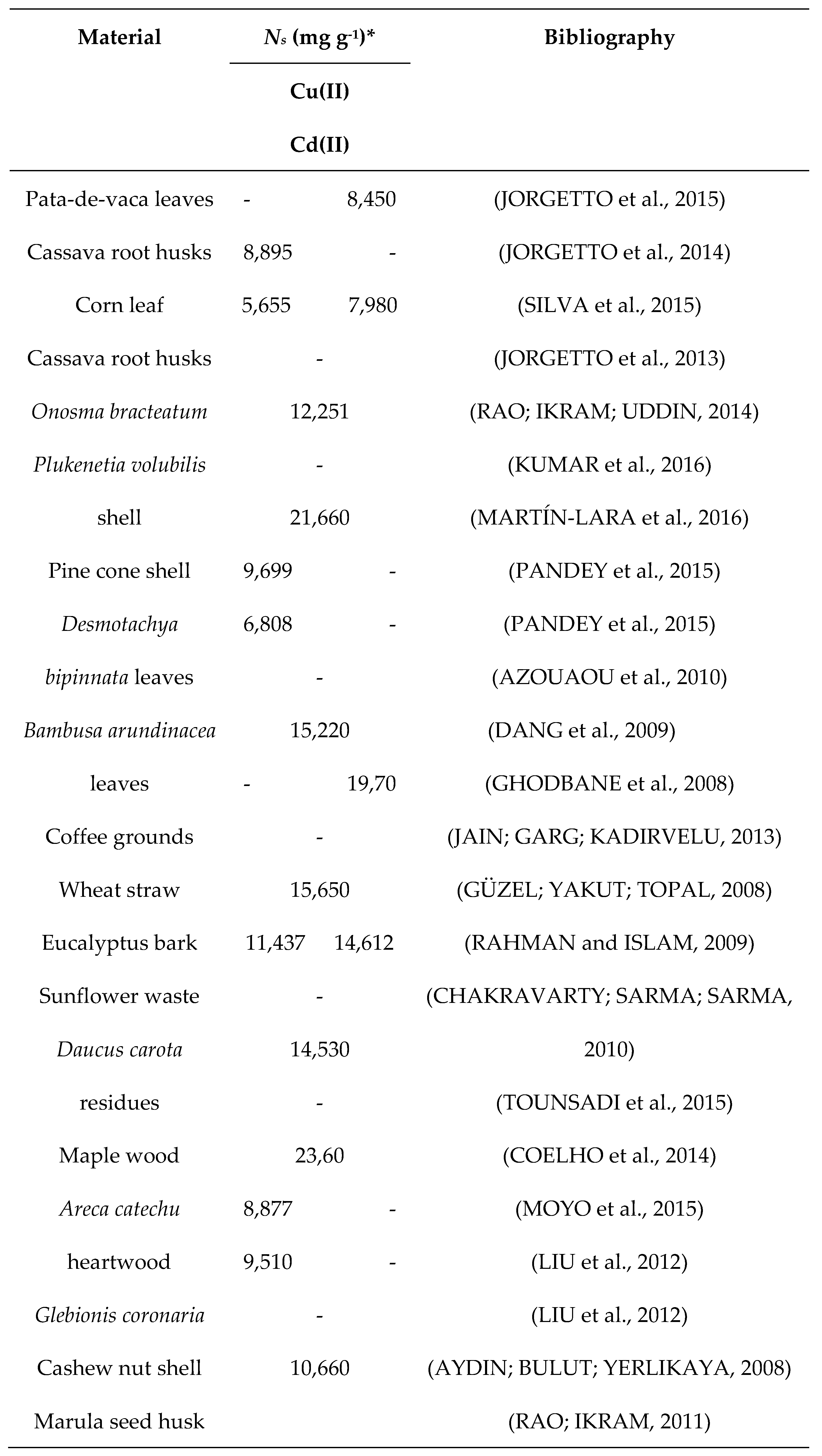
3.2.3. Maximum adsorption capacity
4. Conclusions
Declaration of Interest
References
- AHALYA, N.; RAMACHANDRA, T. V.; KANAMADI, R. D. Biosorption of heavy metals. Research Journal of Chemistry and Environment. 2003, 7, 71–79. [Google Scholar]
- AHMAD, M. A.; AHMAD, N. Effective removal of cadmium and zinc from wastewater using agricultural waste-based activated carbon: adsorption isotherm, kinetic, and thermodynamic studies. Environmental Science and Pollution Research. 2022, 29, 5292–5306. [Google Scholar]
- AHMAD, R.; HASEEB, S. Absorptive removal of Pb2+, Cu2+ and Ni2+ from the aqueous solution by using groundnut husk modified with Guar Gum (GG): Kinetic and thermodynamic studies. Groundwater for Sustainable Development. 2015, 1, 41–49. [Google Scholar] [CrossRef]
- Alcântara, I.L.; Roldan, P.S.; Castro, G.R.; Moraes, F.V.; Silva, F.A.; Padilha, C.C.F.; Oliveira, J.D.; Padilha, P.M. Determination of Cadmium in River Water Samples by Flame AAS after On-line Preconcentration in Mini-Column Packed with 2-Aminothiazole-modified Silica Gel. Anal. Sci. 2004, 20, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- ALMEIDA, E.; SOARES, A. M.; FIGUEIRA, E. Bioaccumulation of cadmium, selenium, and zinc in native oysters (Crassostrea angulata) from a contaminated estuary: Implications for environmental monitoring and human health. Environmental Science and Pollution Research. 2018, 25, 21311–21320. [Google Scholar]
- Alslaibi, T.M.; Abustan, I.; Ahmad, M.A.; Abu Foul, A. Cadmium removal from aqueous solution using microwaved olive stone activated carbon. J. Environ. Chem. Eng. 2013, 1, 589–599. [Google Scholar] [CrossRef]
- Aydın, H.; Bulut, Y.; Yerlikaya, Ç. Removal of copper (II) from aqueous solution by adsorption onto low-cost adsorbents. J. Environ. Manag. 2008, 87, 37–45. [Google Scholar] [CrossRef]
- Azouaou, N.; Sadaoui, Z.; Djaafri, A.; Mokaddem, H. Adsorption of cadmium from aqueous solution onto untreated coffee grounds: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2010, 184, 126–134. [Google Scholar] [CrossRef]
- BAIRD, C.; CANN, M. Química Ambiental, fourth ed., Bookman, Porto Alegre, 2011.
- Carneiro, P.A.; Umbuzeiro, G.A.; Oliveira, D.P.; Zanoni, M.V.B. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J. Hazard. Mater. 2010, 174, 694–699. [Google Scholar] [CrossRef]
- Castro, R.S.D.; Caetano, L.; Ferreira, G.; Padilha, P.M.; Saeki, M.J.; Zara, L.F.; Martines, M.A.U.; Castro, G.R. Banana Peel Applied to the Solid Phase Extraction of Copper and Lead from River Water: Preconcentration of Metal Ions with a Fruit Waste. Ind. Eng. Chem. Res. 2011, 50, 3446–3451. [Google Scholar] [CrossRef]
- Chakravarty, P.; Sarma, N.S.; Sarma, H.P. Biosorption of cadmium(II) from aqueous solution using heartwood powder of Areca catechu. Chem. Eng. J. 2010, 162, 949–955. [Google Scholar] [CrossRef]
- COELHO, G. F.; GONÇALVES Jr, A. C.; TARLEY, C. R. T.; CASARIN, J.; NACKE, H.; FRANCZISKOWSKI, M. A. Removal of metal ions Cd (II), Pb (II), and Cr (III) from water by the cashew nut shell Anacardium occidentale L. Ecological Engineering 2014, 73, 514–525. [Google Scholar] [CrossRef]
- Dang, V.B.H.; Doan, H.D.; Dang-Vu, T.; Lohi, A. Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour. Technol. 2009, 100, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jing, D.; Gong, H.; Zhou, L.; Yang, X. Biosorption of aquatic cadmium(II) by unmodified rice straw. Bioresour. Technol. 2012, 114, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Hu, J.; Wang, J. Radiation-induced grafting of sweet sorghum stalk for copper(II) removal from aqueous solution. J. Hazard. Mater. 2013, 262, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.M.; Elshaarawy, R.F.; Mahmoud, S.A.; El-Moselhy, K.M. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J. Bioresour. Bioprod. 2021, 6, 223–242. [Google Scholar] [CrossRef]
- Ferreira, G.; Caetano, L.; Castro, R.S.D.; Padilha, P.M.; Castro, G.R. Synthesis, characterization, and application of modified silica in the removal and preconcentration of lead ions from natural river water. Clean Technol. Environ. Policy 2010, 13, 397–402. [Google Scholar] [CrossRef]
- Filho, E.C.S.; Lima, L.C.; Silva, F.C.; Sousa, K.S.; Fonseca, M.G.; Santana, S.A. Immobilization of ethylene sulfide in aminated cellulose for removal of the divalent cations. Carbohydr. Polym. 2013, 92, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- FREUNDLICH, H. M. F. About the adsorption in solution. Zeitschrift für Physikalische Chemie. 1906, 57, 385–471. [Google Scholar]
- GARCÍA- SÁNCHEZ, A.; PÉREZ-ESTEBAN, J.; ESCOLÁSTICO, C.; MARTÍNEZ-ÁLVAREZ, R. M.; IBÁÑEZ, R.; MUÑOZ, M.; VILLAFRANCA-SÁNCHEZ, M. Assessment of selenium (IV) and (VI) levels in potable, irrigation, and wastewater samples from an industrial area in southeastern Spain. Environmental Science and Pollution Research. 2022, 29, 4419–4428. [Google Scholar]
- Ghodbane, I.; Nouri, L.; Hamdaoui, O.; Chiha, M. Kinetic and equilibrium study for the sorption of cadmium(II) ions from aqueous phase by eucalyptus bark. J. Hazard. Mater. 2008, 152, 148–158. [Google Scholar] [CrossRef] [PubMed]
- GÖNEN, F.; SERIN, D. S. Adsorption study on orange peel: removal of Ni(II) ions from aqueous solution. African Journal of Biotechnology. 2012, 11, 1250–1258. [Google Scholar]
- Gonzalez, M.H.; Araújo, G.C.; Pelizaro, C.B.; Menezes, E.A.; Lemos, S.G.; de Sousa, G.B.; Nogueira, A.R.A. Coconut coir as biosorbent for Cr(VI) removal from laboratory wastewater. J. Hazard. Mater. 2008, 159, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, A.; Ozdes, D.; Duran, C.; Bulut, V.N.; Soylak, M.; Senturk, H.B. Biosorption of Pb(II) ions from aqueous solution by pine bark (Pinus brutia Ten.). Chem. Eng. J. 2009, 153, 62–69. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—an overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- GUPTA, N.; KHAN, D. K. Distribution, health risk assessment and statistical source identification of heavy metals in surface water of River Ganges Basin, India. Science of The Total Environment. 2020, 706, 135873. [Google Scholar]
- Gurgel, L.V.A.; Gil, L.F. Adsorption of Cu(II), Cd(II) and Pb(II) from aqueous single metal solutions by succinylated twice-mercerized sugarcane bagasse functionalized with triethylenetetramine. Water Res. 2009, 43, 4479–4488. [Google Scholar] [CrossRef] [PubMed]
- Güzel, F.; Yakut, H.; Topal, G. Determination of kinetic and equilibrium parameters of the batch adsorption of Mn(II), Co(II), Ni(II) and Cu(II) from aqueous solution by black carrot (Daucus carota L.) residues. J. Hazard. Mater. 2008, 153, 1275–1287. [Google Scholar] [CrossRef]
- Hajiaghababaei, L.; Tajmiri, T.; Badiei, A.; Ganjali, M.R.; Khaniani, Y.; Ziarani, G.M. Heavy metals determination in water and food samples after preconcentration by a new nanoporous adsorbent. Food Chem. 2013, 141, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Ngo, H.; Guo, W.; Setiadi, T. Adsorption and desorption of copper(II) ions onto garden grass. Bioresour. Technol. 2012, 121, 386–395. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.; Kadirvelu, K. Cadmium(II) sorption and desorption in a fixed bed column using sunflower waste carbon calcium–alginate beads. Bioresour. Technol. 2012, 129, 242–248. [Google Scholar] [CrossRef]
- JORGETTO, A. O.; SILVA, A. C. P.; CAVECCI, B.; BARBOSA, R. C.; UTRERA, M. A.; CASTRO, G. R. Cassava Root Husks as a Sorbent Material for the Uptake and Pre-concentration of Cadmium(II) from Aqueous Media. Orbital: The Electronic Journal of Chemistry. 2013, 5, 206–212. [Google Scholar]
- Jorgetto, A.; Silva, R.; Longo, M.; Saeki, M.; Padilha, P.; Martines, M.; Rocha, B.; Castro, G. Incorporation of dithiooxamide as a complexing agent into cellulose for the removal and pre-concentration of Cu(II) and Cd(II) ions from natural water samples. Appl. Surf. Sci. 2013, 264, 368–374. [Google Scholar] [CrossRef]
- Jorgetto, A.; Silva, R.; Saeki, M.; Barbosa, R.; Martines, M.; Jorge, S.; Silva, A.; Schneider, J.; Castro, G. Cassava root husks powder as green adsorbent for the removal of Cu(II) from natural river water. Appl. Surf. Sci. 2014, 288, 356–362. [Google Scholar] [CrossRef]
- Jorgetto, A.d.O.; da Silva, A.C.; Wondracek, M.H.; Silva, R.I.; Velini, E.D.; Saeki, M.J.; Pedrosa, V.A.; Castro, G.R. Multilayer adsorption of Cu(II) and Cd(II) over Brazilian Orchid Tree ( Pata-de-vaca ) and its adsorptive properties. Appl. Surf. Sci. 2015, 345, 81–89. [Google Scholar] [CrossRef]
- Kobya, M. Adsorption, Kinetic and Equilibrium Studies of Cr(VI) by Hazelnut Shell Activated Carbon. Adsorpt. Sci. Technol. 2004, 22, 51–64. [Google Scholar] [CrossRef]
- KUMAR, B.; SMITA, K.; SÁNCHEZ, E.; STAEL, C.; CUMBAL, L. Andean Sacha inchi (Plukenetia volubilis L. ) shell biomass as new biosorbents for Pb2+ and Cu2+ ions. Ecological Engineering. 2016, 93, 152–158. [Google Scholar]
- LANGMUIR, I. The constitution and fundamental properties of solids and liquids. Journal of the American Chemical Society. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- LANGMUIR, I. Vapor pressures, evaporation, condensation and adsorption. Journal of the American Chemical Society. 1932, 54, 2798–2832. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- LI, J.; FENG, L.; CHEN, J.; DU, Y.; HU, Y.; LI, S.; XIONG, Y. Adsorption behavior of Cd(II) and Zn(II) by modified granular activated carbon in single and binary systems. Journal of Environmental Sciences. 2021, 106, 81–91. [Google Scholar]
- Liu, C.; Ngo, H.H.; Guo, W.; Tung, K.-L. Optimal conditions for preparation of banana peels, sugarcane bagasse and watermelon rind in removing copper from water. Bioresour. Technol. 2012, 119, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Kuang-Wang, M.; Li, Y.-S. Removal of Nickel from Aqueous Solution Using Wine Processing Waste Sludge. Ind. Eng. Chem. Res. 2005, 44, 1438–1445. [Google Scholar] [CrossRef]
- Madrakian, T.; Zolfigol, M.A.; Solgi, M. Solid-phase extraction method for preconcentration of trace amounts of some metal ions in environmental samples using silica gel modified by 2,4,6-trimorpholino-1,3,5-triazin. J. Hazard. Mater. 2008, 160, 468–472. [Google Scholar] [CrossRef] [PubMed]
- MAHMOUD, M. E.; HAFEZ, O. F.; ALREFAAY, A.; OSMAN, M. M. Performance evaluation of hybrid inorganic/organic adsorbents in removal and preconcentration of heavy metals from drinking and industrial wastewater. Desalination. 2010, 253, 9–15. [Google Scholar] [CrossRef]
- MANAHAN, S. E. Environmental Science Technology and Chemistry, CRC Press LLC, Boca Raton, 2000.
- Martín-Lara, M.A.; Blázquez, G.; Calero, M.; Almendros, A.I.; Ronda, A. Binary biosorption of copper and lead onto pine cone shell in batch reactors and in fixed bed columns. Int. J. Miner. Process. 2016, 148, 72–82. [Google Scholar] [CrossRef]
- Martins, A.E.; Pereira, M.S.; Jorgetto, A.O.; Martines, M.A.; Silva, R.I.; Saeki, M.J.; Castro, G.R. The reactive surface of Castor leaf [Ricinus communis L.] powder as a green adsorbent for the removal of heavy metals from natural river water. Appl. Surf. Sci. 2013, 276, 24–30. [Google Scholar] [CrossRef]
- MATSUMOTO, J.; SATO, M. Seasonal Changes in Nutrient Composition of Persimmon (Diospyros kaki L. F.) Leaves. Journal of the Japanese Society for Horticultural Science. 1997, 66, 421–428. [Google Scholar]
- Mellah, A.; Chegrouche, S.; Barkat, M. The removal of uranium(VI) from aqueous solutions onto activated carbon: Kinetic and thermodynamic investigations. J. Colloid Interface Sci. 2006, 296, 434–441. [Google Scholar] [CrossRef]
- Minello, M.C.S.; Paçó, A.L.; Martines, M.A.U.; Caetano, L.; Dos Santos, A.; Padilha, P.M.; Castro, G.R. Sediment grain size distribution and heavy metals determination in a dam on the Paraná River at Ilha Solteira, Brazil. J. Environ. Sci. Heal. Part A 2009, 44, 861–865. [Google Scholar] [CrossRef]
- Moyo, M.; Guyo, U.; Mawenyiyo, G.; Zinyama, N.P.; Nyamunda, B.C. Marula seed husk (Sclerocarya birrea) biomass as a low cost biosorbent for removal of Pb(II) and Cu(II) from aqueous solution. J. Ind. Eng. Chem. 2015, 27, 126–132. [Google Scholar] [CrossRef]
- Naiya, T.; Bhattacharya, A.; Das, S. Removal of Cd(II) from aqueous solutions using clarified sludge. J. Colloid Interface Sci. 2008, 325, 48–56. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorptive removal of Cd(II) ions from aqueous solutions by rice husk ash. Environ. Prog. Sustain. Energy 2009, 28, 535–546. [Google Scholar] [CrossRef]
- Naiya, T.K.; Chowdhury, P.; Bhattacharya, A.K.; Das, S.K. Saw dust and neem bark as low-cost natural biosorbent for adsorptive removal of Zn(II) and Cd(II) ions from aqueous solutions. Chem. Eng. J. 2009, 148, 68–79. [Google Scholar] [CrossRef]
- Pandey, R.; Prasad, R.L.; Ansari, N.G.; Murthy, R.C. Utilization of NaOH modified Desmostachya bipinnata (Kush grass) leaves and Bambusa arundinacea (bamboo) leaves for Cd(II) removal from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 593–602. [Google Scholar] [CrossRef]
- PEARSON, G. R. Hard and soft acids and bases. Journal of the American Chemical Society. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Peng, X.-W.; Zhong, L.-X.; Ren, J.-L.; Sun, R.-C. Highly Effective Adsorption of Heavy Metal Ions from Aqueous Solutions by Macroporous Xylan-Rich Hemicelluloses-Based Hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.G.; Pescara, I.C.; Evangelista, S.M.; Holanda, M.S.; Andrade, R.D.; Suarez, P.A.; Zara, L.F. Adsorption and preconcentration of divalent metal ions in fossil fuels and biofuels: Gasoline, diesel, biodiesel, diesel-like and ethanol by using chitosan microspheres and thermodynamic approach. Talanta 2011, 84, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Islam, M.R. Effects of pH on isotherms modeling for Cu(II) ions adsorption using maple wood sawdust. Chem. Eng. J. 2009, 149, 273–280. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Nandagopal, M.G.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural byproducts. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Ikram, S. Sorption studies of Cu(II) on gooseberry fruit (emblica officinalis) and its removal from electroplating wastewater. Desalination 2011, 277, 390–398. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Ikram, S.; Uddin, M.K. Removal of Cd(II) from aqueous solution by exploring the biosorption characteristics of gaozaban (Onosma bracteatum). J. Environ. Chem. Eng. 2014, 2, 1155–1164. [Google Scholar] [CrossRef]
- REEVE, R. N. Introduction to Environmental Analysis, John Wiley & Sons LTD, Chichester, 2002.
- RIBEIRO, C. M. R.; SOUZA, N. A. General scheme for elucidating the structure of organic compounds using spectroscopic and spectrometric methods. Química Nova. 2007, 30, 1026–1031. [Google Scholar] [CrossRef]
- Ryu, S.; Furihata, K.; Koda, M.; Wei, F.; Miyakawa, T.; Tanokura, M. NMR-based analysis of the chemical composition of Japanese persimmon aqueous extracts. 2015, 54, 213–221. [CrossRef]
- Sepehri, S.; Kanani, E.; Abdoli, S.; Rajput, V.D.; Minkina, T.; Lajayer, B.A. Pb(II) Removal from Aqueous Solutions by Adsorption on Stabilized Zero-Valent Iron Nanoparticles—A Green Approach. Water 2023, 15, 222. [Google Scholar] [CrossRef]
- Silva, A.C.P.; Jorgetto, A.O.; Wondracek, M.H.P.; Saeki, M.J.; Schneider, J.F.; Pedrosa, V.A.; Martines, M.A.U.; Castro, G.R. Characterization of Corn (Zea mays) Leaf Powder and Its Adsorption Properties Regarding Cu(II) and Cd(II) from Aqueous Samples. BioResources 2014, 10, 1099–1114. [Google Scholar] [CrossRef]
- Singha, B.; Das, S.K. Adsorptive removal of Cu(II) from aqueous solution and industrial effluent using natural/agricultural wastes. Colloids Surfaces B Biointerfaces 2013, 107, 97–106. [Google Scholar] [CrossRef] [PubMed]
- SOUZA, E. J.; CRISTANTE, V. M.; PADILHA, P. M.; JORGE, S. M. A.; MARTINES, M. A. U.; SILVA, R. I. V.; CARMO, D. R.; CASTRO, G. R. Attachment of 2,2-bypiridine onto a silica gel for application as a sequestering agent for copper, cadmium and lead ions from an aqueous medium. Polish Journal of Chemical Technology. 2011, 13, 28–33. [Google Scholar] [CrossRef]
- Sousa, F.W.; Oliveira, A.G.; Ribeiro, J.P.; Rosa, M.F.; Keukeleire, D.; Nascimento, R.F. Green coconut shells applied as adsorbent for removal of toxic metal ions using fixed-bed column technology. J. Environ. Manag. 2010, 91, 1634–1640. [Google Scholar] [CrossRef]
- Tan, W.-F.; Lu, S.-J.; Liu, F.; Feng, X.-H.; He, J.-Z.; Koopal, L.K. Determination of the point-of-zero charge of manganese oxides with different methods including an improved salt titration method. Soil Sci. 2008, 173, 277–286. [Google Scholar] [CrossRef]
- Tounsadi, H.; Khalidi, A.; Abdennouri, M.; Barka, N. Biosorption potential of Diplotaxis harra and Glebionis coronaria L. biomasses for the removal of Cd(II) and Co(II) from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 822–830. [Google Scholar] [CrossRef]
- VAGHETTI, J. C. P. Utilização de biossorventes para remediação de efluentes aquo sos contaminados com íons metálicos. Data de defesa: 2009. 84 f. Tese (Doutorado). Universidade Federal do Rio Grande do Sul. Instituto de Química. Porto Alegre. 2009.
- Velazquez-Jimenez, L.H.; Pavlick, A.; Rangel-Mendez, J.R. Chemical characterization of raw and treated agave bagasse and its potential as adsorbent of metal cations from water. Ind. Crop. Prod. 2013, 43, 200–206. [Google Scholar] [CrossRef]
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511. [Google Scholar] [CrossRef]
- XIE, C.; XIE, Z.; XU, X.; YANG, D. Persimmon (Diospyros kaki L. ) leaves: A review on traditional uses, phytochemistry and pharmacological properties. Journal of Ethnopharmacology. 2015, 163, 229–240. [Google Scholar]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef]
- ZHANG, Y.; LI, X.; ZHOU, Y. Application of peanut shell biochar for heavy metal removal from wastewater: A review. Environmental Science and Pollution Research. 2021, 28, 4802–4817. [Google Scholar]
- ZHANG, Z.; WANG, Q.; YAO, L. A review on ecological risk assessment and potential health risk of toxic heavy metals in abandoned mining areas. Environmental Science and Pollution Research. 2021, 28, 10843–10856. [Google Scholar]
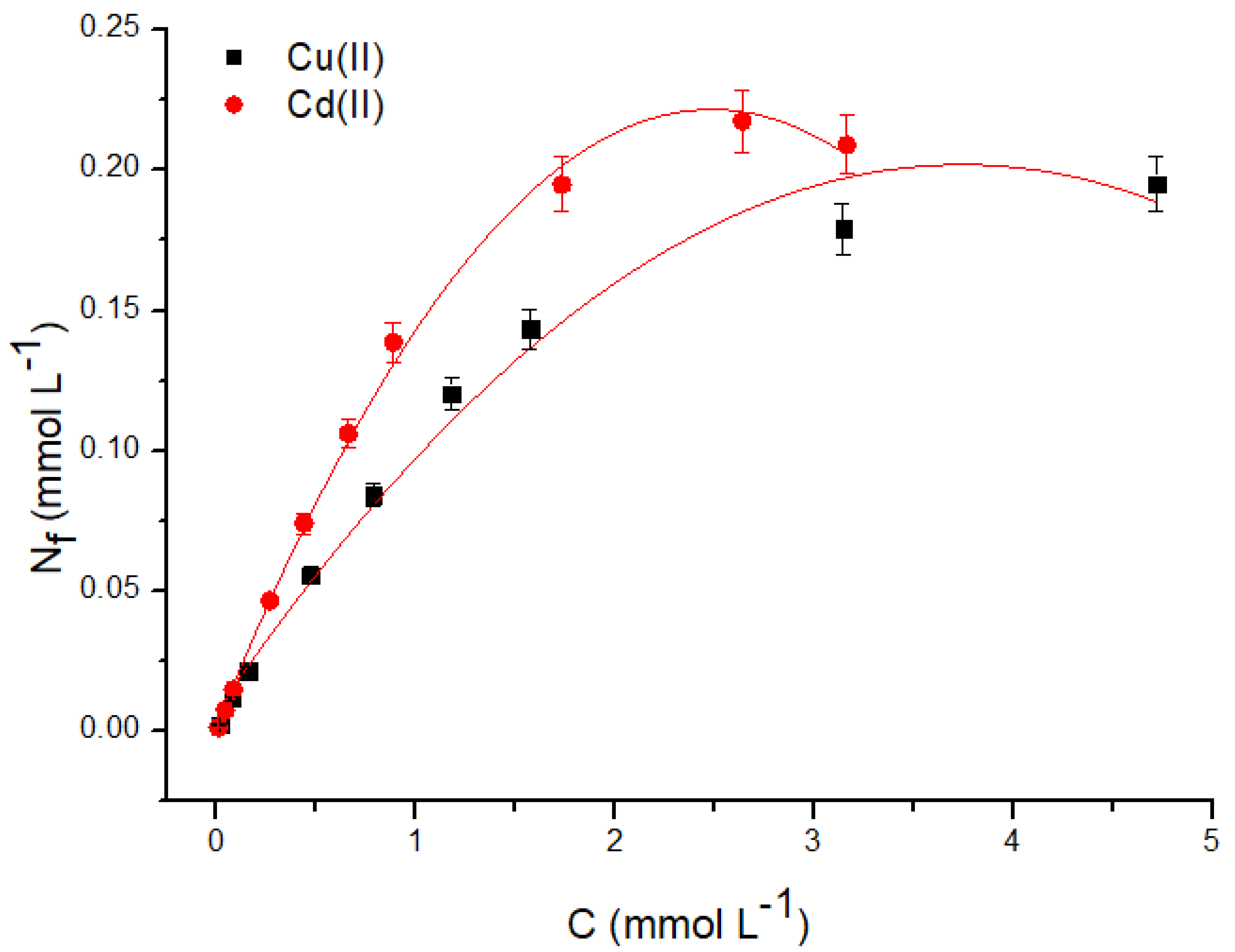
| Metal |
Nfmax (exp.) (mmol g-1) |
Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
|
b (L mmol-1) |
Ns (calc.) (mmol g-1) |
r2 |
Kf (mmol1-(1/n) L1/n g-1) |
n | r2 | ||
| Cu(II) | 0.19 | 2.84 | 0.213 | 0.950 | 0.893 | 1.82 | 0.934 |
| Cd(II) | 0.23 | 32.5 | 0.215 | 0.998 | 4.90 | 2.58 | 0.904 |

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





