Submitted:
18 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology
3. Pathophysiology
3.1. Animal model studies
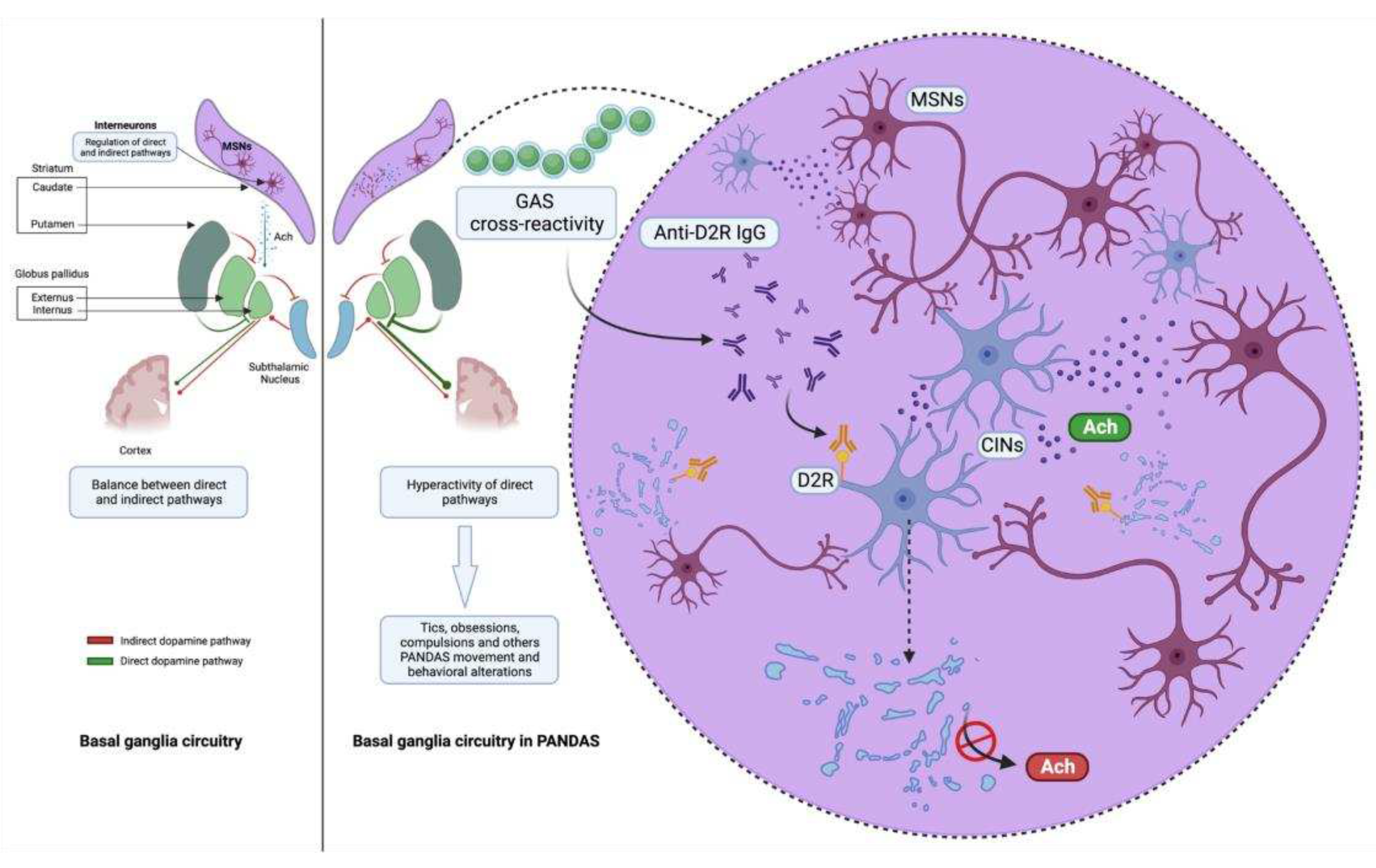
3.2. Striatal cholinergic interneurons: latest evidence of a new key player in PANDAS pathophysiology
3.3. Imaging studies
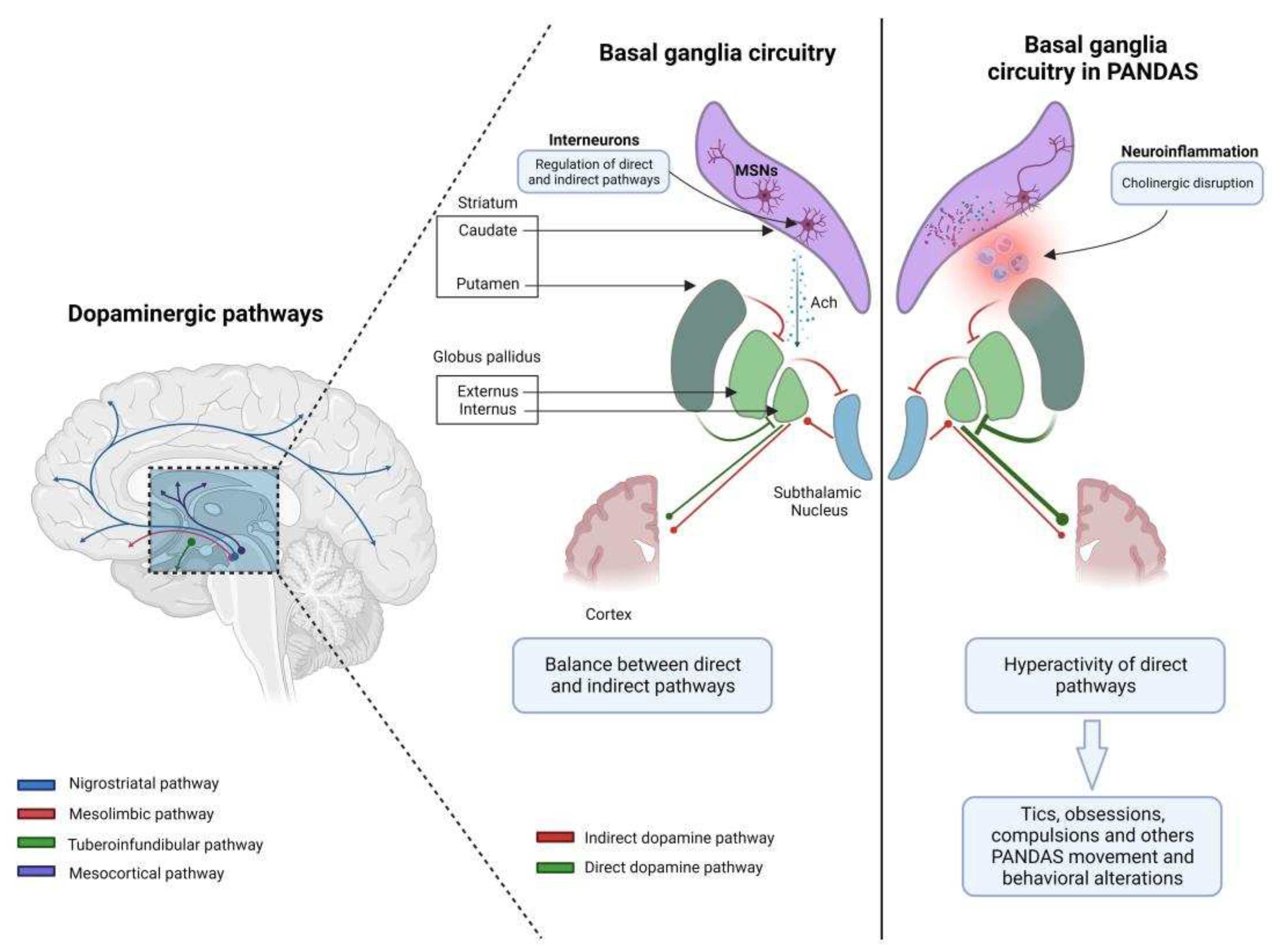
3.4. The complex network of basal ganglia: from physiology to pathophysiology
4. Clinical features and classification criteria
- (1)
- Presence of OCD and/or a tic disorder;
- (2)
- Prepubertal symptom onset;
- (3)
- Acute onset of symptoms with a relapsing/remitting disease course;
- (4)
- A clear temporal association between GAS infection and symptoms’ onset or exacerbation;
- (5)
- Association with other neurological abnormalities (particularly motoric hyperactivity and choreiform movements).
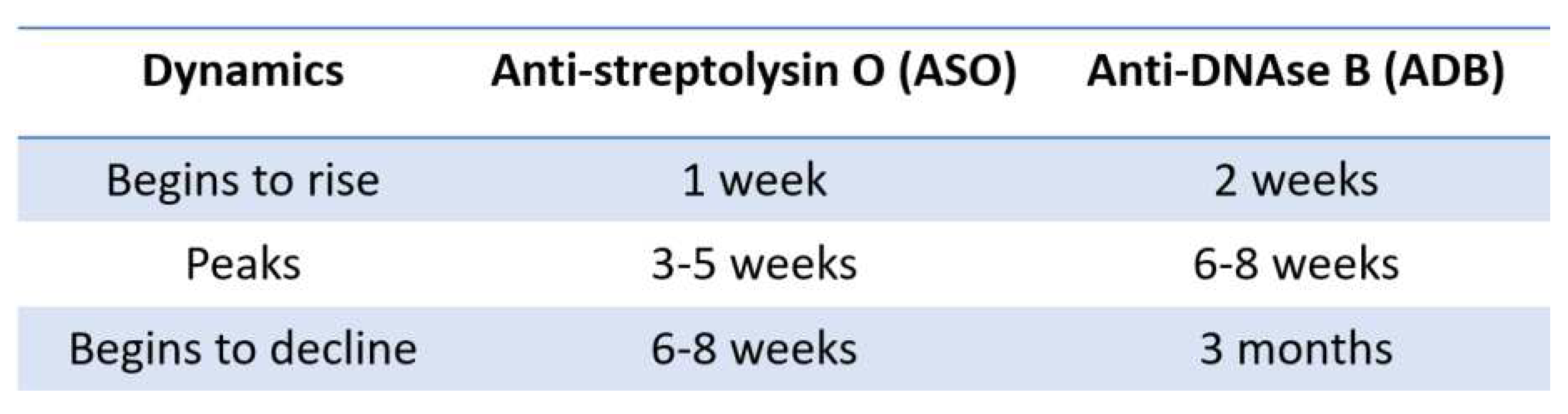
4.1. The causal and temporal relationship associated with GAS infection
- (1)
- GAS infection are common issues among children attending communities and schools;
- (2)
- In a similar way, vocal and/or motor tics and OCD are not uncommon in children;
- (3)
- ASO and ADB may be found elevated also in older GAS infections, resulting in false-positive tests.
- (4)
- ASO may rise due to chronic liver disease, hypergammaglobulinemia, hypercholesterolemia [50].
4.2. Clinical characteristics
5. Treatment options and disease course
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swedo, S.E.; Leonard, H.L.; Garvey, M.; Mittleman, B.; Allen, A.J.; Perlmutter, S.; Dow, S.; Zamkoff, J.; Dubbert, B.K.; Lougee, L. Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections: Clinical Description of the First 50 Cases. Am. J. Psychiatry 1998, 155, 264–271. [Google Scholar] [CrossRef]
- Gewitz, M.H.; Baltimore, R.S.; Tani, L.Y.; Sable, C.A.; Shulman, S.T.; Carapetis, J.; Remenyi, B.; Taubert, K.A.; Bolger, A.F.; Beerman, L.; et al. Revision of the Jones Criteria for the Diagnosis of Acute Rheumatic Fever in the Era of Doppler Echocardiography: A Scientific Statement from the American Heart Association. Circulation 2015, 131, 1806–1818. [Google Scholar] [CrossRef] [PubMed]
- Oosterveer, D.M.; Overweg-Plandsoen, W.C.T.; Roos, R.A.C. Sydenham’s Chorea: A Practical Overview of the Current Literature. Pediatr. Neurol. 2010, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Grant, J.E.; Kim, S.I.; Swanson, T.A.; Bernstein, G.A.; Jaszcz, W.B.; Williams, K.A.; Schlievert, P.M. A Possible Association of Recurrent Streptococcal Infections and Acute Onset of Obsessive-Compulsive Disorder. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F. Sydenham’s Chorea. Handb. Clin. Neurol. 2011, 100, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, M.T.; Campos, M.C.; Marques-Dias, M.J.; Miguel, E.C.; Leckman, J. Vocal Tics in Sydenham’s Chorea. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Perrin, E.M.; Murphy, M.L.; Casey, J.R.; Pichichero, M.E.; Runyan, D.K.; Miller, W.C.; Snider, L.A.; Swedo, S.E. Does Group A β-Hemolytic Streptococcal Infection Increase Risk for Behavioral and Neuropsychiatric Symptoms in Children? Arch. Pediatr. Adolesc. Med. 2004, 158, 848. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, F.; Spitoni, S.; Hollander, E.; Matucci Cerinic, M.; Pallanti, S. An Expert Opinion on PANDAS/PANS: Highlights and Controversies. Int. J. Psychiatry Clin. Pract. 2017, 21, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, C.; Bitnun, A.; Kronenberg, S.; Laxer, R.M.; Levy, D.M.; Logan, W.J.; Shouldice, M.; Yeh, E.A. PANDAS/PANS in Childhood: Controversies and Evidence. Paediatr. Child Health 2019, 24, 85–91. [Google Scholar] [CrossRef]
- Prato, A.; Gulisano, M.; Scerbo, M.; Barone, R.; Vicario, C.M.; Rizzo, R. Diagnostic Approach to Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections (PANDAS): A Narrative Review of Literature Data. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Calaprice, D.; Tona, J.; Parker-Athill, E.C.; Murphy, T.K. A Survey of Pediatric Acute-Onset Neuropsychiatric Syndrome Characteristics and Course. J. Child Adolesc. Psychopharmacol. 2017, 27, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Swedo, S.E.; Leonard, H.L.; Rapoport, J.L. The Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infection (PANDAS) Subgroup: Separating Fact From Fiction. Pediatrics 2004, 113, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W.; Cox, C.J. Autoimmunity against Dopamine Receptors in Neuropsychiatric and Movement Disorders: A Review of Sydenham Chorea and Beyond. Acta Physiol. 2016, 216, 90–100. [Google Scholar] [CrossRef]
- Cox, C.J.; Sharma, M.; Leckman, J.F.; Zuccolo, J.; Zuccolo, A.; Kovoor, A.; Swedo, S.E.; Cunningham, M.W. Brain Human Monoclonal Autoantibody from Sydenham Chorea Targets Dopaminergic Neurons in Transgenic Mice and Signals Dopamine D2 Receptor: Implications in Human Disease. J. Immunol. Baltim. Md 1950 2013, 191, 5524–5541. [Google Scholar] [CrossRef] [PubMed]
- Ben-Pazi, H.; Stoner, J.A.; Cunningham, M.W. Dopamine Receptor Autoantibodies Correlate with Symptoms in Sydenham’s Chorea. PloS One 2013, 8, e73516. [Google Scholar] [CrossRef] [PubMed]
- Kirvan, C.A.; Swedo, S.E.; Heuser, J.S.; Cunningham, M.W. Mimicry and Autoantibody-Mediated Neuronal Cell Signaling in Sydenham Chorea. Nat. Med. 2003, 9, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.L.; Hornig, M.; Yaddanapudi, K.; Jabado, O.; Lipkin, W.I. A Murine Model for Neuropsychiatric Disorders Associated with Group A Beta-Hemolytic Streptococcal Infection. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 1780–1791. [Google Scholar] [CrossRef]
- Yaddanapudi, K.; Hornig, M.; Serge, R.; De Miranda, J.; Baghban, A.; Villar, G.; Lipkin, W.I. Passive Transfer of Streptococcus-Induced Antibodies Reproduces Behavioral Disturbances in a Mouse Model of Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infection. Mol. Psychiatry 2010, 15, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Brimberg, L.; Benhar, I.; Mascaro-Blanco, A.; Alvarez, K.; Lotan, D.; Winter, C.; Klein, J.; Moses, A.E.; Somnier, F.E.; Leckman, J.F.; et al. Behavioral, Pharmacological, and Immunological Abnormalities after Streptococcal Exposure: A Novel Rat Model of Sydenham Chorea and Related Neuropsychiatric Disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Dale, R.C.; Merheb, V.; Pillai, S.; Wang, D.; Cantrill, L.; Murphy, T.K.; Ben-Pazi, H.; Varadkar, S.; Aumann, T.D.; Horne, M.K.; et al. Antibodies to Surface Dopamine-2 Receptor in Autoimmune Movement and Psychiatric Disorders. Brain 2012, 135, 3453–3468. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, R.-J.; Fahey, S.; Frick, L.; Leckman, J.; Vaccarino, F.; Duman, R.S.; Williams, K.; Swedo, S.; Pittenger, C. Antibodies From Children With PANDAS Bind Specifically to Striatal Cholinergic Interneurons and Alter Their Activity. Am. J. Psychiatry 2021, 178, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Bertran-Gonzalez, J.; Chieng, B.C.; Laurent, V.; Valjent, E.; Balleine, B.W. Striatal Cholinergic Interneurons Display Activity-Related Phosphorylation of Ribosomal Protein S6. PLoS ONE 2012, 7, e53195. [Google Scholar] [CrossRef] [PubMed]
- Lennington, J.B.; Coppola, G.; Kataoka-Sasaki, Y.; Fernandez, T.V.; Palejev, D.; Li, Y.; Huttner, A.; Pletikos, M.; Sestan, N.; Leckman, J.F.; et al. Transcriptome Analysis of the Human Striatum in Tourette Syndrome. Biol. Psychiatry 2016, 79, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Kalanithi, P.S.A.; Grantz, H.; Schwartz, M.L.; Saper, C.; Leckman, J.F.; Vaccarino, F.M. Decreased Number of Parvalbumin and Cholinergic Interneurons in the Striatum of Individuals with Tourette Syndrome. J. Comp. Neurol. 2010, 518, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Williams, M.T.; Chugani, H.T. Evaluation of Basal Ganglia and Thalamic Inflammation in Children With Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infection and Tourette Syndrome: A Positron Emission Tomographic (PET) Study Using 11 C-[R]-PK11195. J. Child Neurol. 2015, 30, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Rapoport, J.L.; Garvey, M.A.; Perlmutter, S.; Swedo, S.E. MRI Assessment of Children with Obsessive-Compulsive Disorder or Tics Associated with Streptococcal Infection. Am. J. Psychiatry 2000, 157, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Fazl, A.; Fleisher, J. Anatomy, Physiology, and Clinical Syndromes of the Basal Ganglia: A Brief Review. Semin. Pediatr. Neurol. 2018, 25, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Effects of Acetylcholine in the Striatum: Recent Insights and Therapeutic Implications. Neurology 2012, 79, 274–281. [Google Scholar] [CrossRef]
- DeLong, M.; Wichmann, T. Update on Models of Basal Ganglia Function and Dysfunction. Parkinsonism Relat. Disord. 2009, 15, S237–S240. [Google Scholar] [CrossRef]
- Graybiel, A.M. Neurochemically Specified Subsystems in the Basal Ganglia. In Novartis Foundation Symposia; Evered, D., O’Connor, M., Eds.; Wiley, 1984; pp. 114–149. ISBN 978-0-470-66425-4.
- Van Vulpen, E.H.S.; Van Der Kooy, D. Striatal Cholinergic Interneurons: Birthdates Predict Compartmental Localization. Dev. Brain Res. 1998, 109, 51–58. [Google Scholar] [CrossRef]
- Oldenburg, I.A.; Ding, J.B. Cholinergic Modulation of Synaptic Integration and Dendritic Excitability in the Striatum. Curr. Opin. Neurobiol. 2011, 21, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Aliane, V.; Pérez, S.; Bohren, Y.; Deniau, J.-M.; Kemel, M.-L. Key Role of Striatal Cholinergic Interneurons in Processes Leading to Arrest of Motor Stereotypies. Brain 2011, 134, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kilbertus, S.; Brannan, R.; Sell, E.; Doja, A. No Cases of PANDAS on Follow-Up of Patients Referred to a Pediatric Movement Disorders Clinic. Front. Pediatr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Jaspers-Fayer, F.; Han, S.H.J.; Chan, E.; McKenney, K.; Simpson, A.; Boyle, A.; Ellwyn, R.; Stewart, S.E. Prevalence of Acute-Onset Subtypes in Pediatric Obsessive-Compulsive Disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Gamucci, A.; Uccella, S.; Sciarretta, L.; D’Apruzzo, M.; Calevo, M.G.; Mancardi, M.M.; Veneselli, E.; De Grandis, E. PANDAS and PANS: Clinical, Neuropsychological, and Biological Characterization of a Monocentric Series of Patients and Proposal for a Diagnostic Protocol. J. Child Adolesc. Psychopharmacol. 2019, 29, 305–312. [Google Scholar] [CrossRef]
- Baj, J.; Sitarz, E.; Forma, A.; Wróblewska, K.; Karakuła-Juchnowicz, H. Alterations in the Nervous System and Gut Microbiota after β-Hemolytic Streptococcus Group A Infection—Characteristics and Diagnostic Criteria of PANDAS Recognition. Int. J. Mol. Sci. 2020, 21, 1476. [Google Scholar] [CrossRef] [PubMed]
- Thienemann, M.; Murphy, T.; Leckman, J.; Shaw, R.; Williams, K.; Kapphahn, C.; Frankovich, J.; Geller, D.; Bernstein, G.; Chang, K.; et al. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part I—Psychiatric and Behavioral Interventions. J. Child Adolesc. Psychopharmacol. 2017, 27, 566–573. [Google Scholar] [CrossRef]
- Mell, L.K.; Davis, R.L.; Owens, D. Association between Streptococcal Infection and Obsessive-Compulsive Disorder, Tourette’s Syndrome, and Tic Disorder. Pediatrics 2005, 116, 56–60. [Google Scholar] [CrossRef]
- Murphy, T.K.; Snider, L.A.; Mutch, P.J.; Harden, E.; Zaytoun, A.; Edge, P.J.; Storch, E.A.; Yang, M.C.K.; Mann, G.; Goodman, W.K.; et al. Relationship of Movements and Behaviors to Group A Streptococcus Infections in Elementary School Children. Biol. Psychiatry 2007, 61, 279–284. [Google Scholar] [CrossRef]
- Orlovska, S.; Vestergaard, C.H.; Bech, B.H.; Nordentoft, M.; Vestergaard, M.; Benros, M.E. Association of Streptococcal Throat Infection With Mental Disorders: Testing Key Aspects of the PANDAS Hypothesis in a Nationwide Study. JAMA Psychiatry 2017, 74, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; King, R.A.; Gilbert, D.L.; Coffey, B.J.; Singer, H.S.; Dure, L.S.; Grantz, H.; Katsovich, L.; Lin, H.; Lombroso, P.J.; et al. Streptococcal Upper Respiratory Tract Infections and Exacerbations of Tic and Obsessive-Compulsive Symptoms: A Prospective Longitudinal Study. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kurlan, R.; Johnson, D.; Kaplan, E.L. ; and the Tourette Syndrome Study Group Streptococcal Infection and Exacerbations of Childhood Tics and Obsessive-Compulsive Symptoms: A Prospective Blinded Cohort Study. Pediatrics 2008, 121, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.Ø.; Köhler-Forsberg, O.; Hjorthøj, C.; Benros, M.E.; Nordentoft, M.; Orlovska-Waast, S. Streptococcal Infections and Exacerbations in PANDAS: A Systematic Review and Meta-Analysis. Pediatr. Infect. Dis. J. 2019, 38, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Delice, S. Detection of Anti- DNase B Antibody Upper Normal Values in Children’s Age Groups Who Were Admitted to Hospital with Noninfectious Reasons. North. Clin. Istanb. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.S.; Ramanan, A.V. How to Use Antistreptolysin O Titre. Arch. Dis. Child. - Educ. Pract. Ed. 2014, 99, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Bertille, N.; Cohen, R.; Chalumeau, M. Rapid Antigen Detection Test for Group A Streptococcus in Children with Pharyngitis. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Frankovich, J.; Cooperstock, M.; Cunningham, M.W.; Latimer, M.E.; Murphy, T.K.; Pasternack, M.; Thienemann, M.; Williams, K.; Walter, J.; et al. Clinical Evaluation of Youth with Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS): Recommendations from the 2013 PANS Consensus Conference. J. Child Adolesc. Psychopharmacol. 2015, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Feigin & Cherry’s Textbook of Pediatric Infectious Diseases; Feigin, R.D., Ed.; 6th ed.; Saunders/Elsevier: Philadelphia, PA, 2009. ISBN 978-1-4160-4044-6.
- Shet, A.; Kaplan, E.L. Clinical Use and Interpretation of Group A Streptococcal Antibody Tests: A Practical Approach for the Pediatrician or Primary Care Physician. Pediatr. Infect. Dis. J. 2002, 21, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Kurlan, R.; Leckman, J.; Kaplan, E.L. The Human Immune Response to Streptococcal Extracellular Antigens: Clinical, Diagnostic, and Potential Pathogenetic Implications. Clin. Infect. Dis. 2010, 50, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Jadah, R.H.S.; Mujeeb, A.A. Neuropsychiatric Symptoms Following Sore Throat in a Young Boy. BMJ Case Rep. 2019, 12, e227540. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.K.; Kurlan, R.; Leckman, J. The Immunobiology of Tourette’s Disorder, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcus, and Related Disorders: A Way Forward. J. Child Adolesc. Psychopharmacol. 2010, 20, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.J.; Leonard, H.L.; Swedo, S.E. Case Study: A New Infection-Triggered, Autoimmune Subtype of Pediatric OCD and Tourette’s Syndrome. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, G.A.; Victor, A.M.; Pipal, A.J.; Williams, K.A. Comparison of Clinical Characteristics of Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections and Childhood Obsessive-Compulsive Disorder. J. Child Adolesc. Psychopharmacol. 2010, 20, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Calkin, C.V.; Carandang, C.G. Certain Eating Disorders May Be a Neuropsychiatric Manifestation of PANDAS: Case Report. J. Can. Acad. Child Adolesc. Psychiatry 2007, 16, 132–135. [Google Scholar] [PubMed]
- Williams, K.A.; Swedo, S.E. Post-Infectious Autoimmune Disorders: Sydenham’s Chorea, PANDAS and Beyond. Brain Res. 2015, 1617, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.L.; Pichichero, M.E. Prospective Identification and Treatment of Children With Pediatric Autoimmune Neuropsychiatric Disorder Associated With Group A Streptococcal Infection (PANDAS). Arch. Pediatr. Adolesc. Med. 2002, 156, 356. [Google Scholar] [CrossRef]
- Crealey, M.; Allen, N.M.; Webb, D.; Bouldin, A.; Mc Sweeney, N.; Peake, D.; Tirupathi, S.; Butler, K.; King, M.D. Sydenham’s Chorea: Not Gone but Perhaps Forgotten. Arch. Dis. Child. 2015, 100, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.K.; Goodman, W.K.; Ayoub, E.M.; Voeller, K.K. On Defining Sydenham’s Chorea: Where Do We Draw the Line? Biol. Psychiatry 2000, 47, 851–857. [Google Scholar] [CrossRef]
- Leon, J.; Hommer, R.; Grant, P.; Farmer, C.; D’Souza, P.; Kessler, R.; Williams, K.; Leckman, J.F.; Swedo, S. Longitudinal Outcomes of Children with Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infections (PANDAS). Eur. Child Adolesc. Psychiatry 2018, 27, 637–643. [Google Scholar] [CrossRef]
- Murphy, T.K.; Storch, E.A.; Lewin, A.B.; Edge, P.J.; Goodman, W.K. Clinical Factors Associated with Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. J. Pediatr. 2012, 160, 314–319. [Google Scholar] [CrossRef]
- Leckman, J.F.; King, R.A.; Gilbert, D.L.; Coffey, B.J.; Singer, H.S.; Dure Iv, L.S.; Grantz, H.; Katsovich, L.; Lin, H.; Lombroso, P.J. Streptococcal Upper Respiratory Tract Infections and Exacerbations of Tic and Obsessive-Compulsive Symptoms: A Prospective Longitudinal Study. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Orefici, G.; Cardona, F.; Cox, C.J.; Cunningham, M.W. Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS). In Streptococcus pyogenes : Basic Biology to Clinical Manifestations [Internet]; University of Oklahoma Health Sciences Center, 2016.
- O’Dor, S.L.; Homayoun, S.; Downer, O.M.; Hamel, M.A.; Zagaroli, J.S.; Williams, K.A. A Survey of Demographics, Symptom Course, Family History, and Barriers to Treatment in Children with Pediatric Acute-Onset Neuropsychiatric Disorders and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infections. J. Child Adolesc. Psychopharmacol. 2022, 32, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Burchi, E.; Pallanti, S. Antibiotics for PANDAS? Limited Evidence: Review and Putative Mechanisms of Action. Prim. Care Companion CNS Disord. 2018, 20, 17r02232. [Google Scholar] [CrossRef] [PubMed]
- Garvey, M.A.; Perlmutter, S.J.; Allen, A.J.; Hamburger, S.; Lougee, L.; Leonard, H.L.; Witowski, M.E.; Dubbert, B.; Swedo, S.E. A Pilot Study of Penicillin Prophylaxis for Neuropsychiatric Exacerbations Triggered by Streptococcal Infections. Biol. Psychiatry 1999, 45, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Snider, L.A.; Lougee, L.; Slattery, M.; Grant, P.; Swedo, S.E. Antibiotic Prophylaxis with Azithromycin or Penicillin for Childhood-Onset Neuropsychiatric Disorders. Biol. Psychiatry 2005, 57, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Lau, C.-I.; Lin, C.-C.; Chang, A.; Kao, C.-H. Group A Streptococcal Infections Are Associated With Increased Risk of Pediatric Neuropsychiatric Disorders: A Taiwanese Population-Based Cohort Study. J. Clin. Psychiatry 2016, 77, e848–854. [Google Scholar] [CrossRef]
- Hesselmark, E.; Bejerot, S. Patient Satisfaction and Treatments Offered to Swedish Patients with Suspected Pediatric Acute-Onset Neuropsychiatric Syndrome and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. J. Child Adolesc. Psychopharmacol. 2019, 29, 634–641. [Google Scholar] [CrossRef]
- Pavone, P.; Rapisarda, V.; Serra, A.; Nicita, F.; Spalice, A.; Parano, E.; Rizzo, R.; Maiolino, L.; Di Mauro, P.; Vitaliti, G.; et al. Pediatric Autoimmune Neuropsychiatric Disorder Associated with Group a Streptococcal Infection: The Role of Surgical Treatment. Int. J. Immunopathol. Pharmacol. 2014, 27, 371–378. [Google Scholar] [CrossRef]
- Perlmutter, S.J.; Leitman, S.F.; Garvey, M.A.; Hamburger, S.; Feldman, E.; Leonard, H.L.; Swedo, S.E. Therapeutic Plasma Exchange and Intravenous Immunoglobulin for Obsessive-Compulsive Disorder and Tic Disorders in Childhood. Lancet Lond. Engl. 1999, 354, 1153–1158. [Google Scholar] [CrossRef]
- Frankovich, J.; Swedo, S.; Murphy, T.; Dale, R.C.; Agalliu, D.; Williams, K.; Daines, M.; Hornig, M.; Chugani, H.; Sanger, T.; et al. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part II-Use of Immunomodulatory Therapies. J. Child Adolesc. Psychopharmacol. 2017, 27, 574–593. [Google Scholar] [CrossRef]
- Tucker, D.M.; Leckman, J.F.; Scahill, L.; Wilf, G.E.; LaCamera, R.; Cardona, L.; Cohen, P.; Heidmann, S.; Goldstein, J.; Judge, J.; et al. A Putative Poststreptococcal Case of OCD with Chronic Tic Disorder, Not Otherwise Specified. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Beşiroğlu, L.; Ağargün, M.Y.; Ozbebit, O.; Sözen, M.; Dilek, I.; Güleç, M. [Therapeutic response to plasmapheresis in four cases with obsessive-compulsive disorder and tic disorder triggered by streptococcal infections]. Turk Psikiyatri Derg. Turk. J. Psychiatry 2007, 18, 270–276. [Google Scholar]
- Hachiya, Y.; Miyata, R.; Tanuma, N.; Hongou, K.; Tanaka, K.; Shimoda, K.; Kanda, S.; Hoshino, A.; Hanafusa, Y.; Kumada, S.; et al. Autoimmune Neurological Disorders Associated with Group-A Beta-Hemolytic Streptococcal Infection. Brain Dev. 2013, 35, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Swedo, S.E.; Farmer, C.A.; Grantz, H.; Grant, P.J.; D’Souza, P.; Hommer, R.; Katsovich, L.; King, R.A.; Leckman, J.F. Randomized, Controlled Trial of Intravenous Immunoglobulin for Pediatric Autoimmune Neuropsychiatric Disorders Associated With Streptococcal Infections. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 860–867. [Google Scholar] [CrossRef]
- Bellanti, J.A. The PANDAS/PANS Disorders. Is It Time for More Allergist-Immunologists to Get Involved? Allergy Asthma Proc. 2023, 44, 296–305. [Google Scholar] [CrossRef]
- Cocuzza, S.; Maniaci, A.; La Mantia, I.; Nocera, F.; Caruso, D.; Caruso, S.; Iannella, G.; Vicini, C.; Privitera, E.; Lechien, J.R.; et al. Obsessive-Compulsive Disorder in PANS/PANDAS in Children: In Search of a Qualified Treatment-A Systematic Review and Metanalysis. Child. Basel Switz. 2022, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Cooperstock, M.S.; Swedo, S.E.; Pasternack, M.S.; Murphy, T.K. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part III-Treatment and Prevention of Infections. J. Child Adolesc. Psychopharmacol. 2017, 27, 594–606. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




