1. Introduction
The Tibeto-Himalayan Mountain ranges, which evolved in the Eocene, also known as the “third pole,” have an essential role in global climate dynamics (Mosbrugger et al., 2018) and have a complex and high biodiversity (Rana et al., 2021). The Himalayan ecosystems are among the most sensitive, especially under the climate change scenario, and the most vulnerable global biodiversity hotspots (Myers et al., 2020, Negi and Rawal 2019). Unfortunately, the flora and fauna of the Himalayan regions are among the least studied (Mehta et al., 2023), and the paucity of data on several regions, or even groups of organisms are worrisome, especially of fungi and their hosts (Rana et al., 2021).
Kumar et al., (2018) found that, in general, fungi studies in India are limited and that, to date, only 7-8% of the total diversity has been reported. They also found that the fungi:plant ratio was 10.6:1. They stated the dire need for long-term numerical studies of fungi from the different regions of India, especially because many contain medicinal chemicals important for human welfare. They pointed out that the data for the northwest Himalayas needs to be improved and that it is pertinent to record baseline data because of the fast pace at which the forests are being cut down despite most regions being protected areas.
There are few studies examining or reviewing the biodiversity in the western Indian Himalayan region (see Mosbrugger et al., 2018, Dahal et al., 2021), with most studies in the central region of Nepal (Rana et al., 2021). The western Himalayan region provides essential habitat for various ectomycorrhizal fungi, and fungal diversity is assessed by collecting sporocarps associated with host roots and often associated with pine (Itoo et al.,, 2014). Fungal biodiversity in the Himalayan region is vital for ecosystem services like Ganodermataceae, which provides many ecosystem services to local human communities through food and medicinal uses (Wu and Yang 2021).
Families such as the Ganodermataceae also provide a wide range of ecosystem services. They can significantly impact soil microbial composition, soil properties, tree growth, and medicinal values like antitumor, anti-inflammatory, and detoxifying (Ren et al., 2020). On the other hand, other species of the fungal families, such as Polyporaceae and Hymenochaetaceae, are abundant in this region and regulate ecosystem services such as nutrient cycling as they decompose dead and decaying wood, i.e., they are saprotrophic (Devkota et al., 2023).
In the western Himalayas, in forests of the Sarkaghat region of Mandi, Himachal Pradesh, ectomycorrhizal fungi were recently reported to have several families, including Fomitopsidaceae, Ganodermateceae, Hymeonochaetaceae, Polyporaceae and Schizophyllaceae,. However, the study also noted a need for more data on their geographical distribution (Chander et al., 2017). In Central Europe, wood-inhabiting fungal communities in the Fagus (Beech) and Picea (Spruce) tree communities where saprotrophic fungal families (namely Polyporaceae, Peniophoraceae, Fomitocedaceae, Hymenochaetaceae) of the Basidiomycetes division are typical in the different stages of wood decay (Hoppe et al., 2016). However, studies in southern Europe have not examined wood-dwelling fungi in detail. A recent study shows that higher rainfall positively influences fungal diversity. In contrast, higher temperatures have a negative influence, and tree species richness with structural and ecological heterogeneity has a more significant impact on fungal diversity (Pioli et al., 2018).
It is also essential to understand the range of the species distribution with topography. Some species of Ganodermataceae occur in Central Europe at median altitudes of 500 m but can also reach altitudes of up to 1500m and are usually found in mixed forests as well as in beech and coniferous forests so that altitude and forest type represent important distribution variables (Copoț and Tănase 2017). Families of the order Polyporales, including Fomitopsidaceae and Polyporaceae, were found at 1800-2100 m altitude with a temperature range between 18ºC - 23ºC and a relative humidity of 60-70%. These species occur on leaf litter and wood substrates (Hafizhasando et al., 2021). Forest regions characterized by drier, less disturbed, and warmer forest zones, including tropical and subtropical zones, are dominated by porous macro-fungi, such as the Polyporales group (De Silva et al., 2013). Fungal diversity is also significantly related to the type of host tree species, as the tree species also influences the rhizosphere. For example, the Banj Oak (Quercus leucotrichophora) is associated with moisture- and nutrient-rich soil, while the Chir Pine (Pinus roxburghii) is associated with nutrient- and moisture-poor soils (Kumar and Garkoti 2022). Since Banj Oak is a late successional tree host, Chir Pine is an early successional tree in a forest ecosystem (Singh and Singh 1987). In addition, soil nutrients and elevation play a crucial role in fungal diversity as tree hosts also influence soil microbial biomass, such as mixed Oak-Pine forests in temperate climates that have high microbial biomass, i.e., Carbon (C) and Nitrogen (N) as well as C:N ratios above 5 indicate that fungal communities dominate soil compared to bacterial communities (Manral et al., 2023).
Although previous studies have demonstrated the mycorrhizal fungal associations of about 15 genera of fungi with Himalayan tree species like West Himalyan Fir (Abies pindrow), Deodar Cedar (Cedrus deodara), Himalayan Spruce (Picea smithiana ), Chir Pine, Bhutan Pine (Pinus wallichiana), Tree Rhododendron (Rhododendron arboreum), White Oak (Quercus incana) and Brown Oak (Quercus semicarpifolia)(Manoharachary et al., 2005 ), their comparative analysis regarding the Shannon diversity and species richness has not been comprehensively analyzed.
Therefore, we analyzed the richness, diversity, and distribution of fungal species and the fungal host tree species in the western Himalayan region.
2. Materials and Methods
2.1 Data Collection
The dataset used for this study included specimens from the Forest Research Institute (FRI) fungariumin, Dehradun, India. The FRI fungarium was established in 1906, and the oldest Indian specimen date back to 1878, making this one of the oldest and largest in India. Over 12,000 fungal specimens, including mushrooms, molds, and yeasts, are stored. The specimens were collected mainly from the western Himalayan Region, the central highlands, and the Karakoram Mountains in the Kashmir region. A large sample size was selected (N = 2066) based on the tree species of interest and with confirmed geolocations and taxonomic details. Based on the abundance in the datasets, the top ten fungal families were selected for analysis to limit bias for host tree and fungal family comparisons of diversity and richness.
2.2 Topographic and Climatic Variables
For the analysis of fungal families, we selected four types of variables. We used Worldclim version 2.1 historical climate datasets based on geolocations, including the SRTM 30 arc seconds altitude dataset. In addition, mean temperature, precipitation, and solar radiation with a resolution of 30 arc seconds each, which has a temporal time range of 30 years, i.e., from 1970 to 2000 (Fick and Hijmans 2017).
As for rainfall, the average precipitation for July was considered the primary rainy season in India, known as the monsoon. Understanding the significant impact of monsoon onset on phenology is imperative, especially regarding the peak growth rate of many species in the coniferous forests in the Himalayan region (Singh and Parida 2018).
2.3 Statistical Analysis
We conducted the statistical analysis in the R 4.3.1 statistical environment. First, we conducted PCA (Principal Component Analysis) with ANOVA (Analysis of variance) to understand the variations and distribution of fungal families concerning climatic and environmental conditions. It is critical to understand the fungal families’ phenological and environmental characteristics using violin plots showing density distributions to gain more knowledge about these dominant fungal families. Using ANOVA-PCA, F-statistics with variances are derived, and 6.63 is the threshold at a significance level of 0.01, i.e., F values above 6.63 indicate significant differences between fungal families.
In addition, the Shannon Diversity Index (H) was used to measure diversity for both fungal families and host tree species. Additionally, species richness was calculated and plotted against the Shannon Diversity Index to compare richness and diversity. Where ‘n’ is the Shannon index, 'p' represents the ratio of individuals of a particular species, and ‘N’ is the total number of individuals observed. The 'ln' denotes the natural logarithm, 'Σ' signifies the sum of these calculations, and 's' denotes the number of different species (Vidakovic 2011).
Also, we included species richness, which provides a primary count of the number of different species in a given area without considering the abundance of each species or the distribution of their relative abundance.
3. Results
3.1. Host Tree - Fungal Species Diveristy and Richness
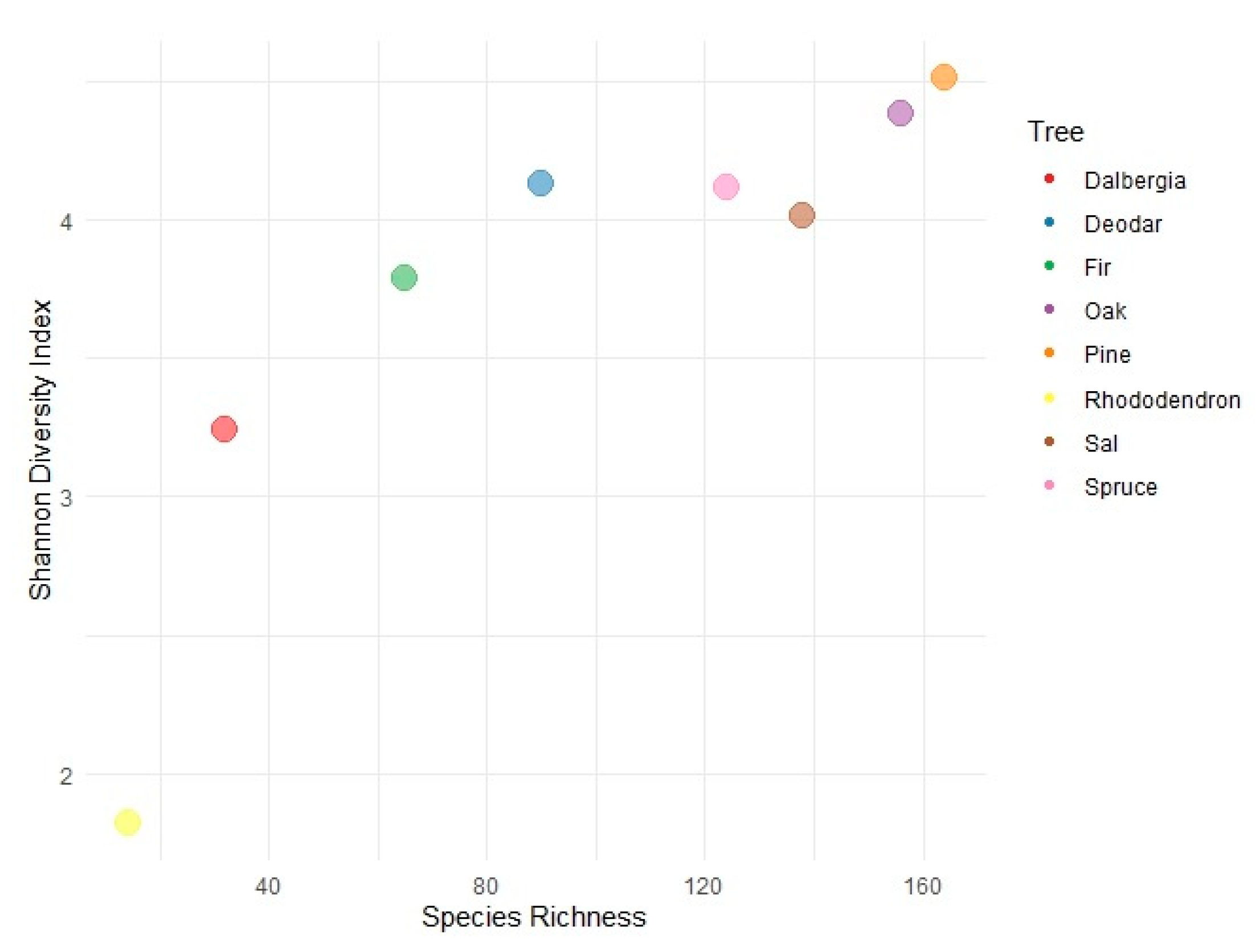
A separate analysis was performed for host tree species and fungal families to determine the diversity and richness of fungal species. Among the host tree species, pine (H’ = 4.5) and oak (H’ = 4.38) recorded a very high diversity and species richness, i.e., ssp. 165 and ssp. 156. In addition, Deodar (H’ = 4.13) and Spruce (H’ = 4.11) also recorded very high diversity and relatively moderate species richness, i.e., ssp. 90 and 124, respectively. Dalbergia (H = 3.2) and Rhododendron (H = 1.81) showed moderate to low species biodiversity and very low richness, i.e., ssp. 32 and ssp. 14, respectively. However, bias can also occur for Rhododendrons due to the small sample size. In addition, Sal, which occurs at relatively lower altitudes than conifer species, has high diversity (H’ = 4.01) and species richness ssp. 138 (
Figure 1).
3.2. Fungal Family- PCA-ANOVA, Species Diveristy and Richness
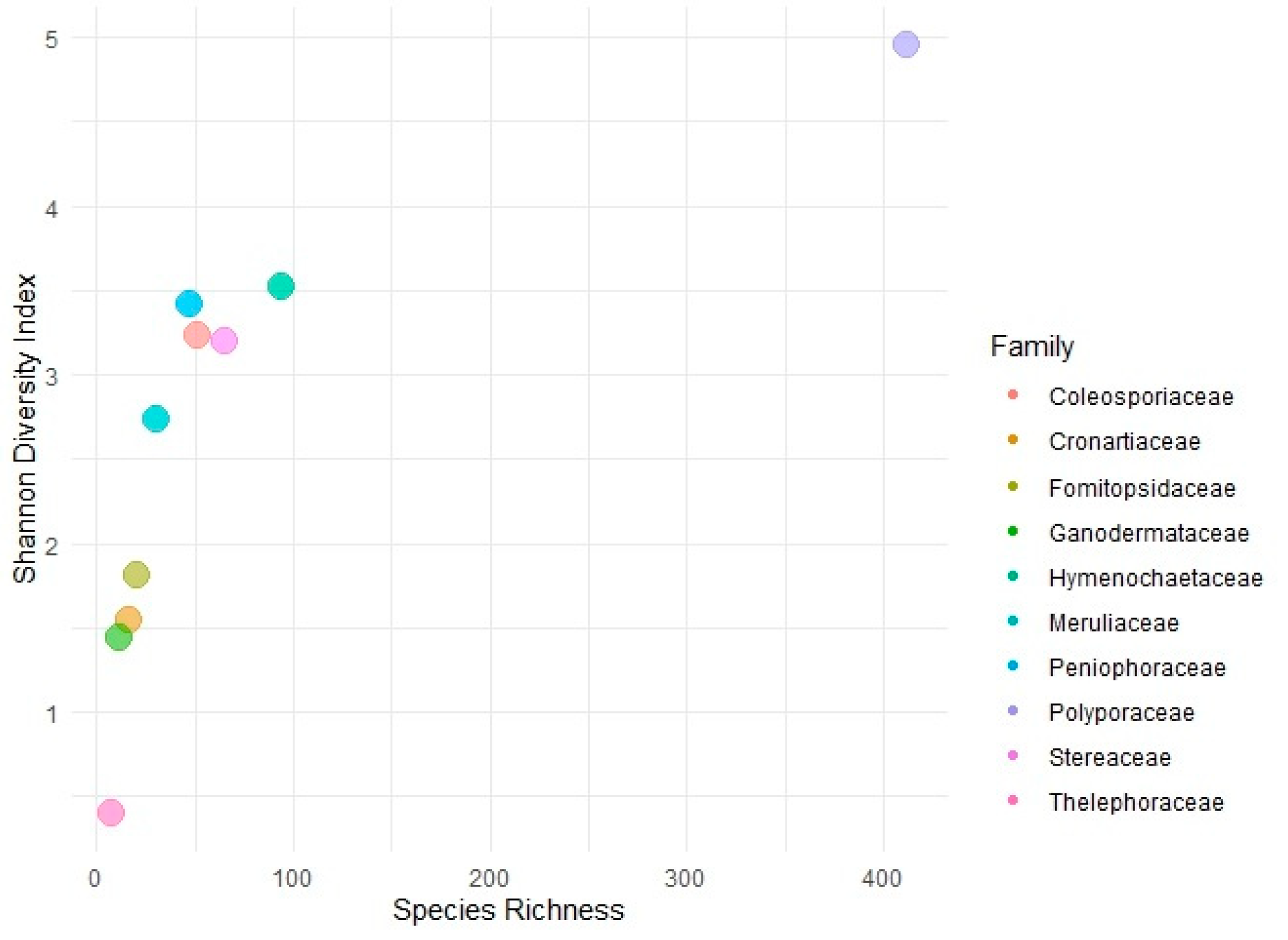
Polyporaceae had the highest diversity and species richness among all fungal families (H’ = 4.95; ssp. 412). Hymenochaetaceae, Peniophoraceae, Coleosporiaceae, and Stereaceae also showed high diversity, i.e., H’ = 3.52, H’ = 3.41, H’ = 3.23 and H’ = 3.19, respectively, with moderate species richness (ssp. > 40). Other families, including Fomitopsidaceae, Cronartiaceae, Ganodermataceae, and Thelephoraceae, have low species diversity, i.e., H’ = 1.81, H’ = 1.53, H’ = 1.43 and H’ = 0.39 respectively. In addition, species richness is low (ssp. < 25) (
Figure 2).
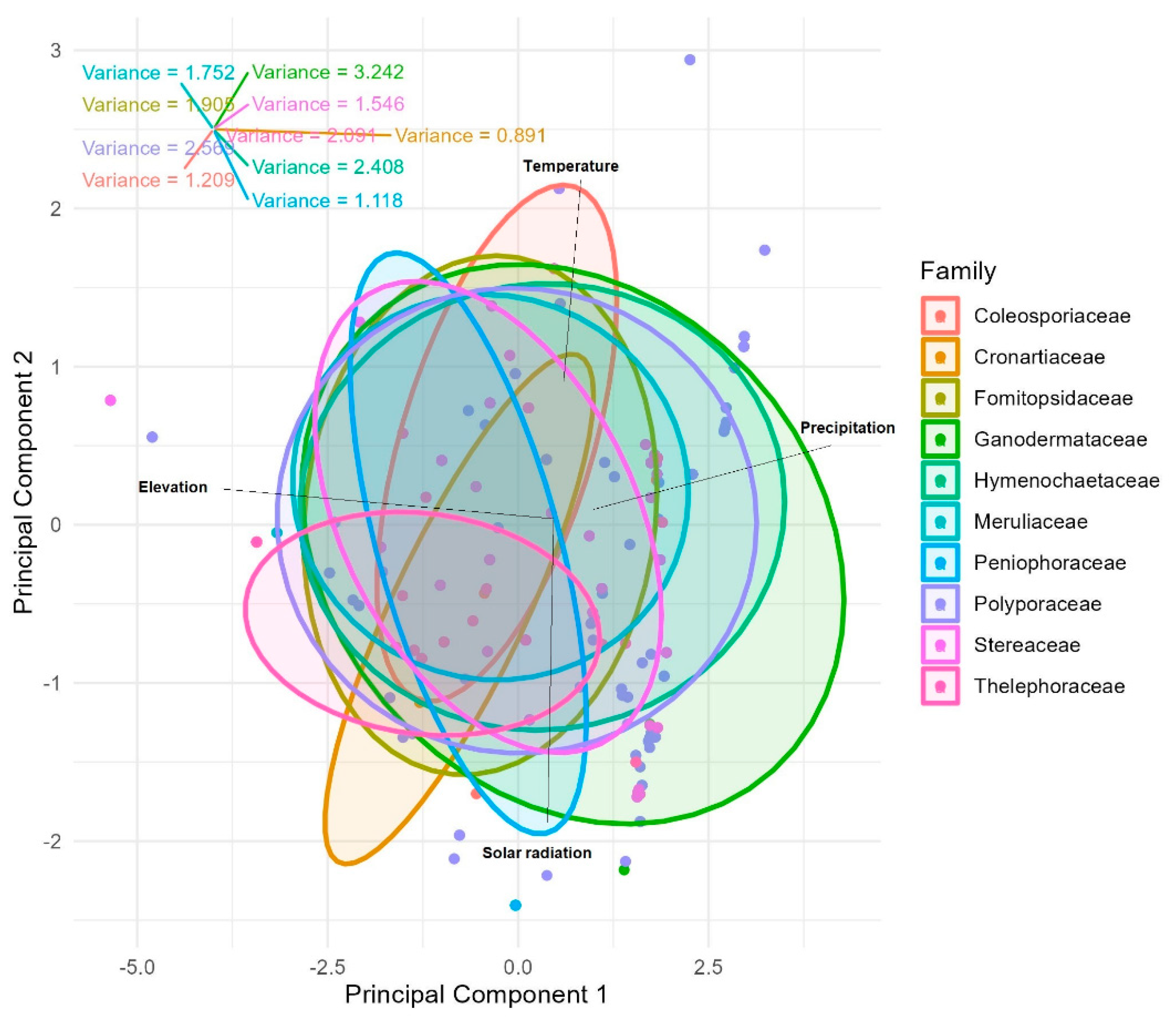
The p-values for the fungal families that show significant differences between the fungal families include Hymenochaetaceae (σ² = 2.4, F = 11.6, p < 0.01 ), Stereaceae (σ² = 1.5, F = 10.5, p < 0.01 ), Thelephoraceae (σ² = 2.09, F = 28.4, p < 0.01), Peniophoraceae (σ² = 1.1, F = 20.7 p < 0.01) and Cronartiaceae (σ² = 0.89, F = 14.5, p < 0.01). The variances also indicate the variability within families found in the PCA-ANOVA, with Ganodermataceae (σ² = 3.24, F = 2.09) and Polyporaceae (σ² = 2.56, F = 3.72) having higher variances (
Figure 3).
3.3. Spatio-Climatic Distribution of Fungal Families
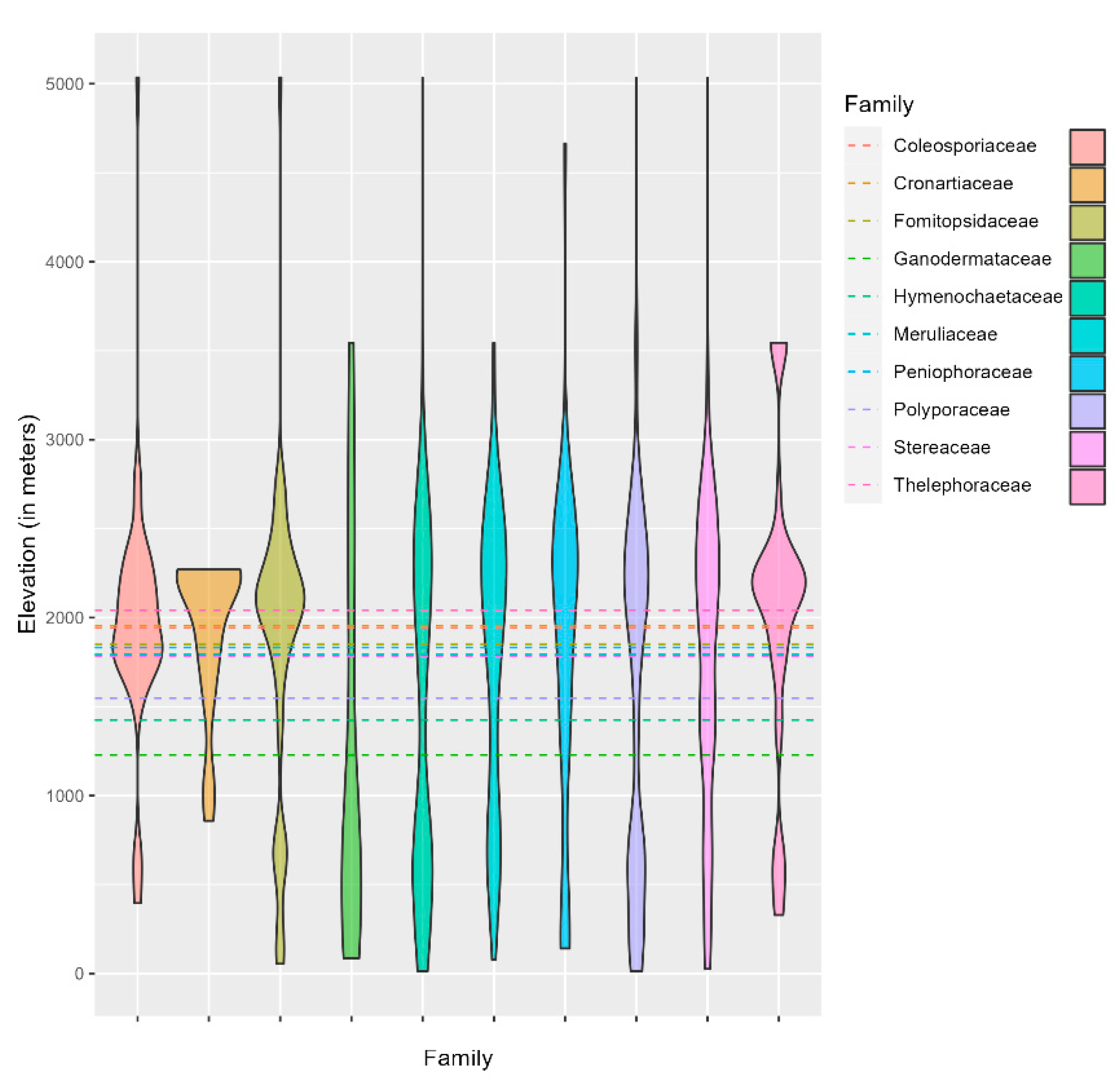
The family Thelesporaceae is one of the families distributed above 2000 m mean altitude, in contrast to families such as Ganodermateceae and Hymenochaetaceae, which, although distributed below 1000 m mean altitude, have more comprehensive distribution ranges, namely 0 - 3500 m and 0 - 5000 m, respectively. The family Polyporaceae shows diverse variations in species distribution concerning altitude ranges and occurs between 0 and 4500 m, with the mean altitude slightly above 1500 m. Colesporaceae and Cronartiaceae have a mean altitude of slightly below 2000 m with slight variation. The distribution is usually around 2000 m. In addition, the four families, i.e., Stereaceae, Meruliaceae, Peniophoraceae, and Fomitopsidaceae, occur between 1700 and 1800 m mean elevation, with all Stereaceae and Fomitopsidaceae having different altitude distribution ranges up to 5000 m (
Figure 4).
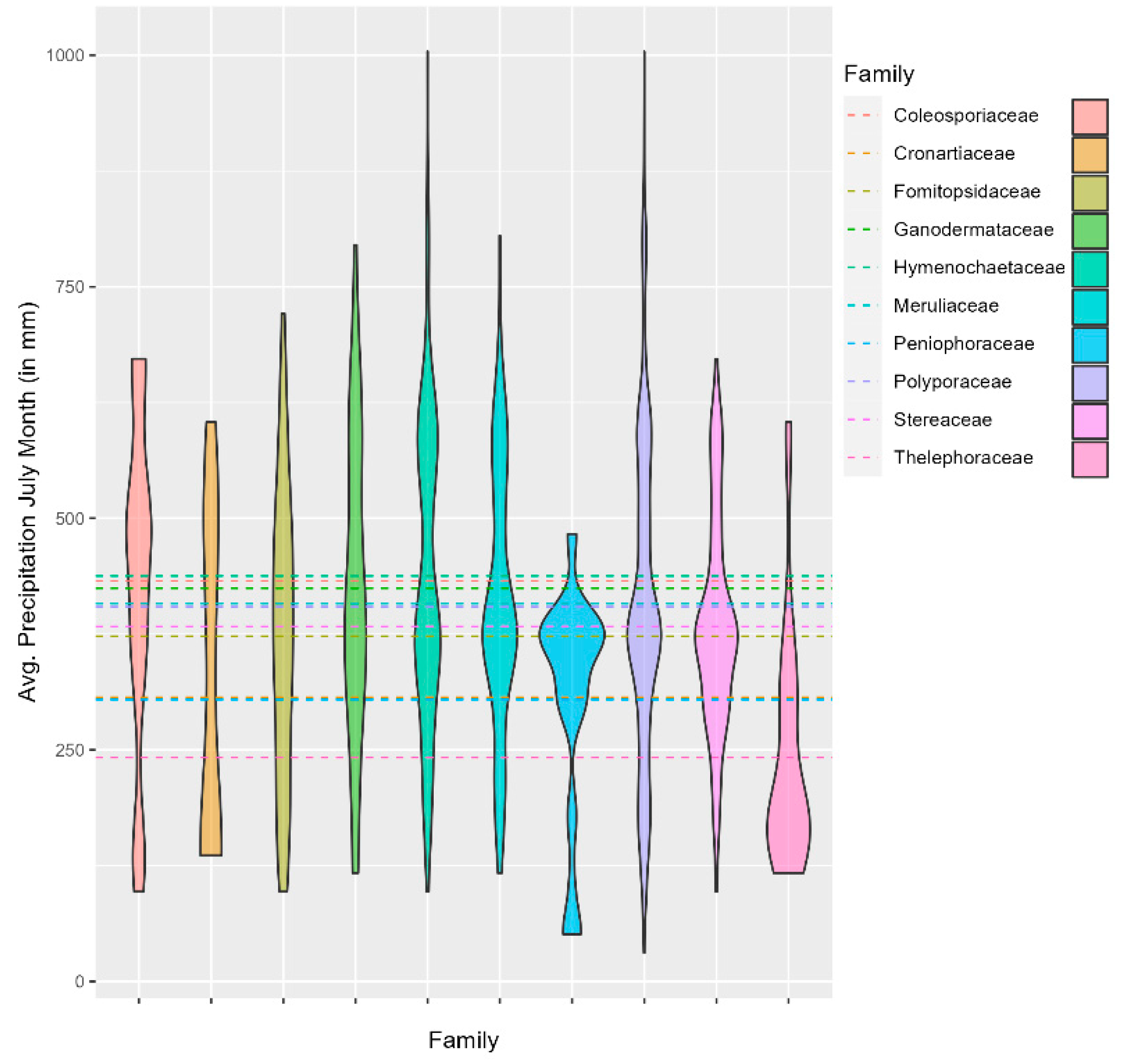
Furthermore, Hymenochaetaceae, Coleosporiaceae, and Ganodermateceae have some of the highest mean precipitation, namely 438 mm, 432 mm, and 424 mm, respectively. In comparison, Thelephoraceae are found among the lowest average rainfall at 242 mm, along with others such as Peniophoraceae and Cronartiaceae at 304 mm and 307 mm, respectively (
Figure 5,
Table 1).
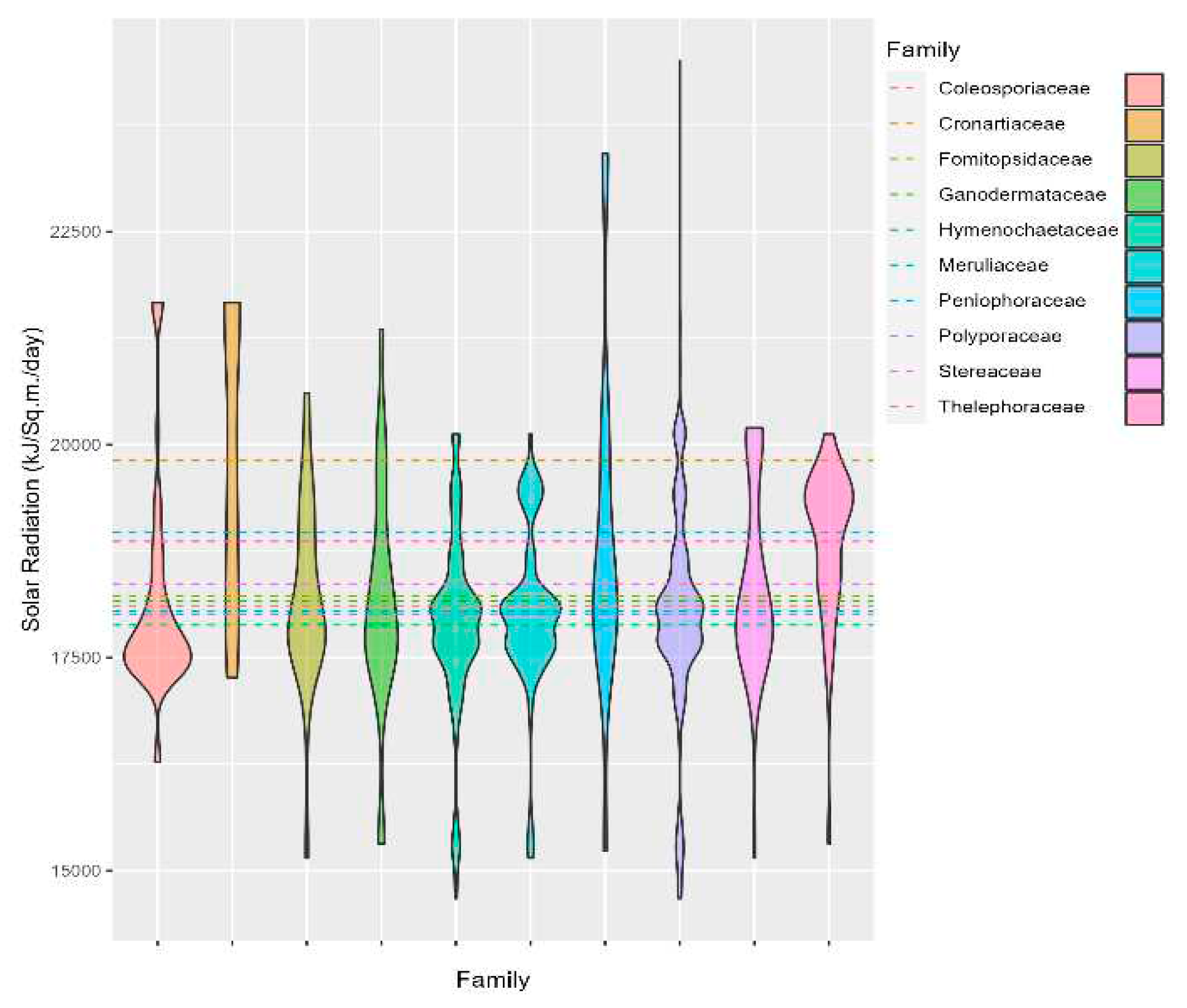
In contrast, the three species, Thelephoraceae, Peniophoraceae, and Cronartiaceae, have relatively higher mean solar radiation of 18,864, 18,968, and 19,811 kJ m
-2 day
-1 respectively (
Figure 6,
Table 1). Furthermore, Hymenochaetaceae had the lowest mean solar radiation of 17,880 kJ m
-2 day
-1.
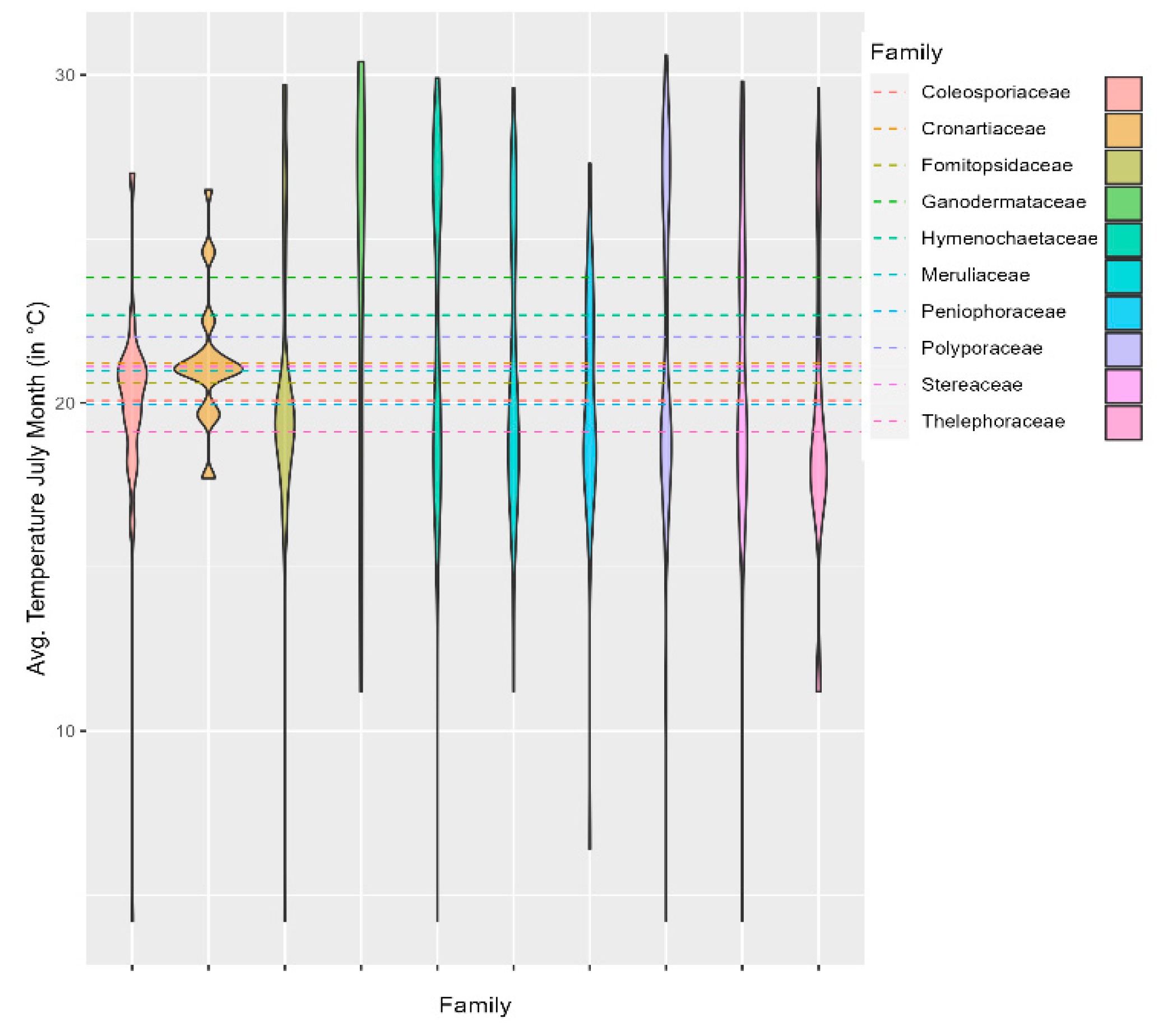
Regarding temperature distribution, Thelephoraceae and Peniophoraceae were found to have a mean temperature of less than 20°C, while Thelephoraceae display a skew toward a temperature lower than 20°C. In contrast, Ganodermateceae has a temperature range of 10 to 30°C with an average temperature of 23.8°C (±6), which is the highest among the families, followed by Hymenochaetaceae and Polyporaceae at 22.6°C (±4.7) and 22°C (±4.9). However, they have a more comprehensive temperature distribution range of 0 to 30°C. In addition, Coleosporaceae also has a relatively lower average temperature, namely 20°C (±3.1), with a temperature range of 0-25°C. Cronartiaceae has a mean temperature of about 21.2°C (±1.9) with a minimal temperature range of 18-26°C (
Figure 7,
Table 1).
4. Discussion
Our study demonstrates that high diversity and species richness occur in forests dominated by oaks, including ectomycorrhizal fungal species from families such as Polyporaceae, Hymenochaetaceae, Peniophoraceae, Ganodermataceae, Thelephoraceae, in the western Himalayas. This is consistent with other studies (Lakhandpal 1997), including one that identified 24 fungal species as host-specific and associated only with oaks (Deshmukh 2004). One study also found ectomycorrhizal hotspots in oak forests worldwide, with southwestern Mexico as a hotspot for Oak forest ectomycorrhizal diversity (García-Guzmán et al., 2017).
Another important finding of the study is that host tree species such as spruce, deodar, and sal also have high fungal diversity. Sal, a woody species used for timber production, has a high species richness and ectomycorrhizal and endomycorrhizal fungal associations, previously reported only from the eastern Himalayas (Tapwal et al., 2015). Hence, the continued removal of Sal for the timber industry can impact the fungal species it hosts.
As obligatory dependents on plants, plant diversity dictates fungi diversity and populations (Kumar et al., 2018). Hence, although the sample sizes from Rhododendrons are small, the threat to the species in the study region (Singh et al., 2022) and the phenological changes documented in recent years (Singh et al., 2019, Negi et al., 2019), can impact future diversity. Similarly, conifers, especially Pine, are host to many fungi species. Many of these are endemic to the arc of Himalayan hotspots, and there is a need for sustainable conservation strategies for these species at the national level (Singh et al., 2018). Our results underline the need for species-specific studies, especially the little-studied ones before it becomes too late.
Similarly, Cronatiaceae, Peniophoraceae, and Thelephoraceae are in areas with relatively low rainfall compared to all other families and have lower species richness. In contrast, Hymenochaetaceae and Polyporaceae, which are in higher rainfall environments, recorded a high species richness, which is also confirmed by a study of oak forests in the Mediterranean region, which showed a positive relationship between higher precipitation and species richness, especially in the dry season (Reis et al., 2018).
Furthermore, fungal families such as Polyporaceae and Hymenochaetaceae occur at altitudes of about 1500 m and have very high species richness in this Himalayan region. This result concurs with a study focusing on the Mid-domain effect (MDE) for ectomycorrhizal fungi. It conforms to Miyamoto et al., (2014) because MDEs show the highest species richness in mid-domain geographical locations (Colwell and Lees 2000).
Also, the families found in environments with high solar radiation, Cronartiaceae and Thelesporaceae, have lower diversity and species richness, except for Peniophoraceae, which have high diversity and moderate richness. However, the variance in the later, i.e., Peniophoraceae, compared to these two families is higher, indicating adaptation to higher solar radiation, as fungal communities are known to tolerate higher solar radiation and extreme desiccation and adaptations such as melanin accumulation (Santiago et al., 2018; Fernandez and Koide 2013).
Nevertheless, the abundance of parasitic Fomitopsidaceae is significant in this study as they are classified in the order Polyporales, with the majority of their species having a parasitic affinity for woody plants and a propensity to cause brown rot (Joshi et al., 2022). Species from the Cronartiaceae and Coleosporaceae families are also known to cause rust diseases on conifers and are, therefore, parasitic by nature (Zwetko and Blanz 2018).
The world faces climatic and other environmental challenges because of the exacerbated rate of climate change and the unprecedented impact of anthropogenic activities. Mapping biodiversity at all levels is challenging in extreme geophysical habitats like the Tibeto-Himalayan mountain range. Although the more easily studied species, like vertebrates and plants, are documented (Mosbrugger et al., 2018, Singh et al., 2018, Dorji 2019, Negi and Rawal 2019), the less prominent species, like fungi, are neglected. Kumar et al. (2018) stated that there is no current record of the number of macro-wood-decaying fungi in India. The importance of our study is the analysis of the long-term collection in the national fungarium and the host plants. Continued exploitation of these little-studied species, which play an essential ecological role in the health of the forests, can have detrimental effects for all involved (Singh et al., 2019).
Our study in the western Indian Himalayan region emphasizes the importance of conducting similar studies in the central and eastern parts of the range. Mehta et al. (2023) reported that the diversity of plant species was highest in the east. Nevertheless, more information about the ecology of the related fungal populations must be available. Bijayalaxmi Devi et al. (2023) also reported the degradation of Himalayan agro-forestry systems wherein human population pressures threaten innumerable ecosystem services.
We conclude that there is high diversity and species richness of fungi in forests dominated by oaks, spruce, deodar, and sal in the western Himalayan region. We also found that areas with relatively low rainfall had lower species richness and vice versa. High solar radiation also negatively impacted fungal diversity and species richness. Similar studies need to be undertaken in other parts of the Himalayas, and the importance of fungi in ethno-botanical needs to be understood for mitigating sustainable use of host plants by the local communities. Also, authorities must accordingly include fungi in the RED lists of the region.
Author Contributions
Conceptualization, N.Y., S.R. and R.Y.; methodology, S.R..; software, S.R., N.A.; validation, N.Y., N.A. and S.R.; formal analysis, S.R.; investigation, S.R, R.Y..; resources, N.Y..; data curation, N.Y., S.R., N.A.; writing—original draft preparation, S.R.; writing—review and editing, S.R., R.Y.; visualization, S.R.; supervision, R.Y.; project administration, S.R.; funding acquisition, N.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study did not require ethical approval.
Data Availability Statement
All data are included in the text and as supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chander, H.; Thakur, S.; Sharma, S. Investigations on diversity of wood inhabiting fungi in Sarkaghat region of district Mandi, Himachal Pradesh, North-Western Himalaya. J. Biol. Chem. Chron 2017, 3, 41–54. [Google Scholar]
- Colwell, R.K.; Lees, D.C. The mid-domain effect: geometric constraints on the geography of species richness. Trends in ecology & evolution 2000, 15, 70–76. [Google Scholar] [CrossRef]
- Copoț, O.; Tănase, C. Maxent modelling of the potential distribution of Ganoderma lucidum in north-eastern region of Romania. Journal of Plant Development 2017, 24. [Google Scholar]
- Dahal, N.; Lamichhaney, S.; Kumar, S. Climate change impacts on Himalayan biodiversity: evidence-based perception and current approaches to evaluate threats under climate change. Journal of the Indian Institute of Science 2021, 101, 195–210. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Sudarman, E.; Stadler, M.; Xu, J.; Aisyah Alias, S.; Hyde, K.D. Bioactive metabolites from macrofungi: ethnopharmacology, biological activities and chemistry. Fungal Diversity 2013, 62, 1–40. [Google Scholar] [CrossRef]
- Deshmukh, S. K. Biodiversity of tropical basidiomycetes as sources of novel secondary metabolites. Microbiology and Biotechnology for Sustainable Development ,121-140. New Delhi : CBS Publishers and Distributors https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55.))/reference/referencespapers.aspx?referenceid=1863308. 2004. [Google Scholar]
- Devi, N.B.; Lepcha, N.T.; Bhutia, P.T.; Rocky, P.; Sahoo, U.K.; Pandey, R.; Nath, A.J. Biodiversity and Ecosystems Services of the Agroforestry Systems of the Himalayan Region: An Overview. Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa 2023, 487–513. [Google Scholar] [CrossRef]
- Devkota, S.; Fang, W.; Arunachalam, K.; Phyo, K. M. M.; Shakya, B. Systematic review of fungi, their diversity and role in ecosystem services from the Far Eastern Himalayan Landscape (FHL). Heliyon 2023. [Google Scholar] [CrossRef]
- Dorji, S.; Vernes, K.; Rajaratnam, R. Mapping Conservation Priorities and Assessing Connectivity Pathways for Threatened Mammals Under Future Climate Change in the Eastern Himalayan Biodiversity Hotspot of Bhutan. [Doctoral thesis, University of New England]. Research UNE. https://hdl.handle.net/1959.11/41384. 2019. [Google Scholar]
- Fernandez, C.W.; Koide, R.T. The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecology 2013, 6, 479–486. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International journal of climatology 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- García-Guzmán, O.M.; Garibay-Orijel, R.; Hernández, E.; Arellano-Torres, E.; Oyama, K. Word-wide meta-analysis of Quercus forests ectomycorrhizal fungal diversity reveals southwestern Mexico as a hotspot. Mycorrhiza 2017, 27, 811–822. [Google Scholar] [CrossRef]
- Hafizhasando, R.; Rahayuningsih, M.; Parmin, S. S. Fungi in Selo hiking trail of mount Merbabu national park Central Java. In Journal of Physics: Conference Series; IOP Publishing, 2021; Vol. 1918, p. 052033. [Google Scholar]
- Hoppe, B.; Purahong, W.; Wubet, T.; Kahl, T.; Bauhus, J.; Arnstadt, T.; Krüger, D. Linking molecular deadwood-inhabiting fungal diversity and community dynamics to ecosystem functions and processes in Central European forests. Fungal Diversity 2016, 77, 367–379. [Google Scholar] [CrossRef]
- Itoo, Z.A.; Basharat, Q.; Majeed, S.T.; Andrabi, K.I.; Reshi, Z.A. Ectomycorrhizal fungal species of Kashmir Himalaya: identification and characterization by ITS analysis. Brazilian Journal of Botany 2014, 37, 531–542. [Google Scholar] [CrossRef]
- Joshi, M.; Bhargava, P.; Bhatt, M.; Kadri, S.; Shri, M.; Joshi, C.G.; Joshi, C.G. Fomitopsidaceae. Mushrooms of Gujarat 2021, 125–126. [Google Scholar] [CrossRef]
- Kumar, M.; Harsh NS, K.; Prasad, R. Fungal diversity with special reference to wood decaying fungi in India: status, conservation and prospects. In Plant diversity in the Himalayan hotspot Region; M/s Bishen Singh Mahendra Pal Singh Publishers & Distributors of Scientific Books: Dehradun; Vol. 1.
- Kumar, S.; Garkoti, S.C. Rhizosphere infl uence on soil microbial biomass and enzyme activity in banj oak, chir pine and banj oak regeneration forests in the central Himalaya. Geoderma 2022, 409, 115626. [Google Scholar] [CrossRef]
- Lakhanpal, T. N. Diversity of mushroom mycoflora in the North-West Himalaya. Recent researches in ecology, environment and pollution. Today and Tomorrow’s Printers and Publishers, New Delhi, 1997. [Google Scholar]
- Manoharachary, C.; Sridhar, K.; Singh, R.; Adholeya, A.; Suryanarayanan, T.S.; Rawat, S.; Johri, B.N. Fungal biodiversity: distribution, conservation and prospecting of fungi from India. Current Science 2005, 58–71. [Google Scholar]
- Manral, V.; Bargali, K.; Bargali, S.S.; Karki, H.; Chaturvedi, R.K. Seasonal dynamics of soil microbial biomass C, N and P along an altitudinal gradient in central Himalaya, India. Sustainability 2023, 15, 1651. [Google Scholar] [CrossRef]
- Mehta, P.; Bisht, K.; Sekar, K.C.; Tewari, A. Mapping biodiversity conservation priorities for threatened plants of Indian Himalayan Region. Biodiversity and Conservation 2023, 1–37. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Nakano, T.; Hattori, M.; Nara, K. The mid-domain effect in ectomycorrhizal fungi: range overlap along an elevation gradient on Mount Fuji, Japan. The ISME journal 2014, 8, 1739–1746. [Google Scholar] [CrossRef]
- Mosbrugger, V.; Favre, A.; Muellner-Riehl, A.N.; Päckert, M.; Mulch, A. Cenozoic evolution of geo-biodiversity in the Tibeto-Himalayan region. Mountains, climate, and biodiversity 2018, 429, 448, ISBN:9781119159896, 111915989X. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Negi GC, S.; Rawal, R.S. Himalayan biodiversity in the face of climate change. Tropical Ecosystems: Structure, Functions and Challenges in the Face of Global Change 2019, 263–277. [Google Scholar] [CrossRef]
- Pioli, S.; Antonucci, S.; Giovannelli, A.; Traversi, M.L.; Borruso, L.; Bani, A.; Tognetti, R. Community fingerprinting reveals increasing wood-inhabiting fungal diversity in unmanaged Mediterranean forests. Forest Ecology and Management 2018, 408, 202–210. [Google Scholar] [CrossRef]
- Rana, S. K.; Rawal, R. S.; Dangwal, B.; Bhatt, I. D.; Price, T. D. 200 years of research on Himalayan biodiversity: trends, gaps, and policy implications. Frontiers in Ecology and Evolution 2021, 8, 603422. [Google Scholar] [CrossRef]
- Reis, F.; Valdiviesso, T.; Varela, C.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Ectomycorrhizal fungal diversity and community structure associated with cork oak in different landscapes. Mycorrhiza 2018, 28, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhang, Y.; Yu, H.; Zhang, Y.A. Ganoderma lucidum cultivation affect microbial community structure of soil, wood segments and tree roots. Scientific Reports 2020, 10, 3435. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I.F.; Gonçalves, V.N.; Gómez-Silva, B.; Galetovic, A.; Rosa, L.H. Fungal diversity in the Atacama Desert. Antonie Van Leeuwenhoek 2018, 111, 1345–1360. [Google Scholar] [CrossRef]
- Singh, J.S.; Singh, S.P. Structure and functioning of central Himalayan chirpine forest ecosystem. Current Science 1987, 56, 383–391. [Google Scholar]
- Singh, N.; Parida, B.R. Environmental factors associated with seasonal variations of night-time plant canopy and soil respiration fluxes in deciduous conifer forest, Western Himalaya, India. Trees 2019, 33, 599–613. [Google Scholar] [CrossRef]
- Singh, N.; Bhargava, S.; Singh, J.; Singh, R. Threats To Rhododendron Biodiversity In Indian Himalayan Regions. International Journal of Research - Granthaalayah 2022, 10, 10–13. [Google Scholar] [CrossRef]
- Singh, R.; Biswas, J.; Bisht, S. Gymnosperms diversity of the Himalaya Biodiversity Hotspot. Plant diversity in the Himalaya hotspot region 2018, 1, 129–161. [Google Scholar]
- Singh, S.P.; Pandey, A.; Singh, V. Nature and extent of Forest degradation in Central Himalayas. Tropical Ecosystems: Structure, Functions and Challenges in the Face of Global Change 2019, 27–43. [Google Scholar] [CrossRef]
- Tapwal, A.; Kumar, R.; Borah, D. Effect of mycorrhizal inoculations on the growth of Shorea robusta seedlings. Nusantara bioscience 2015, 7. [Google Scholar] [CrossRef]
- Vidakovic, B. Statistics for bioengineering sciences: with MATLAB and WinBUGS support; pringer Science & Business Media: New York, 2011; ISBN: 9781461403944; 1461403944. [Google Scholar]
- Wu, G.; Yang, Z. Medicinal Mushrooms and Fungi from Yunnan Province, Part 1: Resources and Diversity. In Medicinal Plants and Mushrooms of Yunnan Province of China; Clara, B.-S.L., Chun-Lin, L., Eds.; CRC Press, 2021; pp. 81–118. [Google Scholar] [CrossRef]
- Zwetko, P.; Blanz, P. Distinctiveness of aecia and aeciospores in rust fungi on conifers. Biodiversity and ecology of fungi, lichens, and mosses-biosystematics and ecology series 2018, 34, 271–287. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).