1. Introduction
The determination of the fetal sex is often performed due to parental request and for various medical reasons such as: in families that are carriers of X-linked disorders, Duchenne muscular dystrophy, and fragile X syndrome [
1], and in cases where there is a question regarding the fetal phenotype and the development of the external genitalia [
2].
In cases of fetuses with congenital adrenal hyperplasia (CAH), early gender assignment and antenatal treatment with corticosteroids can prevent the masculinization of the female fetus [
3].
Specifically in twin pregnancies, the determination of fetal sex can also help in the evaluation of chorionicity and labeling of the fetuses [
4]. Ongoing technological progress, including high-resolution probes for abdominal and vaginal ultrasound scanning, contribute to routine first-trimester ultrasound examinations between 11 weeks +0 days to 13 weeks +6-day gestation and has long become an established part of antenatal care [
5]. This first-trimester scan is now a common practice in fetal evaluation and gender assessment during the first trimester [
6].
Sonographic determination of the fetal sex in the first trimester is performed by mid-sagittal probe plane and assessing the angle between the genital tubercle and the body axis. Fetuses with assigned male gender display a cranially directed tubercle and a higher angle; in female fetuses, the tubercle is pointed caudally with a shallow angle [
7].
Another method for detecting fetal gender in the first trimester is a non-invasive prenatal screen (NIPS) using circulating free fetal DNA in the maternal blood. Gender assignment by NIPS is performed using either real-time PCR or Y chromosome read counting. The NIPS test assigns fetal gender based on whether Y chromosome material is detected or not [
8]. The American College of Medical Genetics and Genomics (ACMG) recommends the use of NIPS as a first tire for screening for fetal aneuploidy, including sex chromosome aneuploidies (SCA). This approach is adopted in the Netherlands. Most genetic laboratories report fetal gender in a singleton pregnancy and the detection of the Y chromosome in twin pregnancies, upon parental request or medical needs [
9,
10].
In the current study, we aimed to ultrasonographical assess the accuracy of fetal gender determination during 11-14 weeks’ gestational age.
We further subdivided gestational age into three different cutoffs of crown-rump length (CRL) CRL 45-54 mm, CRL 55-67 mm, and CRL 67-87 mm, and accordingly, we assessed sex assignment accuracy in each group.
A secondary aim was to determine the sensitivity and specificity of Y chromosome detection using NIPS in these twin pregnancies.
2. Results
2.1. Cohort Flow Chart
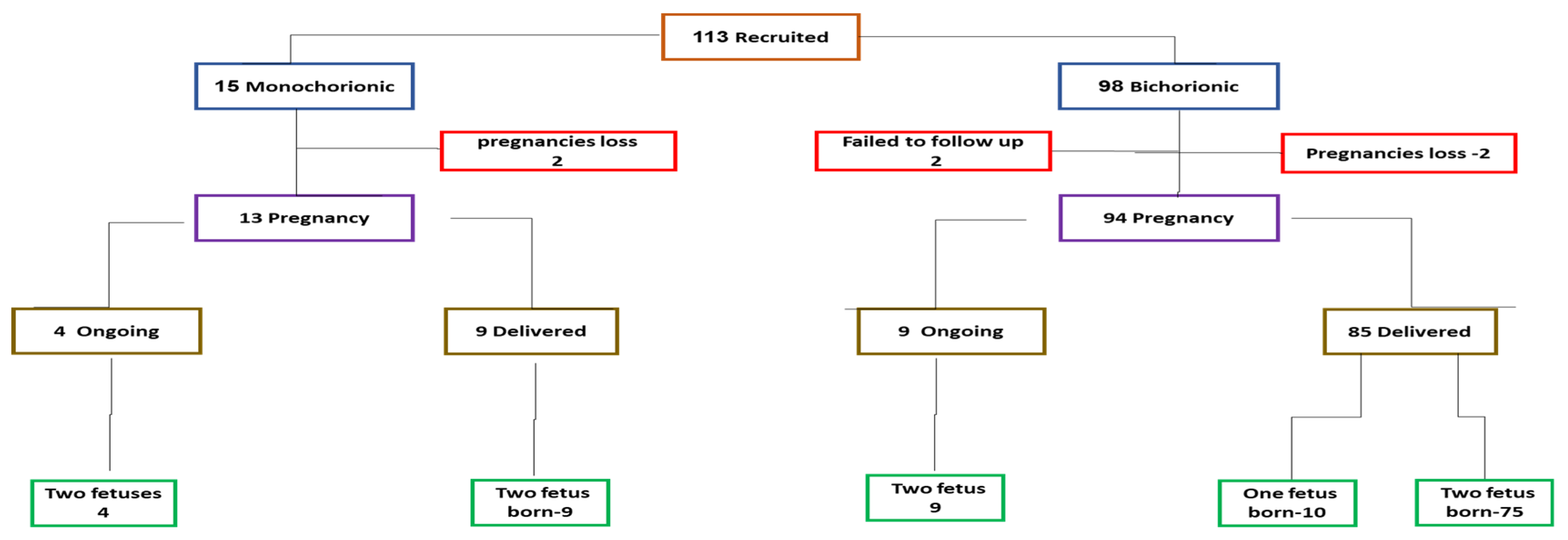
A total of 113 fetuses from twin pregnancies were enrolled from December 2020 to March 2023. Of these, 98 were from dichorionic diamniotic (DCDA) pregnancies and 15 from monochorionic diamniotic (MCDA) pregnancies (
Figure 1). Of the 15 monochorionic diamniotic twins enrolled (left), two lost their pregnancy before the 2nd trimester, and of the 13 continued 9 delivered twins each, and 4 are ongoing with 2 fetuses.
Of the 98 with dichorionic diamniotic pregnancies (right,) 2 were lost to follow-up, 2 loss their entire pregnancies, and of the 94 pregnancies continued, 85 were delivered of which 75 had two live-born babies and either one baby spontaneous loss or undergone selective reduction due to fetal anomalies. (left) Nine are still ongoing with twins.
2.2. Cohort Characteristics
The median maternal age at enrolment was 34.1 years, the median gestational week at enrollment was 12.1 weeks, and the median BMI was 24.9 (all values were centered on the medians). Most of the participants (95.6%) were Caucasian, and 38.9% were nulliparous. Conception was spontaneous for most (93.3%) MCDC and 47.8% of the DCDA (p<0.001). The rest were conceived through assisted reproduction technologies (ART), mainly in-vitro fertilization (IVF).
Table 1 summarizes the maternal and pregnancy characteristics. All these features correspond to the general characteristics of our Twin Clinic population [10, 11]
2.3. Gender determination by non-invasive prenatal screening (NIPS) and by Ultrasound scanning in the first trimester
Circulating cell-free DA (cffDNA) determined by non-invasive prenatal screening (NIPS) was considered accurate in cases of Y chromosome-positive in which at least one of the fetuses was a male fetus (M) and in cases of Y chromosome negative, when both of the fetuses were females (F). Accordingly, there was a 100% sensitivity and specificity of gender determination in the first trimester using the Y chromosome detection with cffDNA (NIPS) for both monochorionic and dichorionic twin pregnancies, regardless of the gestational week. Testing with kits and analysis performed by Invitae ® (San Francisco, California, USA) and Medicover Genetics ® (Nicosia, Cyprus) gave similar accuracy.
As for first-trimester ultrasound: The first trimester accuracy in gender assignment of all cases was 79.7%. When individual first trimester gestational weeks were examined, the accuracy reached 91.5% in CRL 67-87 mm corresponding to gestational week 13 wks and 0 days to 13wks and 6 days but was lower for CRL 55-67 mm (87.7%) corresponding to 12 wks and 0 days to 12 wks and 6 days and further lower for CRL 45-54 (0nly 54.2%) for gestational week 11 wks and 0 days to 11 wks and 6 days. For every elevation of 1 mm in the CRL length, the chances for error were reduced by a factor of 0.826. Above CRL of 55 mm, the sex assignment was accurate in around 90% of the examined fetuses.
Male fetus had a significantly higher chances of a gender assignment error compared to female fetuses, odds ratio = 23.57 (95% Confidence I interval (95% CI) = 7.35–75.66).
Table 2 summarizes the gender sex assignment results. Other factors such as maternal age, parity, mode of conception, and BMI had no effect on gender assignment accuracy.
3. Discussion
To the best of our knowledge, this is the first study in twin pregnancy were gender accuracy was compared using sonographic imaging and NIPS. There was 100% sensitivity and specificity in Y chromosome detection using ccfDNA for both monochorionic and dichorionic twin pregnancies regardless of the gestational weeks between 11 wks ad 0 days and 13 wks and 6 days as determined by the CRL The accuracy of kits of both and Medicover Genetics was the same as defined by the presence of the Y chromosome when at least one of the fetuses was a male, and in cases of absent Y chromosome, when both of the fetuses were females.
In comparison, the ultrasound scanning was less accurate in gender assignment in the first trimester and reached 79.7% when all fetuses were examined. However, for gestation week 13, when the CRL was larger (between 67-87 mm), gender determination was higher and reached 91.5% accuracy. For every elevation of 1 mm in the CRL length, the chances for error were reduced by a factor of 0.83, so from 54.2% in CRL of 45-54 mm it increased to 87.7% in CRL 55-67 mm and 91.5% in CRL 67-87 mm.
NIPS is an established powerful screening tool for trisomy 21, 18, and 13 in both singleton and twin pregnancies [
13]. However, there are relatively few studies regarding its roll in gender assignment in twin pregnancies. Villela D et al. [
14] in a retrospective study developed a model that determines fetal gender with 100% sensitivity and specificity when both twins are female, and with 98% sensitivity and 95% specificity when a male fetus is present [
14]. In our prospective study there was 100% sensitivity and specificity in Y chromosome detection using cffDNA. Determined by NIPS. Accordingly, our study results support the idea that first trimester NIPS at gestational week 11 and 0 days and 13 and 6 days (above CRL of 55 mm) is an accurate for gender assignment in the first trimester of twin pregnancy. This s meaningful for certain clinical scenarios such as X-linked disorders and congenital adrenal hyperplasia (CAH), NIPS can help both the parents and their physicians in their decision regarding the necessity and timing of invasive procedures and need for medical treatment. In the case of monochorionic diamniotic twins, when the first trimester NIPS alone shows no presence of the Y chromosome, further invasive test for X-linked disorders can be avoided, which in certain cases of a precious pregnancy conceived through IVF appears to be very important to the parents. In our data it appears to be good also for dichorionic diamniotic twins, but larger the accuracy is less and the parents may still need to vote for conducting the invasive testing.
The case of ultrasound scan is way less accurate. In general, it shoes tat accuracy >90% is inly achieved from the midst of the gestational week 12. This is another reason to aim for first trimester evaluation from 12 weeks on since gender and also defects (our personal data, in preparation) indicated the accuracy of ultrasound examination increases if taken from the mid 12 week gestation and not from the 11th week.
3.1. Study Limitations
Our study has a few limitations. We enrolled 113 twin pregnancy from a single center, and only 10 mono-chorionic diamniotic twins were included. Thus. A larger multi-center cohort is warranted. Also, the study was conducted in the public health setting in which the conditions allow for only one sonographer to examine each patient, and the results presented involves three different sonographers. Obtaining a second examiner analysis might be biased as it is linked to the use of the first examiner’s stored images while the patient herself already left.
3.2. Conclusion
In twin pregnancies, the prospective testing of cffDNA by NIPS in the first trimester is highly sensitive and specific in determining fetal gender according to the presence of the Y chromosome. This first trimester accuracy is enough to avoid CVS in the first trimester in cases where X0linked gene defects is suspected, at least for mono chorionic mono amniotic twins.
First trimester gender determination accuracy by ultrasound is less but at CRL 67-87 mm (gestational week 13+0 to 13+6) ultrasound scanning offers a highly accurate determination of fetal gender (98%) in twin pregnancies, and the accuracy is >90 from the mid 12 wee of gestation.
Further larger multi-center studies are warranted.
4. Materials and Methods
4.1. Sample and Patients
This prospective study was approved by the Hospital Ethics Committee (Trial # 0043-20-ASF, Israel Ministry of Health Authorization # 2020166325). Women were enrolled between December 7th 2020 to May 31, 2023. The cohort included all pregnant women aged eighteen and above, carrying two live fetuses with CRL that was measured between 45 -84 mm. All women gave their written informed consent. Excluded were women with triplet pregnancies that were reduced to twins, cases of vanished twin, and cases of mono chorionic mono amniotic twins. Women who subsequently lost one fetus or underwent twin reduction to singleton due to fetal defects were included.
All precipitants underwent first (11-13 weeks), second (20-22 weeks), and third (28-32 weeks) trimesters ultrasound scan with a complete anatomical evaluation included the determination of the fetal gender and blood drawing for other pregnancy complications.
They also add additional evaluation by ultrasound in 15-16, 24-26, and 34-36 weeks of gestation. Monochorionic twins were evaluated every two weeks.
The delivery records and fetal gender at delivery were retrieved from the hospital medical records and the neonatal unit records and, when necessary, from interviews with patients if they delivered in other hospitals
4.2. Gender determination during the first trimester
Sonographic sex assessment was carried out by two experienced sonographers, either transabdominal or trans vaginally by examining the genital tubercle in a midsagittal plane with the fetus horizontal (parallel) to the probe in a supine position. The angle of the genital tubercle was then assessed and compared to an imaginary horizontal line through the lumbosacral skin surface. As previously published by Efrat et al. [
7], a male gender was assigned in cases where the sonographer estimated that the angle was higher than 30°, and female if the phallus was parallel or less than 30°.
Assessing for the presence of the Y chromosome: During the first-trimester scan, all women were offered NIPS screening for the common trisomy and the presence of the Y chromosome. Once signing on the additional annex to the informed consent requesting this test, blood samples were drawn and submitted for tests using either Invitae® or Medicover Genetics ® NIPS test for Twin Pregnancies.
Fetal gender sex was subsequently ascertained in most cases by the delivery records or telephone interviews, and in the few cases that didn’t deliver, it was determined by the second and third-trimester scan.
4.3. Statistical analysis
The data were analyzed using the SPSS version 28 (IBM). For descriptive statistics, the categorical variables are presented as frequencies (n) and percentages, and the continuous variables are presented as medians and interquartile ranges [IQR]. For the inferential statistics, differences between groups for the continuous variables were examined using a Mann-Whitney non-parametric test. Depending on sample size, relationships between groups and the categorical variables were calculated using Chi-square tests or the Fisher exact test.
SPSS statistic package was also used to conduct a generalized linear mixed model that considered a link between the fetuses of the same mother to determine factors that influenced the accuracy of gender assignment.
Author Contributions
This study was initiated by R.M. H.M and R.S. who conceptualized the study on all fronts and designed its flow. R.S.. A.O., and RM enrolled the patients and conducted all the sonographic analysis and data acquisition of all type of testing, N.K. built the study data base, and he with T.S., R.S, and H.M. audited the database and verified its accuracy. The NIPS was supervised by R.S. and H.M. H.M. and R.S. worked with A.S.-N., who conducted the statistical analyses. All authors participated in the manuscript writing and editing, and also read and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of Era PerMed JCT-2019-061 funded by the ERAPERMED program and by Israel Ministry of Health Project # 2020166 and for the project titled “Develop a multi-disciplinary approach for personalized prenatal diagnostics and care for twin pregnancies [11].
Data Availability Statement
At Shamir Medical Center through Contact with Dr. Nadav Kugler.
Acknowledgments
The authors thanks Revital Yager, Anat Gayer, and Orit Baram for their clinical support in patient scheduled, lobotomy services, patients enrolment, follow up and clinical support.
Conflicts of Interest
The authors declare no conflict of interest that could have influence its outcome.
Abbreviations
| BMI |
Body mass index |
| BP |
Blood pressure |
| cffDNA |
Circulating cell free DNA |
| GA |
Gestational Age |
| CRL |
Crown Rump Length |
| IVF |
In Vitro Fertilization |
| MAP |
Mean arterial blood pressure |
| NIPS |
Non-Invasive prenatal screening |
| SMC |
Shamir Medical Center |
| US |
Ultrasound |
References
- Moser, H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet. 1984, 66, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Witchel, SF. Disorders of sex development. Best Pract Res Clin Obstet Gynaecol. 2018, 48, 90–102. [Google Scholar] [CrossRef]
- El-Maouche D, Arlt W, Merke DP. Congenital adrenal hyperplasia. Lancet 2017, 390, 2194–2210. [Google Scholar] [CrossRef]
- Khalil A, Rodgers M, Baschat A, Bhide A, Gratacos E, Hecher K, Kilby MD, Lewi L, Nicolaides KH, Oepkes D, Raine-Fenning N, Reed K, Salomon LJ, Sotiriadis A, Thilaganathan B, Ville Y. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef]
- Salomon LJ, Alfirevic Z, Bilardo CM, Chalouhi GE, Ghi T, Kagan KO, Lau TK, Papageorghiou AT, Raine-Fenning NJ, Stirnemann J, Suresh S, Tabor A, Timor-Tritsch IE, Toi A, Yeo G. ISUOG practice guidelines: performance of first-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2013, 41, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Efrat Z, Akinfenwa OO, Nicolaides KH. First-trimester determination of fetal gender by ultrasound. Ultrasound Obstet Gynecol. 1999, 13, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Devaney SA, Palomaki GE, Scott JA, Bianchi DW. Noninvasive fetal sex determination using cell-free fetal DNA: a systematic review and meta-analysis. JAMA 2011, 10, 627–636. [Google Scholar] [CrossRef]
- Dungan JS, Klugman S, Darilek S, Malinowski J, Akkari YMN, Monaghan KG, Erwin A, Best RG; ACMG Board of Directors. Electronic address: documents@acmg.net. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: An evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2023, 25, 100336. [Google Scholar] [CrossRef] [PubMed]
- Gregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG, Klugman S, Watson MS. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016, 18, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Svirsky R, Levinsohn-Tavor O, Feldman N, Klog E, Cuckle H, Maymon R. First- and second-trimester maternal serum markers of pre-eclampsia in twin pregnancy. Ultrasound Obstet Gynecol. 2016, 47, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Svirsky R, Maymon R, Melcer Y, Klog E, Cuckle H. First and second trimester maternal serum inhibin A levels in twins with pre-eclampsia. Prenat Diagn. 2016, 36, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Schaefer EC, McKenna DS, Sonek JD. First trimester identification of fetal sex by ultrasound. Arch Gynecol Obstet. 2023. [CrossRef]
- Khalil A, Archer R, Hutchinson V, Mousa HA, Johnstone ED, Cameron MJ, Cohen KE, Ioannou C, Kelly B, Reed K, Hulme R, Papageorghiou AT. Noninvasive prenatal screening in twin pregnancies with cell-free DNA using the IONA test: a prospective multicenter study. Am J Obstet Gynecol. 2021, 225, e1–e79. [Google Scholar] [CrossRef]
- Villela D, Che H, Van Ghelue M, Dehaspe L, Brison N, Van Den Bogaert K, Devriendt K, Lewi L, Bayindir B, Vermeesch JR. Fetal sex determination in twin pregnancies using non-invasive prenatal testing. NPJ Genom Med. 2019, 4, 15. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).