Submitted:
19 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Experimental materials
2.2. Ethical statement
2.3. Determination of LD50 of bifenthrin
2.4. Experimental design
2.5. Collection of samples and processing
2.6. Acetyl cholinesterase activity in the brain region:
2.7. Histological examination of tissues:
2.8. Neurochemical studies:
2.9. Oxidative stress activity in brain regions:
2.10. Statistical analysis:
3. Results and Discussion
3.1. LD50 of bifenthrin
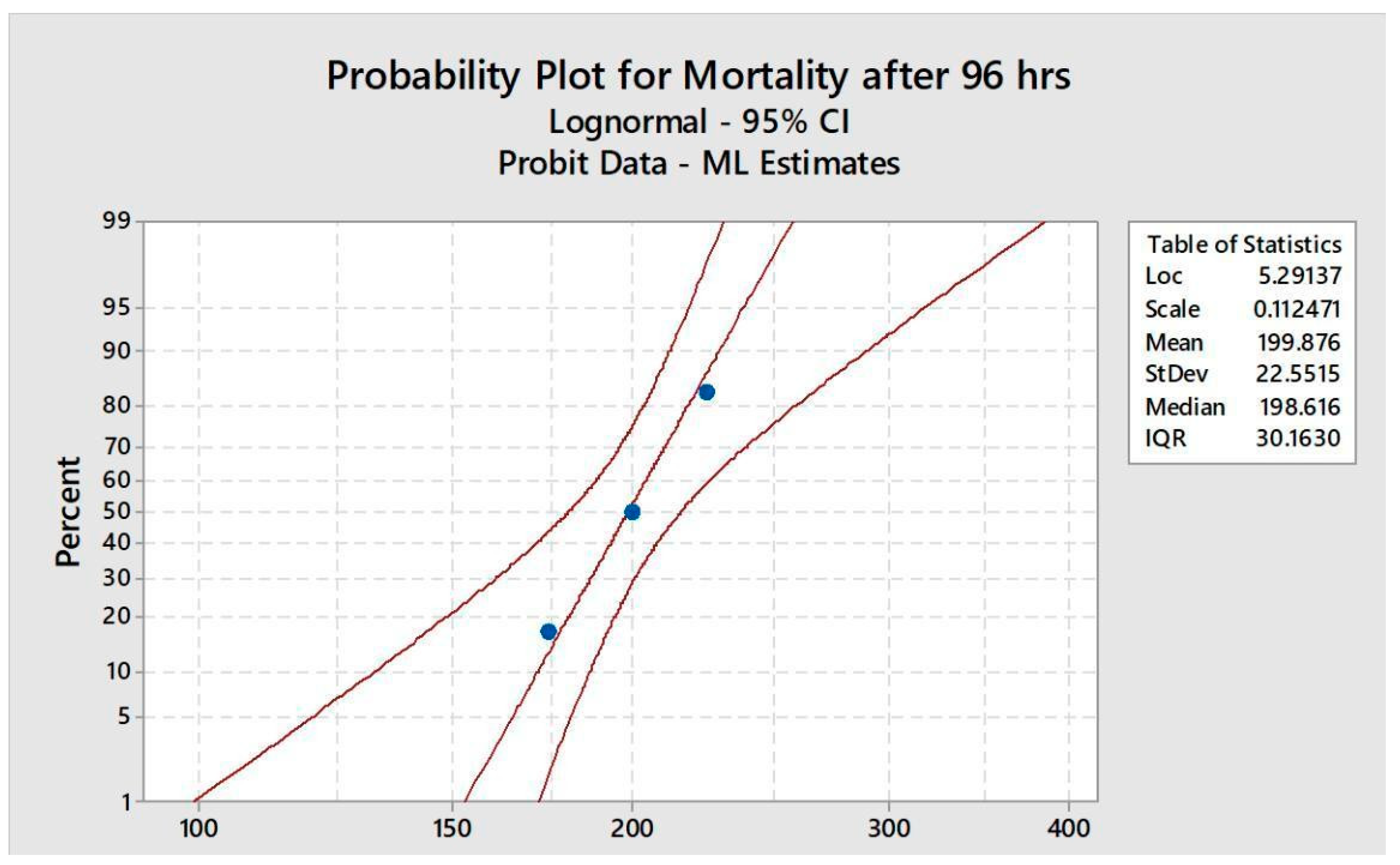
3.2. Body Weight and Somatic Index
3.3. Acetyl cholinesterase activity
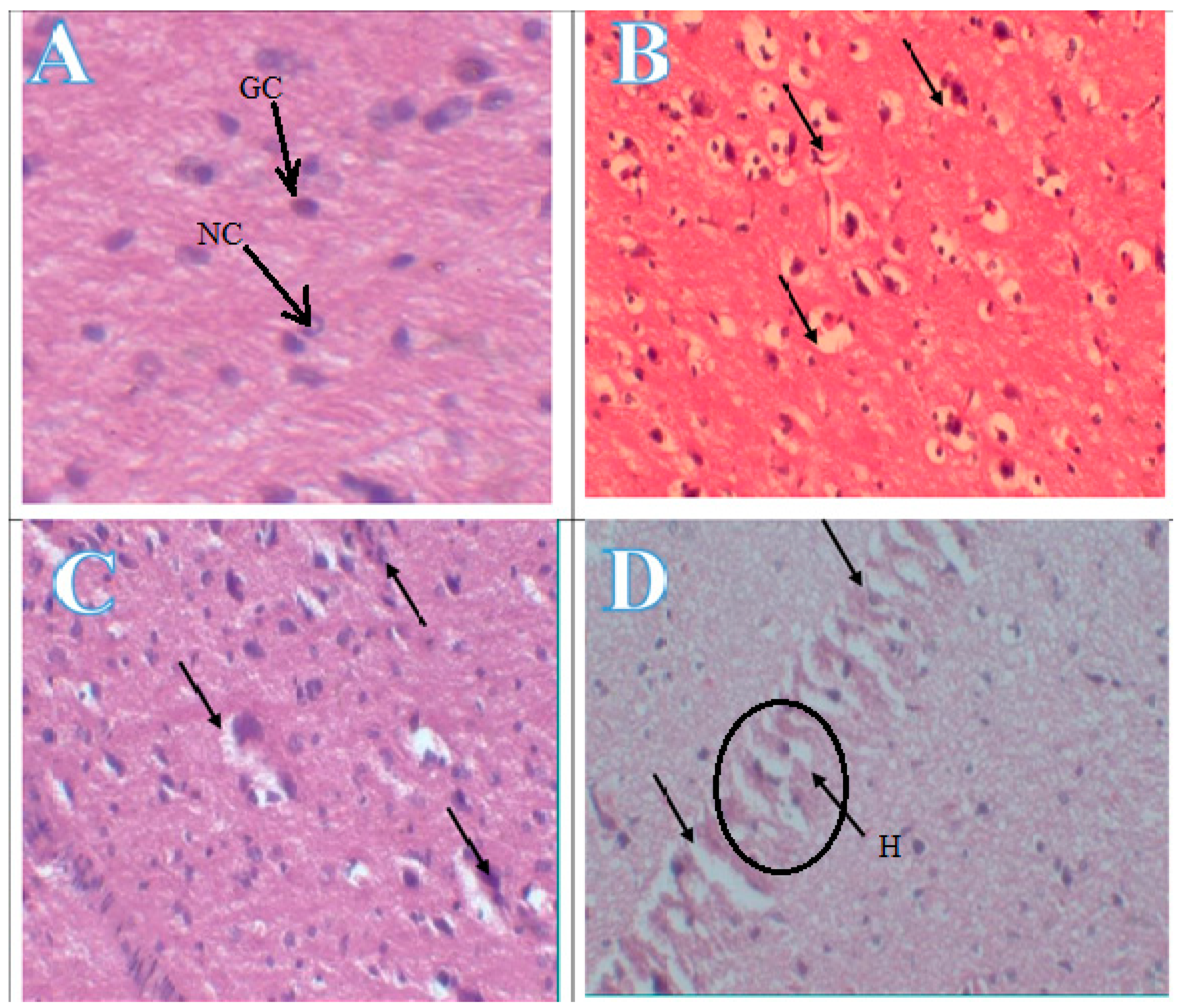
3.4. Histopathological study
4. Discussion
5. Conclusion:
Conflicts of Interest
References
- Younas, A.; Naqvi, S. A.; Khan, M. R.; Shabbir, M. A.; Jatoi, M. A.; Anwar, F.; Aadil, R. M. Functional food and nutra-pharmaceutical perspectives of date (Phoenix dactylifera) fruit. Journal of Food Biochemistry. 2020, 44, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ismail, W. I. W.; Radzi, M. N. F. M. Evaluation on the benefits of date palm (Phoenix dactylifera) to the brain. Alternative and Integrative Medicine. 2013, 5, 1–3. [Google Scholar]
- Weston, K. S.; Wisløff, U.; Coombes, J. S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. British Journal of Sports Medicine. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Wolansky, M. J.; McDaniel, K. L.; Moser, V. C.; Crofton, K. M. Influence of dosing volume on the neurotoxicity of bifenthrin. Neurotoxicology and Teratology. 2007, 29, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, N.; Wang, C. Toxicity of the pyrethroid bifenthrin insecticide. Environmental Chemistry Letters, 2020, 16, 1377–1391. [Google Scholar] [CrossRef]
- Cao, Z.; Shafer, T. J.; Crofton, K. M.; Gennings, C.; Murray, T. F. Additivity of pyrethroid actions on sodium influx in cerebrocortical neurons in primary culture. Environmental Health Perspectives. 2011, 119, 1239–1246. [Google Scholar] [CrossRef]
- Wolansky, M. J.; Gennings, C.; Crofton, K. M. Relative potencies for acute effects of pyrethroids on motor function in rats. Toxicological Sciences. 2006, 89, 271–277. [Google Scholar] [CrossRef]
- Mohan, G. K.; Pallavi, E.; Kumar, R.; Ramesh, M.; Venkatesh, S. Hepatoprotective activity of F. carica Linn leaf extract against carbon tetrachloride-induced hepatotoxicity in rats. Journal of Pharmaceutical Sciences, 2007, 15, 162–166. [Google Scholar]
- Dar, M. A.; Khan, A. M.; Raina, R.; Verma, P. K.; Wani, N. M. Effect of bifenthrin on oxidative stress parameters in the liver, kidneys, and lungs of rats. Environmental Science and Pollution Research, 2019, 26, 9365–9370. [Google Scholar] [CrossRef]
- Holt, M. P.; Ju, C. Mechanisms of drug-induced liver injury. The AAPS Journal. 2006, 8, 48–54. [Google Scholar] [CrossRef]
- Jaeschke, H.; Gores, G. J.; Cederbaum, A. I.; Hinson, J. A.; Pessayre, D.; Lemasters, J. J. Mechanisms of hepatotoxicity. Toxicological Sciences. 2002, 65, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Brander, S. M.; He, G.; Smalling, K. L.; Denison, M. S.; Cherr, G. N. The in vivo estrogenic and in vitro anti-estrogenic activity of permethrin and bifenthrin. Environmental Toxicology and Chemistry. 2012, 31, 2848–2855. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M. B.; Krstic, D. Z.; Lazarevic-Pasti, T. D.; Bondzic, A. M.; Vasic, V. M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Current Neuropharmacology. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Malomouzh, A.; Ilyin, V.; Nikolsky, E. Components of the GABAergic signaling in the peripheral cholinergic synapses of vertebrates: a review. Amino Acids. 2019, 51, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Zandona, A.; Katalinić, M. Nicotinic acetylcholine receptors: Diversity and physiological importance for neurodegenerative disorders and development of organophosphate antidotes. Periodicum Biologorum. 2020, 121, 115–128. [Google Scholar] [CrossRef]
- Osteen, C. D.; Fernandez-Cornejo, J. Economic and policy issues of US agricultural pesticide use trends. Pest Management Science. 2013, 69, 1001–1025. [Google Scholar] [CrossRef]
- Mofunanya, A. O. The effect of a common household pesticide, bifenthrin on neuronal cell survival and differentiation. Adelphi University. 2003.
- APHA. Standard methods for the examination of water and waste waters, 20th ed. American Public Health Association, Washington DC. 1998.
- Cao, Z.; Shafer, T. J.; Murray, T. F. Mechanisms of pyrethroid insecticide- induced stimulation of calcium influx in neocortical neurons. Journal of Pharmacology and Experimental Therapeutics. 2011, 336, 197–205. [Google Scholar] [CrossRef]
- Ellman, G. L.; Courtney, K. D.; Andres Jr, V.; Featherstone, R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Takashima, F.; Hibiya, T. An atlas of fish histology: normal and pathological features. 1995.
- Syed, F.; Awasthi, K. K.; Chandravanshi, L. P.; Verma, R.; Rajawat, N. K.; Khanna, V. K.; Soni, I. Bifenthrin-induced neurotoxicity in rats: involvement of oxidative stress. Toxicology Research. 2018, 7, 48–58. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods in Enzymology. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D. E.; Valentine, W. N. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. Journal of Laboratory and Clinical Medicine. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Ross, R. G.; Selvasubramanian, S.; Jayasundar, S. Immunomodulatory activity of Punica granatum in rabbits a preliminary study. Journal of Ethnopharmacology, 2001, 78, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Silva, A. P. B.; Santos, J. M. M.; Martins, A.J. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids–a review. Parasite and Vectors. 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Agrawal, A.; Sharma, B. Pesticides induced oxidative stress in mammalian systems. International Journal of Current Biological and Medical Science. 2010, 1, 90–104. [Google Scholar]
- Mekircha, F.; Chebab, S.; Gabbianelli, R. The possible ameliorative effect of Olea europaea L. oil against deltamethrin-induced oxidative stress and alterations of serum concentrations of thyroid and reproductive hormones in adult female rats. Ecotoxicology and Environmental Safety. 2018, 161, 374–382. [Google Scholar] [CrossRef]
- Gargouri, B.; Boukholda, K.; Kumar, A. Bifenthrin insecticide promotes oxidative stress and increases inflammatory mediators in human neuroblastoma cells through NF-kappa B pathway. Toxicology in Vitro. 2020, 65, 104792. [Google Scholar] [CrossRef]
- Leutner, S.; Eckert, A.; Müller, W. E. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. Journal of Neural Transmission. 2001, 108, 955–967. [Google Scholar] [CrossRef]
- Shah, D.; Gupta, P. Fast updating algorithms for TCAM. IEEE Micro. 2001, 21, 36–47. [Google Scholar] [CrossRef]
- Rao, G. V.; Rao, J. K. S. Modulation in acetylcholinesterase of rat brain by pyrethroids in vivo and an in vitro kinetic study. Journal of Neurochemistry. 1995, 65, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Bond, C. E.; Greenfield, S. A. Multiple Cascade Effects of Oxidative Stress on Astroglia. Glia. 2007, 55, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Noeman, S. A.; Hamooda, H. E.; Baalash, A. A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetology and Metabolic Syndrome, 2011, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dar, M. A.; Khan, A. M.; Raina, R. Effect of repeated oral administration of bifenthrin antioxidant status and acetylcholinesterase activity in brain of rats. Environmental Toxicology and Chemistry, 2015, 97, 961–967. [Google Scholar] [CrossRef]
- Khan, A. M.; Rampal, S. Effects of repeated oral administration of pazufloxacin mesylate and meloxicam on the antioxidant status in rabbits. Journal of the American Association for Laboratory Animal Science, 2014, 53, 399–403. [Google Scholar]
- Yang, L.; Li, L. Actions of the pyrethroid insecticide bifenthrin on sodium channels expressed in rat cerebral cortical neurons. Toxicology Mechanisms and Methods. 2015, 25, 63–69. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Abdel Moneim, A.E.; Qayed, M.M.; El-Yamany, N.A. Potential role of N-acetylcysteine on chlorpyrifos-induced neurotoxicity in rats. Environmental Science and Pollution Research, 2019, 26, 20731–20741. [Google Scholar] [CrossRef]
- Britt, J. O.; Martin, J. L.; Okerberg, C. V.; Dick, E. J. Histopathologic changes in the brain, heart, and skeletal muscle of rhesus macaques, ten days after exposure to soman (an organophosphorus nerve agent). Comparative Medicine, 2002, 50, 133–139. [Google Scholar]
| Weight in grams | Experimental Group | ||||
|---|---|---|---|---|---|
| G1 (Control) | G2 (Saline) | G3 | G4 | G5 | |
| Initial weight | 881±9.5 | 880±9.3 | 877±9.3 | 875±9.8 | 872±8.8 |
| 1stweeks | 945±9.7 | 942±9.2 | 832±9.2 | 818±8.1 | 802±9.3 |
| 2nd week | 1011±9.5 | 1005±9.1 | 782±9.1 | 758±8.2 | 705±8.5 |
| Brain region of hippocampus | Treatment for 14 days | P value | ||||
|---|---|---|---|---|---|---|
| G1 (Control) |
G2 (Saline) |
G3 (1/15th of LD50) | G4 (1/10th of LD50) | G5 (1/5th of LD50) | ||
| Norepinephrine | 317±0.66 | 315±0.65 | 327±0.73 | 336±0.44 | 349±0.44 | 0.00 |
| Epinephrine | 276.73±0.5 | 271.72±0.6 | 252.66±0.30 | 220.66±0.55 | 190.55±0.50 | 0.00 |
| Dopamine | 500.54±0.11 | 501.52±0.1 | 404.6±0.08 | 370.8±0.12 | 347.44±0.66 | 0.00 |
| 3,4 Dihydroxyphe nylacetic acid |
40.01±0.24 | 39.41±0.22 | 38.06±0.22 | 26.07±0.20 | 17.09±0.17 | 0.00 |
| Homovanillic acid | 30.66±0.44 | 31.86±0.43 | 33.37±0.5 | 45.55±0.55 | 61.29±0.66 | 0.00 |
| Serotonin | 505.66±0.77 | 503.96±0.6 | 465.67±0.7 | 395.73±0.6 | 324.88±0.57 | 0.00 |
| Parameters | Experimental Groups | P values | ||||
| G1 (Control) | G2 (Saline) | G3 | G4 | G5 | ||
| Erythrocyte | 4.0±0.01 | 3.86±0.02 | 3.6±0.05 | 3.2±0.04 | 2.9±0.03 | 0.00 |
| Plasma | 0.60±0.05 | 0.58±0.04 | 0.55±0.06 | 0.50±0.03 | 0.48±0.05 | 0.00 |
| Brain | 6.8±0.04 | 6.9±0.03 | 6.2±0.05 | 5.7±0.06 | 5.0±0.05 | 0.00 |
| Parameters | Experimental Groups | P value | ||||
|---|---|---|---|---|---|---|
| G1 (Control) | G2 (Saline) |
G3 | G4 | G5 | ||
| GPx (U/L) | 2.04±0.05 | 2.00±0.04 | 1.31±0.06 | 1.06± 0.04 | 0.57±0.03 | 0.00 |
| SOD (U/ml) | 1.75±0.06 | 1.71±0.05 | 1.10±0.05 | 0.89± 0.04 | 0.43±0.03 | 0.00 |
| GST (U/L) | 74.99±0.07 | 72.89±0.05 | 48.74±0.07 | 40.49±0.06 | 20.24±0.04 | 0.00 |
| CAT (U/ml) | 20.40±0.04 | 19.72±0.02 | 13.05±0.03 | 11.83±0.04 | 5.71±0.03 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




