Submitted:
19 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study sites and species
2.2. Soil characteristics
2.3. Coralloid root surface sterilisation
2.4. Extracellular enzyme activities
2.5. Leaf nutrient composition
2.6. Percentage N Derived from the atmosphere (%NDFA)
2.7. Statistical analysis
3. Results
3.1. Soil characteristics
| Study site | Oceanview | Rhebu | ||
| Parameter | Rhizosphere | Non-rhizosphere soils | Rhizosphere | Non-rhizosphere soils |
| P K |
6.1±2.9a 139.5±33.6a |
3.86±1.9a 81.9±12.5 a |
22.95±5.9 a 330.0±100.9 a |
13.23±3.7 a 179.1±54.9 a |
| N | 3093.7±741.9 a | 2638.0±438.2 a | 5189.3±297.9 a | 3473.3±1396.3 a |
| Ca | 1555.3±274.8 a | 1379.2±153.5 a | 1534.9±974.2 a | 1229.4±1087.2 a |
| Mg | 454.7±46.1a | 382.12±35.3b | 838.5±549.3 a | 697.1±548.2 a |
| Mn | 55.3±21.1 a | 44.8±15.9 a | 89.7±58.0 a | 48.1±20.2 a |
| Zn | 2.2±0.1c | 1.3±0.4d | 4.1±1.6 a | 2.8±1.2 a |
| Cu | 1.1±0.2 a | 0.6±0.1 a | 0.9±0.1 a | 0.6±0.2 a |
| Exchange acidity (cmol/L) Total cations (cmol/L) pH |
0.05±0.01 a 11.53±1.65 a 5.43±0.41e |
0.05±0.01 a 11.65±1.62 a 4.97±0.57f |
1.46±1.42 a 15.26±8.13 a 4.28±0.61 a |
1.37±1.19 a 15.10±9.48 a 4.14±0.58 a |
| In each row, different letters denote significant differences in the nutrient concentrations and relative acidity of soil samples within study sites (independent samples t-test, p≤0.05, means±SE, n=32). | ||||
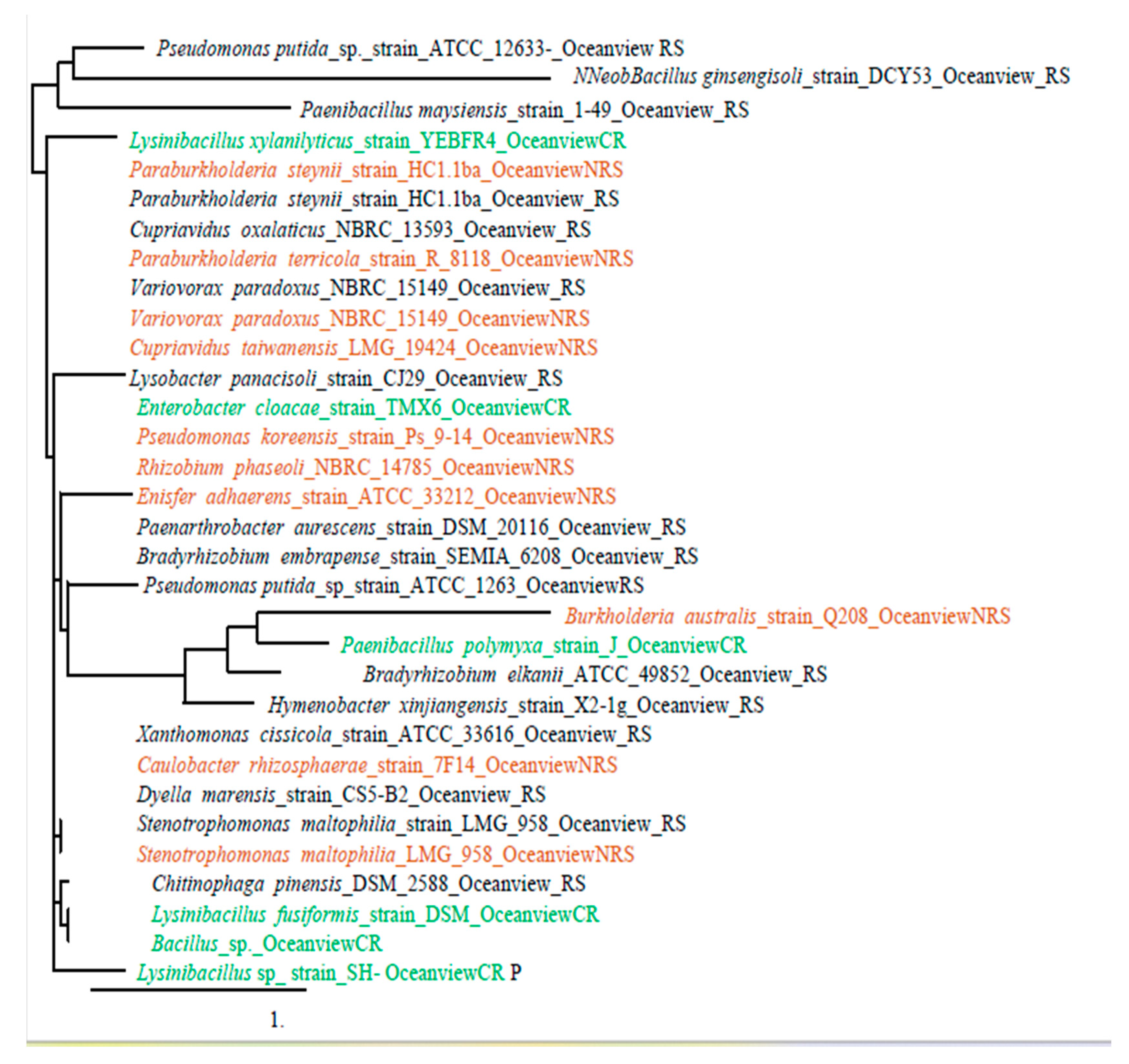
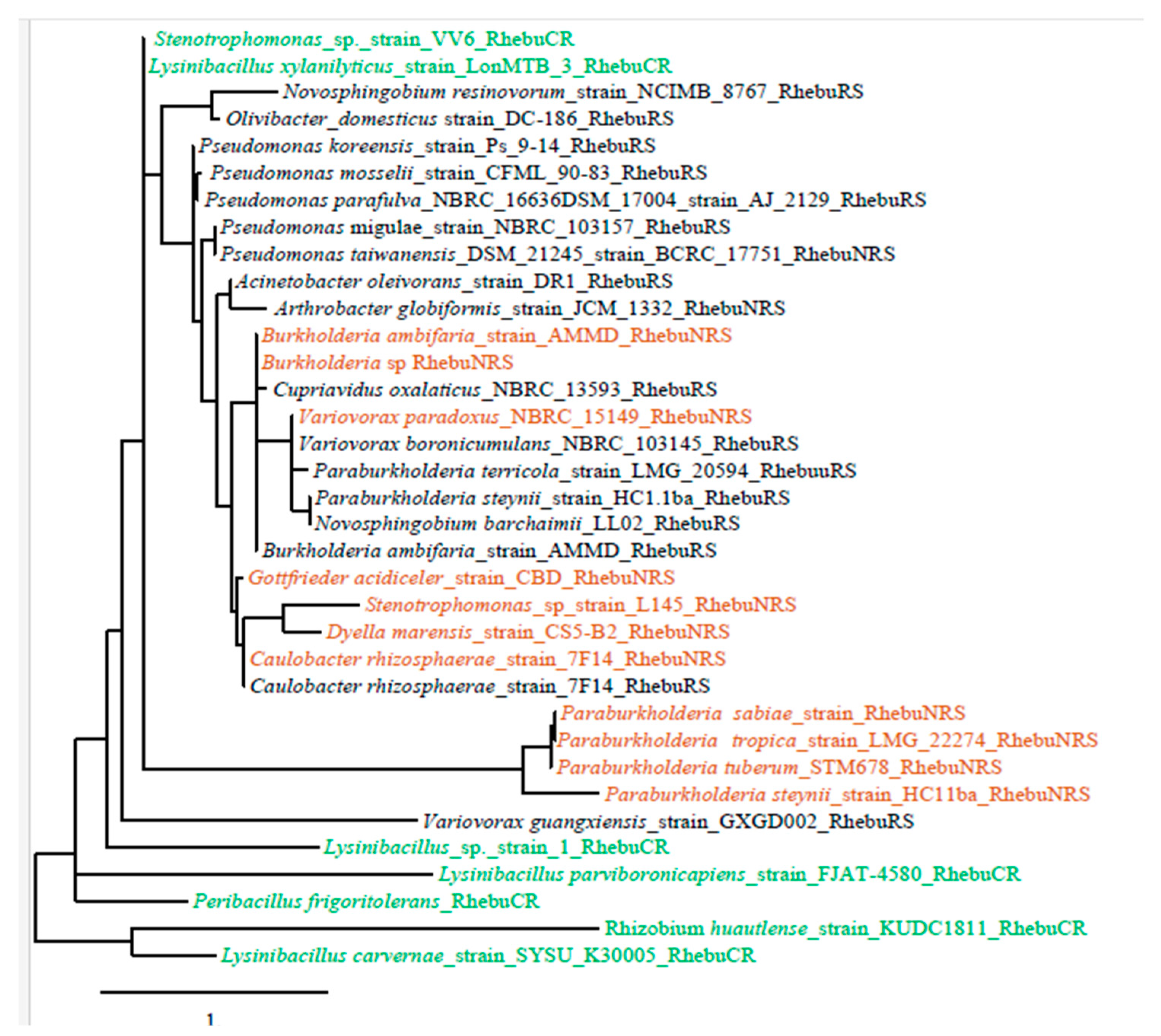
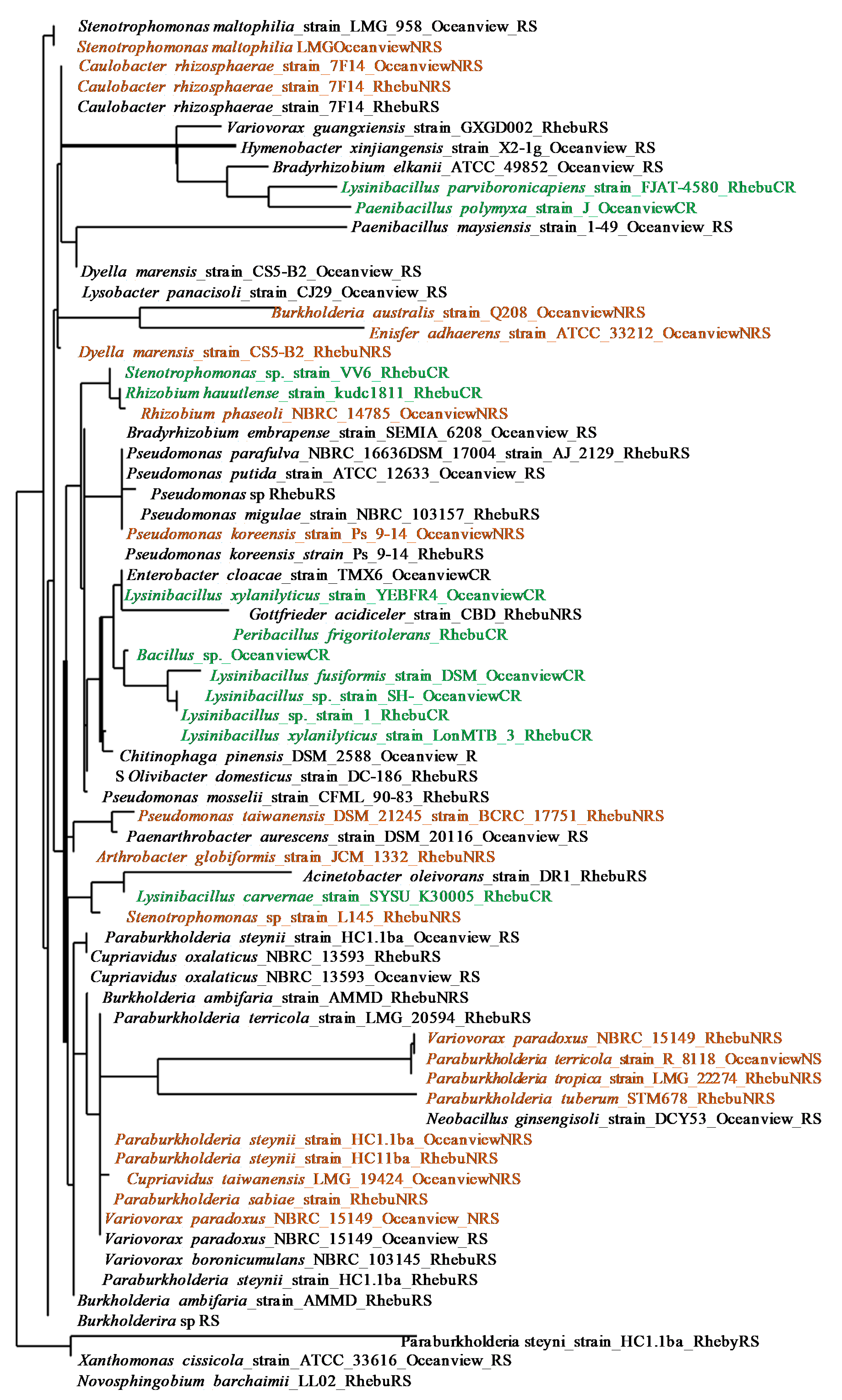
3.2. Bacterial identification
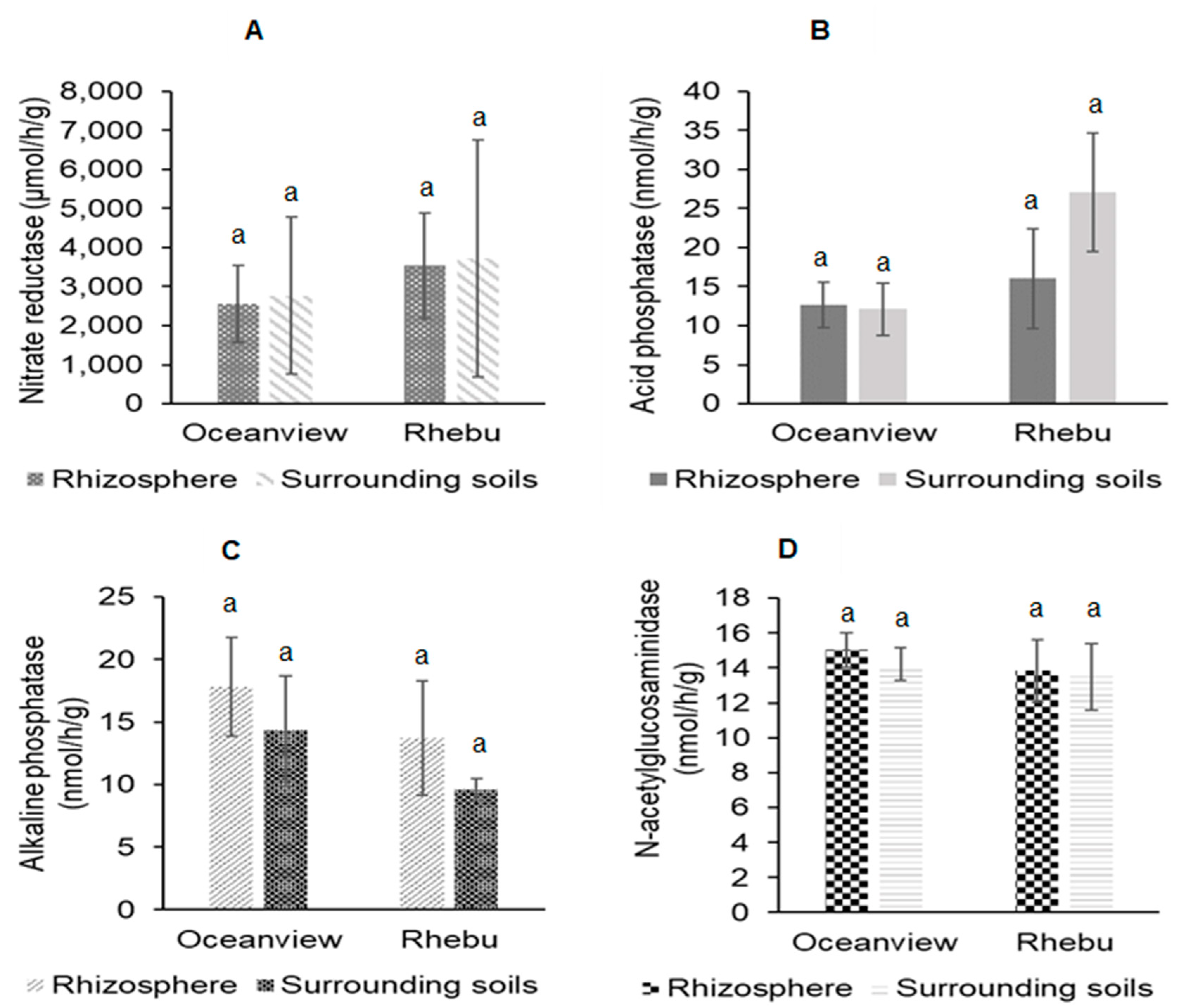
3.3. Extracellular enzyme activities
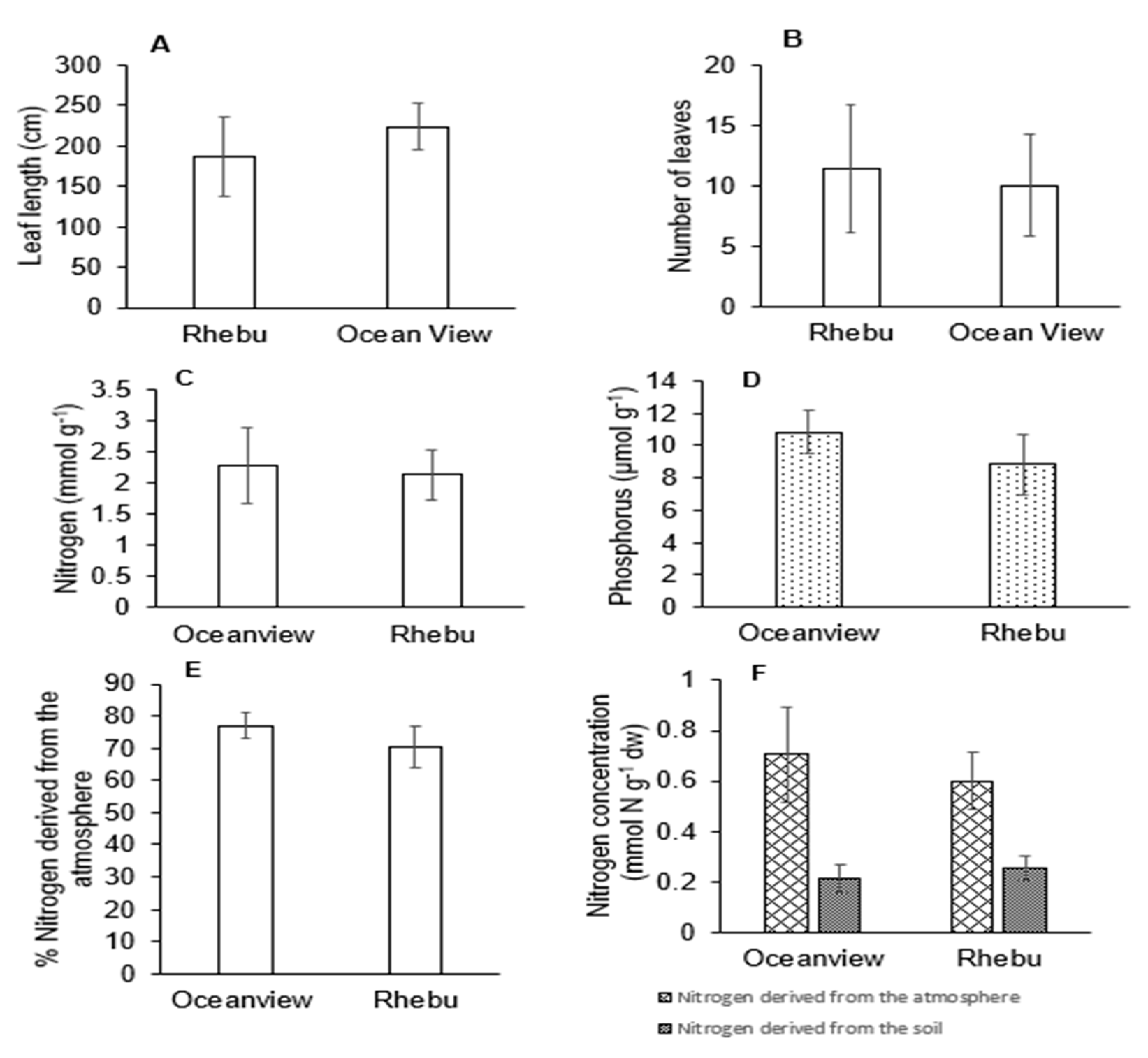
3.4. Leaf nutrition and N source reliance
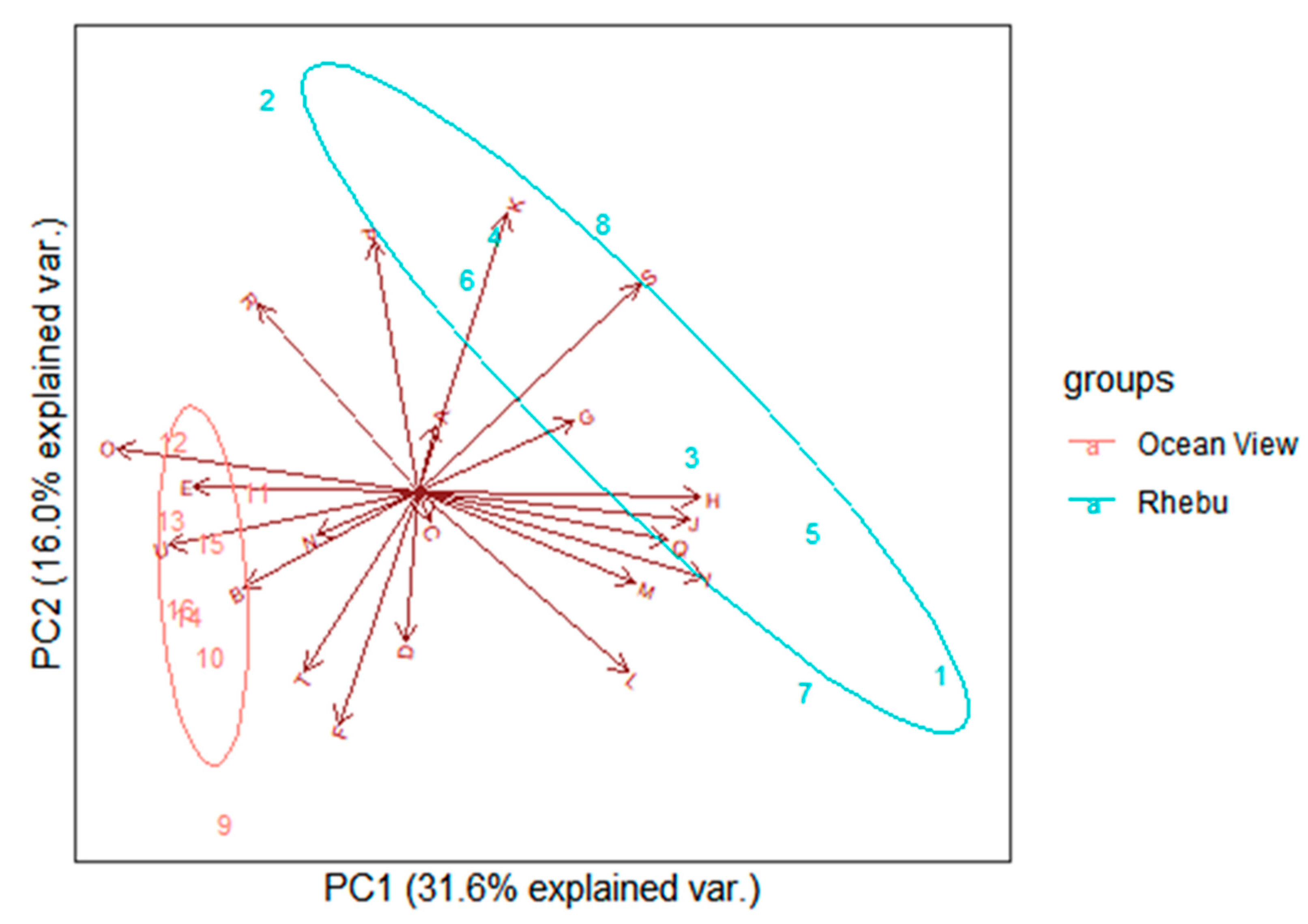
3.5. Correlations between rhizosphere soil characteristics, extracellular enzyme activities, leaf nutrition, and nitrogen source reliance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, E.D.; Stevenson, D.W.; Twigg, R.W. Cycads: Evolutionary innovations and the role of plant-derived neurotoxins. Trends Plant Sci. 2003, 8, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Krishnapillai, M.V. Cycas micronesica trees alter local soil traits. Forests. 2018, 9, 565. [Google Scholar] [CrossRef]
- Erdei, B.; Coiro, M.; Miller, I.; Johnson, K.R.; Griffith, P.M.; Murphy, V. First cycad seedling foliage from the fossil record and inferences for the Cenzoic evolution cycads. Biol. Lett. 2019, 15, 1–6. [Google Scholar] [CrossRef]
- Cousins, S.R.; Williamson, V.L.; Witkowski, E.T.F. Sifting through cycads: A guide to identifying the stem fragments of six South African medicinal Encephalartos species. S. Afr. J. Bot. 2013, 84, 115–123. [Google Scholar] [CrossRef]
- Donaldson, J.S. Cycads status survey and conservation action plan; IUCN/SSC Cycad Specialist Group. IUCN Gland, Switzerland and Cambridge: United Kingdom, 2003; pp. 26–89. [Google Scholar]
- Raimondo, D.; von Staden, L.V.; Foden, W.; Victor, J.E.; Helmen, N.A.; Turner, R.C.; Kamundi, D.A.; Manyama, P.A. Red list of South African plants, Strelitzer 25; South African National Biodiversity Institute: Pretoria, 2009. [Google Scholar]
- Condamine, F.L.; Nagalingum, N.S.; Marshall, C.R.; Morlon, H. 2015. Origin and diversification of living cycads: A cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evol. Biol. 2015, 15, 1–5. [Google Scholar] [CrossRef]
- Cousins, S.R.; Williamson, V.L.; Witkowski, E.T.F. Uncovering the cycad taxa (Encephalartos species) traded for traditional medicine in Johannesburg and Durban, South Africa. S. Afr. J. Bot. 2012, 78, 129–138. [Google Scholar] [CrossRef]
- Yessoufou, K.; Bamigboye, S.O.; Daru, B.H.; van der Bank, M. Evidence of constant diversification punctuated by a mass extinction in the African cycads. Ecol. Evol. 2014, 4, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Yepiz, J.C.; Cueva, A.; Dovciak, M.; Teece, M.; Enrico, A.; Yepez, E.A. 2014. Ontogenic resource-use strategies in a rare long-lived cycad along environmental gradients. Conserv. Physiol. 2014, 2, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Mucina, L.; Scott-Shaw, C.R.; Rutherford, M.C.; Camp, K.G.T.; Matthews, W.S.; Powrie, L.W.; Hoare, D.B. Indian Ocean coastal belt. Mucina, L., Rutherford, M.C., Eds.; In The vegetation of South Africa, Swaziland and Lesotho. Strelitzia 19; Pretoria: South African National Biodiversity Institute, 2006. [Google Scholar]
- Marler, T.E.; Calonje, M. Two cycad species affect the Carbon, Nitrogen, and Phosphorus content of soils. Horticulturae. 2020, 6, 24. [Google Scholar] [CrossRef]
- Ndlovu, S.; Suinyuy, T.N.; Pérez-Fernández, M.A.; Magadlela, A. Encephalartos natalensis, Their Nutrient-Cycling Microbes and Enzymes: A Story of Successful Trade-Offs. Plants. 2023, 12, 1034. [Google Scholar] [CrossRef]
- Smith, J.; Johnson, M.; Williams, D. The impact of cycads on the environment. J. Environ. Stud. 2018, 27, 123–135. [Google Scholar]
- Smith, J.; Johnson, M.; Williams, D. The impact of cycads on arid environments. J. Arid Environ. 2017, 105, 123–135. [Google Scholar]
- García, M.; Hernandez, R.; Lopez, P. The role of cycads in forest restoration efforts. Forestry Chronicle. 2019, 95, 12–23. [Google Scholar]
- Brown, T.; Davis, R.; Rodriguez, L. The economic value of cycads in developing countries. Economic Botany. 2018, 72, 34–45. [Google Scholar]
- Cenciani, K.; dos Santos Freitas, S.; Critter, S.A.M.; Airoldi, C. Microbial enzymatic activity and thermal effect in tropical soil treated with organic material. Soil Sci. Plant Nutr. 2008, 65, 1–7. [Google Scholar] [CrossRef]
- Latha, P.; Anand, T.; Prakasam, V.; Jonathan, E.I.; Paramathma, M.; Samiyappan, R. Combining Pseudomonas, Bacillus and Trichoderma stains with organic amendments and micronutrient to enhance suppression of collar and root rot disease in physic nut. Appl. Soil Ecol. 2011, 49, 215–223. [Google Scholar] [CrossRef]
- Meena, A.; Rao, K.S. Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semi-arid region, India Ecol. Process. 2021, 10, 1–10. [Google Scholar]
- Merino, C.; Godoy, R.; Matus, F. Soil enzymes and biological activity at different levels of organic matter stability. J Soil Sci. Plant Nutr. 2016, 16, 14–30. [Google Scholar]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase, and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Grobbelaar, N.; Hattingh, W.; Marshall, J. The occurrence of coralloid roots on the South African species of the Cycadales and their ability to fix nitrogen symbiotically. S. Afr. J. Bot. 1986, 52, 467–471. [Google Scholar] [CrossRef]
- Barea, J.; Pozo, M.J.; Azcòn-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-García, K.; Bustos-Díaz, E.D.; Corona-Gómez, J.A.; Ramos-Aboites, H.E.; Sélem-Mojica, N.; Cruz-Morales, P.; Pérez-Farrera, M.A.; Barona-Gómez, F.; Cibrián-Jaramillo, A. Cycad coralloid roots contain bacterial communities including Cyanobacteria and Caulobacter spp. that encode niche-specific biosynthetic gene clusters. Genome Biol. Evol. 2019, 11, 319–334. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northern, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Lindstrӧm, A. Leaf nutrient relations of cycads in common garden. Trop. Conserv. Sci. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Deloso, B.E.; Krishnapillai, M.V.; Ferreras, U.F.; Lindström, A.J.; Calonje, M.; Marler, T.E. Chemical element concentrations of cycad leaves: Do we know enough? Horticulturae. 2020, 6, 85. [Google Scholar] [CrossRef]
- Gehringer, M.M.; Pengelly, J.J.L.; Cuddy, W.S.; Fieker, C.; Forster, P.I.; Neilan, B.A. Host selection of symbiotic cyanobacteria in 31 species of the Australian cycad genus: Macrozamia (Zamiaceae). Molecular Plant Microbe Interactions. 2010, 23, 811–82. [Google Scholar] [CrossRef]
- Suárez-Moo, P.J.; Vovides, A.P.; Griffith, M.P.; Barona-Gòmez, F.; Cibrián-Jaramillo, A. Unlocking a high bacterial diversity in the coralloid root microbiome from the cycad genus Dioon. PLoS ONE. 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Kipp, M.A.; Stüeken, E.E.; Gehringer, M.M.; Sterelny, K.; Scott, J.K.; Forster, P.I.; Strӧmberg, C.A.E.; Buick, R. Exploring cycad foliage as an archive of the isotopic composition of atmospheric nitrogen. Gebiology. 2019, 18, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Lindstrӧm, A. Inserting cycads into global climate nutrient relations datasets. Plant Signal. Behav. 2018, 13, e15475781–e15475786. [Google Scholar] [CrossRef]
- Marler, T.E.; Ferreras, U.F. Disruption of leaf nutrient remobilization in coastal Cycas trees by tropical cyclone damage. Journal Geography and Natural Disasters. 2015, 5, 142. [Google Scholar]
- Manson, A.D.; Roberts, V.G. Analytical methods used by the soil fertility and analytical services section. KZN Agri-report no. N/A/2001/04, Pietermaritzburg, South Africa. 2000. [Google Scholar]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in legume–Rhizobium technology; Springer-Verlag: New York, USA., 1994. [Google Scholar]
- Ndabankulu, N.; Tsvuura, Z.; Magadlela, A. Soil microbe and associated extracellular enzymes largely impact nutrient bioavailability in acidic and nutrient poor grassland ecosystem soil. Scientific Reports. 2022, 12, 12601. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Him, S.J.; Dymock, D.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998, 64, 795–9. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11, Molecular Evolutionary Genetics Analysis Version 11. Molecular Biology and Evolution. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Tyler, H.L.; Millar, J.J. Determination of microbial extracellular enzyme activity in waters, soils, and sediments using high throughput microplate assays. JoVE. 2013, 80, e5039. [Google Scholar]
- Kandeler, E. Potential Nitrification. In Schinner F et al. (eds.) Methods in Soil Biology; Spinger-Verlag Berlin Heidelberg: Berlin, 1995; pp. 146–149. [Google Scholar]
- Marler, T.E.; Krishnapillai, M.V. Incident light and leaf age influence leaflet element concentrations of Cycas micronesica trees. Horticulturae. 2019, 5, 58. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H. N2-fixation in field settings: estimations based on natural 15N abundance. Funct. Plant Biol. 1986, 13, 699–756. [Google Scholar]
- Johnson, S.L.; Holt, R.D. The evolution of protein function. Science. 1989, 246, 1293–1296, Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1228813/. [Google Scholar]
- Sparks, D.W.; et al. The evolutionary origins of biodiversity. Science. 2017, 358, 692–697. [Google Scholar]
- Kropinski, M.; et al. The hologenome: a novel concept for understanding the organization and dynamics of prokaryotic genomes. PLOS Computational Biology. 2017, 13, e1005647. [Google Scholar]
- Simonsen, A.K.; Dinnage, R.; Barrett, L.G.; Prober, S.M.; Thrall, P.H. Symbiosis limits establishment of legumes outside their native range at a global scale. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Ulkrike, M. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol Sep. 2022, 76, 153765. [Google Scholar] [CrossRef]
- Motsomane, N.; Suinyuy, T.N.; Pérez-Fernández, M.A.; Magadlela, A. How the right evolved partners in Cycads and Legumes drive enhanced growth in a harsh environment. Symbiosis. 2023, in press.
- Jinek, M.; Chylinski, K.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Nature. 2005, 337, 816–82. [Google Scholar] [CrossRef]
- Charpentier, E.; Valgepea, T. The holobiont: A New Paradigm for Life on Earth. Dialogues in Human Geography. 2017, 1–14. [Google Scholar] [CrossRef]
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate solubilizing fungi. Adv. Agron. 2000, 69, 99–151. [Google Scholar]
- Igual, J.M.; Valverde, A.; Cervantes, E.; Velázquez, E. Phosphate-solubilizing bacteria as inoculants for agriculture: use of updated molecular techniques in their study. Agronomie. 2001, 21, 561–568. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Crowley, D.E.; Marschner, P.; Greiner, R.; Fernández, M.T.; Romero, D.; Menezes-Blackburn, D.; De La Luz Mora, M. Identification of b-propeller phytase-encoding genes in culturable Paenibacillus and Bacillus sp. from the rhizosphere of pasture plants on volcanic soils. FEMS Microbiol Ecol. 2011, 75, 163–172. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of Phosphatase Enzymes in Soil. In Phosphorus in Action; Springer, 2011; pp. 215–243. [Google Scholar]
- Wouter, J.T.M.; Buijsman, P.J. Secretion of alkaline phosphatase by Bacillus licheniformis 749/C during growth in batch and chemostat cultures. FEMS Microbiol. 1980, 7, 91–95. [Google Scholar] [CrossRef]
- Magadlela, A.; Lembede, Z.; Egbewale, S.O.; Olaniran, A.O. The metabolic potential of soil microorganisms and enzymes in phosphorus deficient KwaZulu Natal grassland ecosystem soil. Appl. Soil Ecol. 2023, 181, 1–11. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Moorhead, D.L. Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol. Biochem. 1994, 26, 1305–1311. [Google Scholar] [CrossRef]
- Adnan, A.; Mavinic, D.S.; Koch, F.A. Pilot-scale study of phosphorus recovery through struvite crystallization-examining to process feasibility. J. Environ. Health Sci. Eng. 2003, 2, 315–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




