Submitted:
19 September 2023
Posted:
21 September 2023
You are already at the latest version
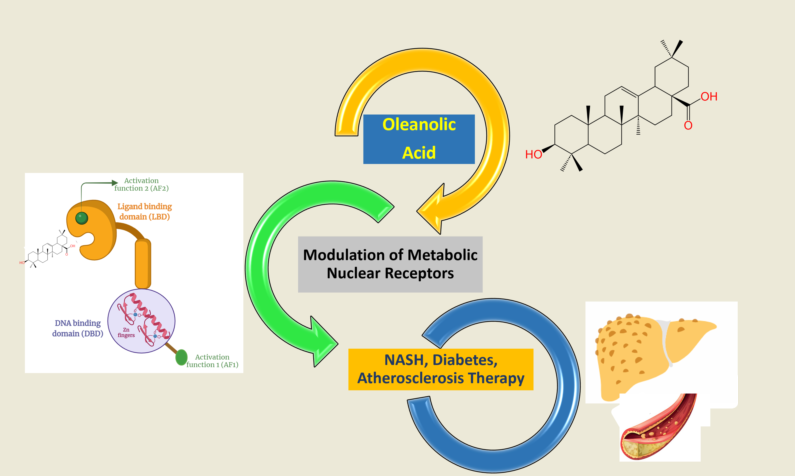
Abstract
Keywords:
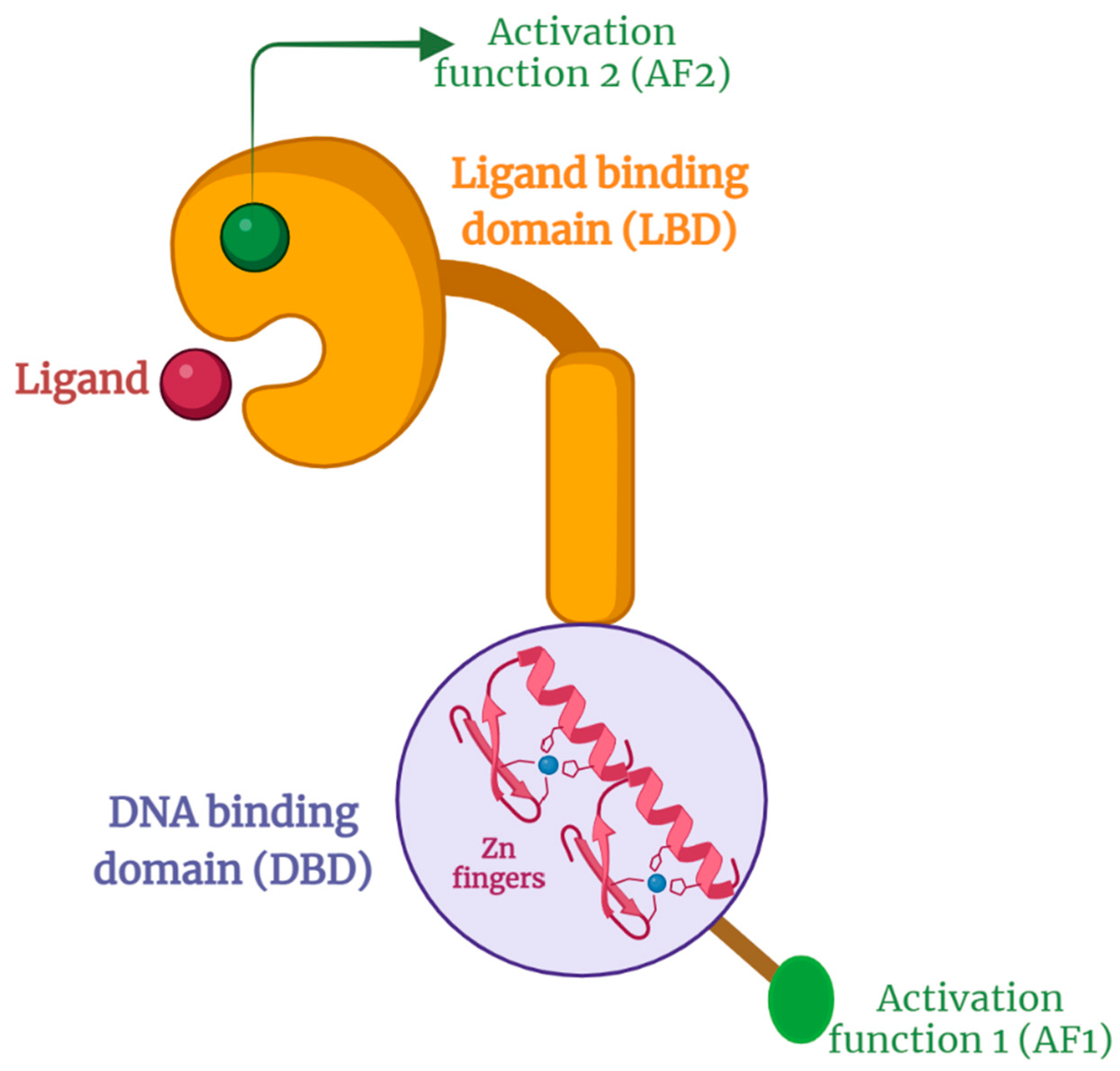
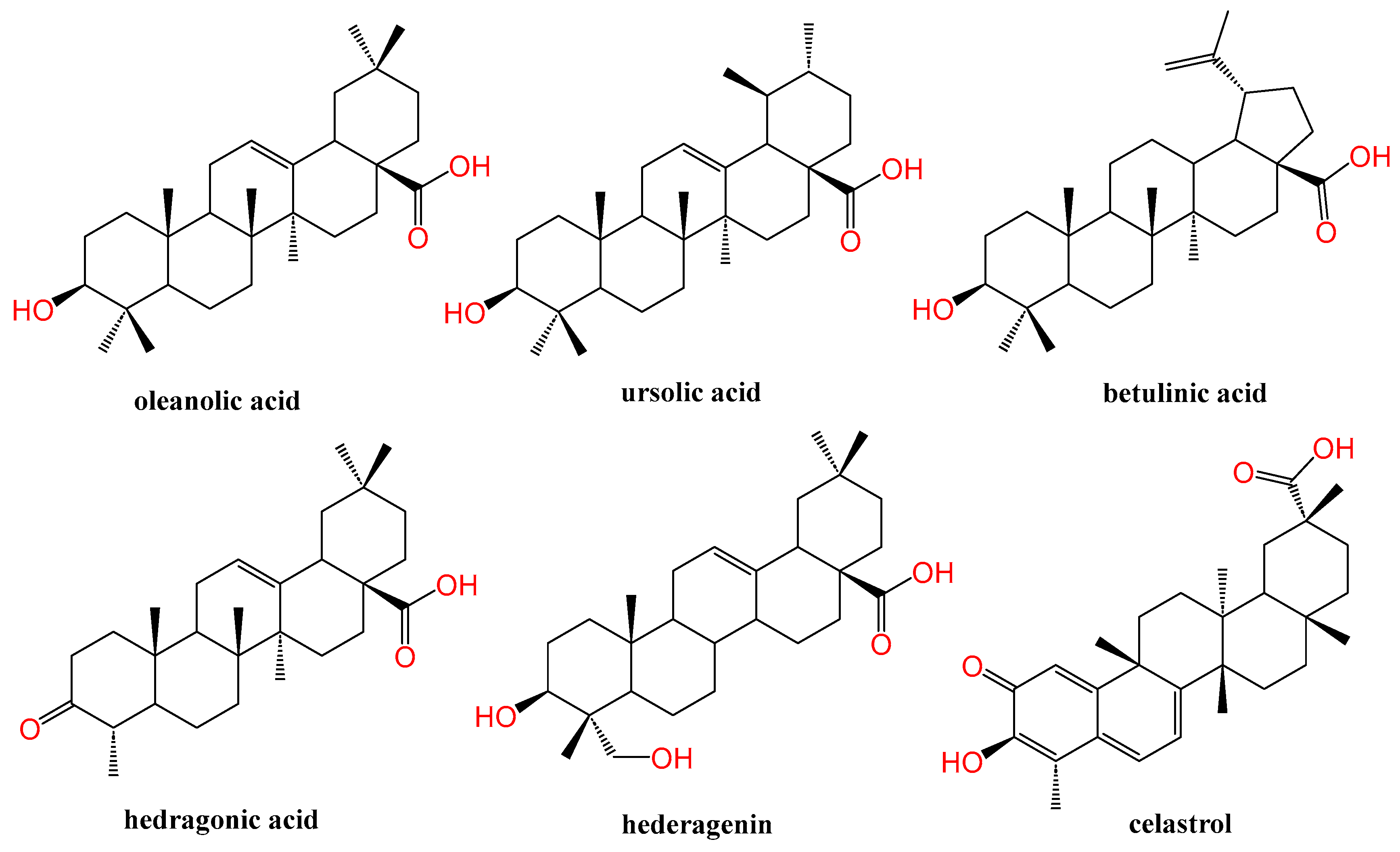
1. Introduction
2. Methodology
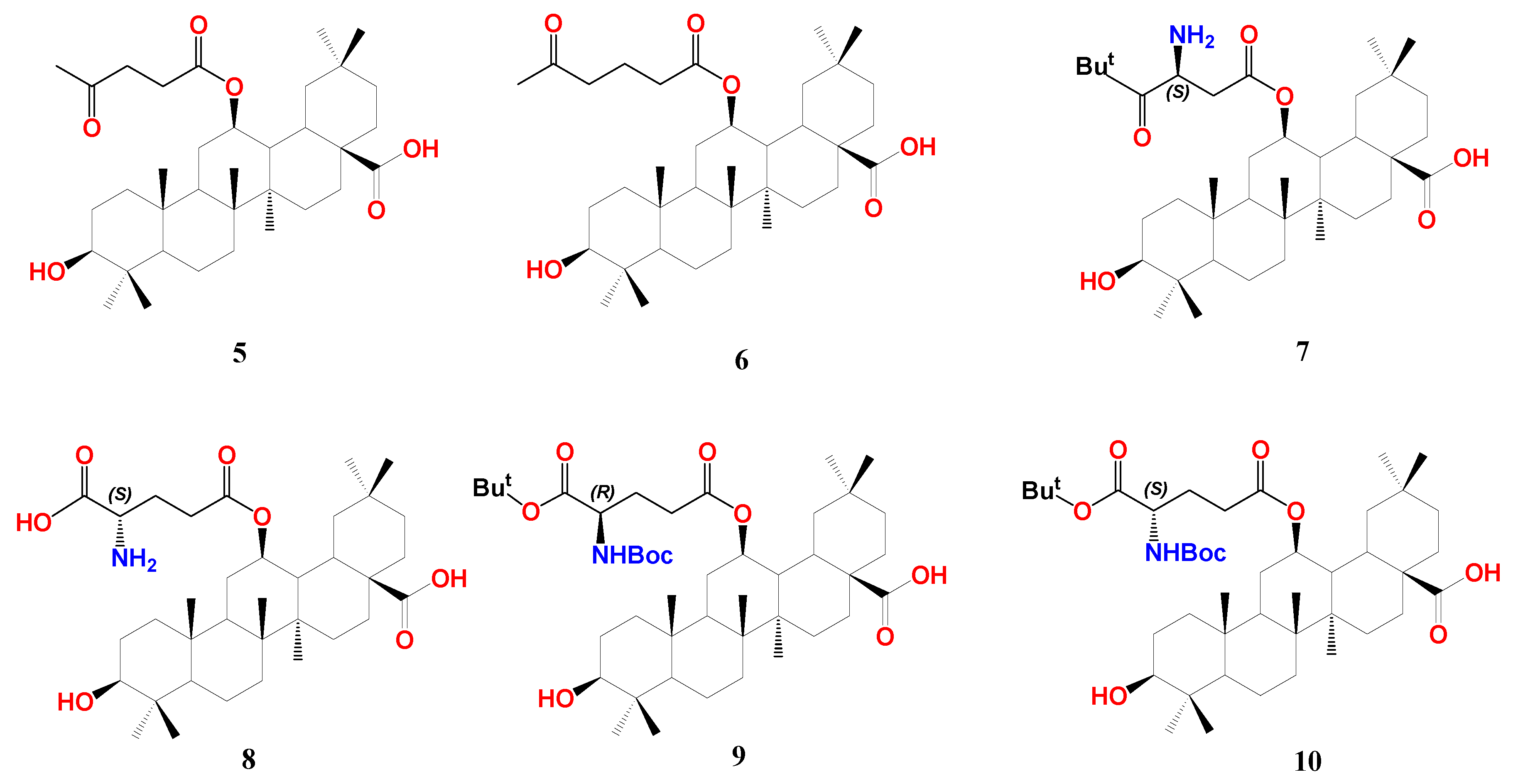
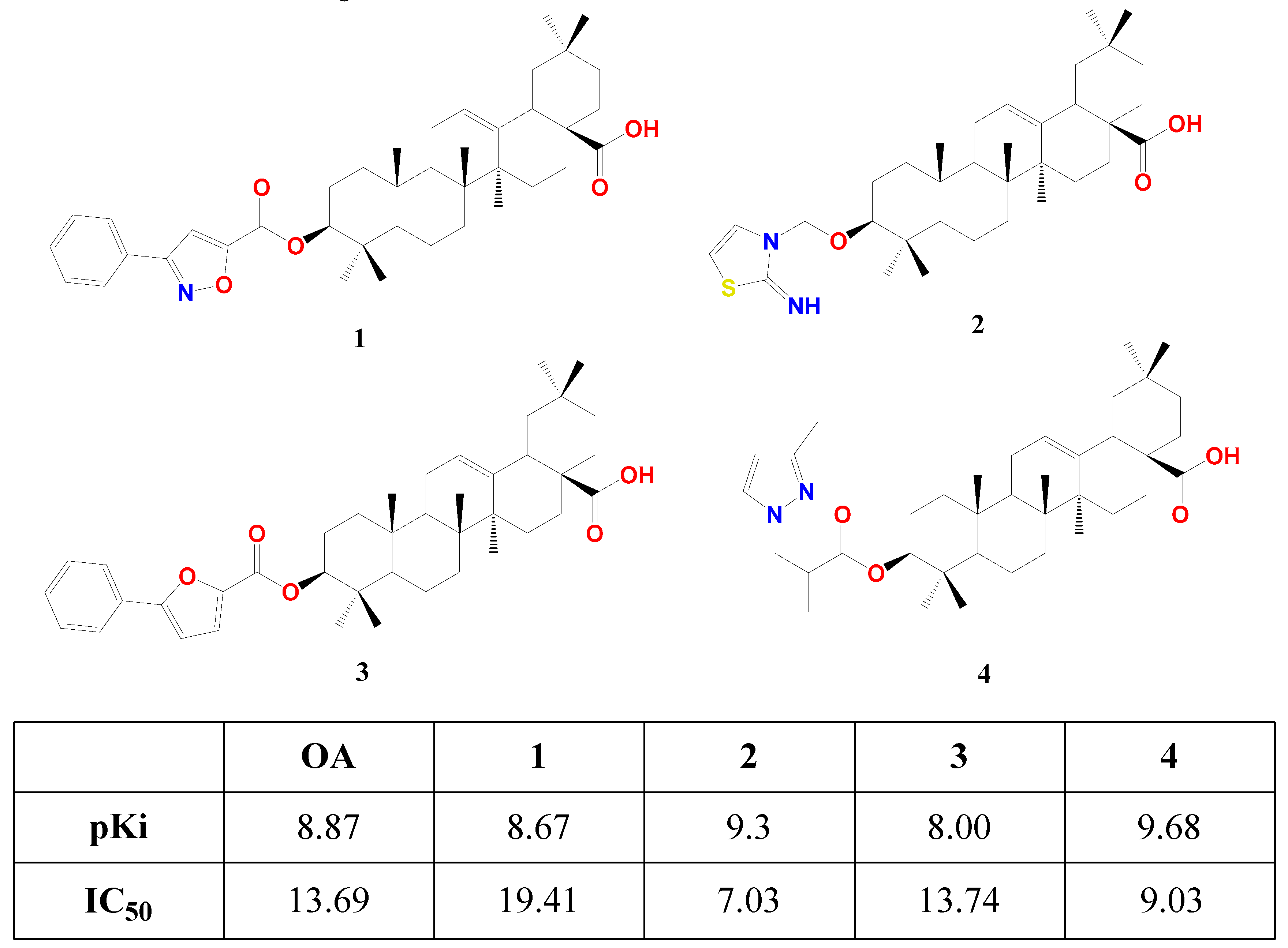
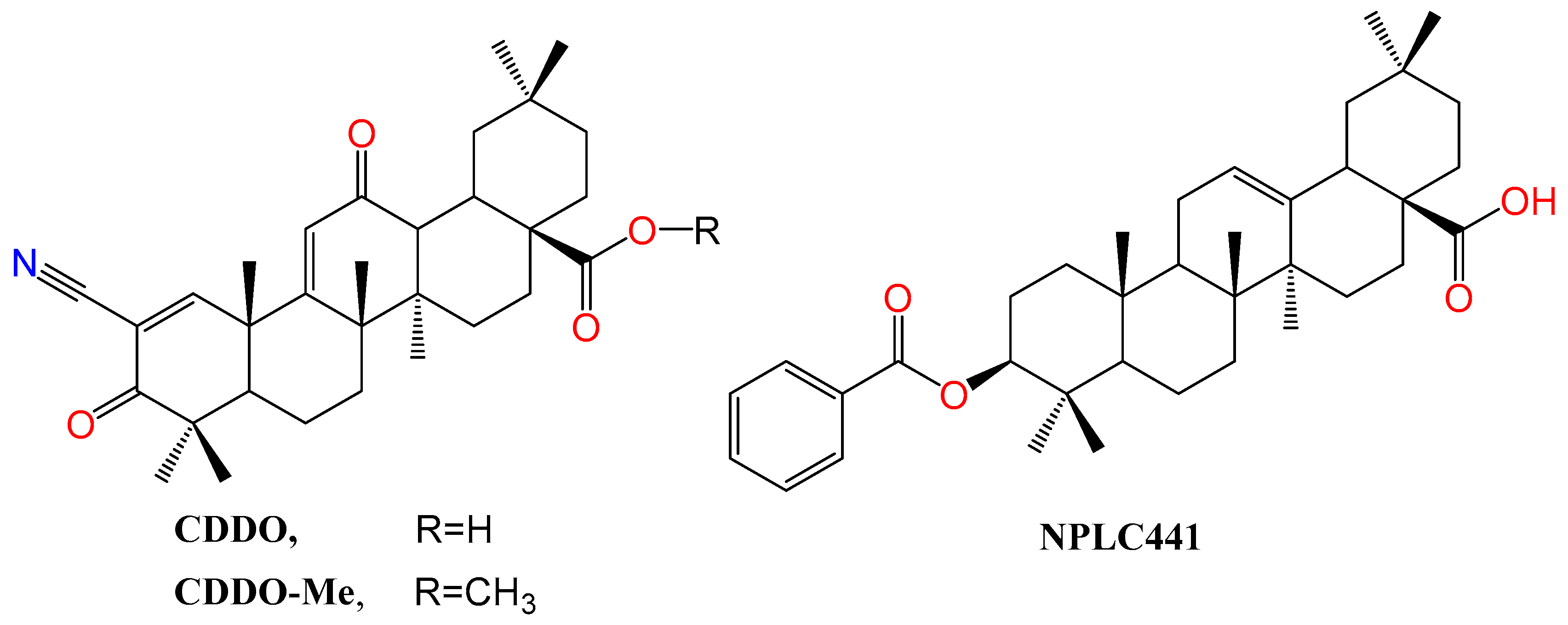
3. Oleanolic acid and its derivatives as nuclear receptors’ modulators
3.1. Modulation of FXR
3.2. Modulation of PPARs
3.3. Modulation of LXR
3.4. Modulation of RXR
3.5. Modulation of PXR and CAR
3.6. Modulation of ROR
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T. Nuclear Receptor Drug Discovery. Curr Opin Chem Biol 2008, 12, 418–426. [Google Scholar] [CrossRef]
- Burris, T. P.; Solt, L. A.; Wang, Y.; Crumbley, C.; Banerjee, S.; Griffett, K.; Lundasen, T.; Hughes, T.; Kojetin, D. J. Nuclear Receptors and Their Selective Pharmacologic Modulators. Pharmacol Rev 2013, 65, 710–778. [Google Scholar] [CrossRef]
- Mangelsdorf, D. J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; Evans, R. M. The Nuclear Receptor Superfamily: The Second Decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Frigo, D. E.; Bondesson, M.; Williams, C. Nuclear Receptors: From Molecular Mechanisms to Therapeutics. Essays Biochem 2021, 65, 847–856. [Google Scholar] [CrossRef]
- Fang, Y.; Hegazy, L.; Finck, B. N.; Elgendy, B. Recent Advances in the Medicinal Chemistry of Farnesoid X Receptor. J. Med. Chem. 2021, 64, 17545–17571. [Google Scholar] [CrossRef]
- Lith, S. C.; de Vries, C. J. M. Nuclear Receptor Nur77: Its Role in Chronic Inflammatory Diseases. Essays in Biochemistry 2021, 65, 927–939. [Google Scholar] [CrossRef]
- Penvose, A.; Keenan, J. L.; Bray, D.; Ramlall, V.; Siggers, T. Comprehensive Study of Nuclear Receptor DNA Binding Provides a Revised Framework for Understanding Receptor Specificity. Nat Commun 2019, 10, 2514. [Google Scholar] [CrossRef]
- Pan, P.; Chen, X. Nuclear Receptors as Potential Therapeutic Targets for Myeloid Leukemia. Cells 2020, 9, 1921. [Google Scholar] [CrossRef]
- Forman, B. M.; Ruan, B.; Chen, J.; Schroepfer, G. J.; Evans, R. M. The Orphan Nuclear Receptor LXRalpha Is Positively and Negatively Regulated by Distinct Products of Mevalonate Metabolism. Proc Natl Acad Sci U S A 1997, 94, 10588–10593. [Google Scholar] [CrossRef]
- Giguère, V. Orphan Nuclear Receptors: From Gene to Function. Endocr Rev 1999, 20, 689–725. [Google Scholar] [CrossRef]
- Kliewer, S. A.; Lehmann, J. M.; Willson, T. M. Orphan Nuclear Receptors: Shifting Endocrinology into Reverse. Science 1999, 284, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Solt, L. A.; Conkright, J. J.; Wang, Y.; Istrate, M. A.; Busby, S. A.; Garcia-Ordonez, R. D.; Burris, T. P.; Griffin, P. R. The Benzenesulfoamide T0901317 [N-(2,2,2-Trifluoroethyl)-N-[4-[2,2,2-Trifluoro-1-Hydroxy-1-(Trifluoromethyl)Ethyl]Phenyl]-Benzenesulfonamide] Is a Novel Retinoic Acid Receptor-Related Orphan Receptor-Alpha/Gamma Inverse Agonist. Mol Pharmacol 2010, 77, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D. J.; Evans, R. M. The RXR Heterodimers and Orphan Receptors. Cell 1995, 83, 841–850. [Google Scholar] [CrossRef]
- Sever, R.; Glass, C. K. Signaling by Nuclear Receptors. Cold Spring Harb Perspect Biol 2013, 5, a016709. [Google Scholar] [CrossRef] [PubMed]
- Sladek, F. M. What Are Nuclear Receptor Ligands? Molecular and Cellular Endocrinology 2011, 334, 3–13. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Y.; Zhang, Y.; Shen, H.; Hu, L.; Jiang, H.; Shen, X. Oleanolic Acid Derivative NPLC441 Potently Stimulates Glucose Transport in 3T3-L1 Adipocytes via a Multi-Target Mechanism. Biochem Pharmacol 2008, 76, 1251–1262. [Google Scholar] [CrossRef]
- Baes, M.; Gulick, T.; Choi, H. S.; Martinoli, M. G.; Simha, D.; Moore, D. D. A New Orphan Member of the Nuclear Hormone Receptor Superfamily That Interacts with a Subset of Retinoic Acid Response Elements. Mol Cell Biol 1994, 14, 1544–1552. [Google Scholar]
- Wada, T.; Gao, J.; Xie, W. PXR and CAR in Energy Metabolism. Trends Endocrinol Metab 2009, 20, 273–279. [Google Scholar] [CrossRef]
- Hiebl, V.; Ladurner, A.; Latkolik, S.; Dirsch, V. M. Natural Products as Modulators of the Nuclear Receptors and Metabolic Sensors LXR, FXR and RXR. Biotechnol Adv 2018, 36, 1657–1698. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Li, Y. Targeting Nuclear Receptors with Marine Natural Products. Mar Drugs 2014, 12, 601–635. [Google Scholar] [CrossRef]
- She, J.; Gu, T.; Pang, X.; Liu, Y.; Tang, L.; Zhou, X. Natural Products Targeting Liver X Receptors or Farnesoid X Receptor. Front Pharmacol 2021, 12, 772435. [Google Scholar] [CrossRef] [PubMed]
- Krasowski, M. D.; Ni, A.; Hagey, L. R.; Ekins, S. Evolution of Promiscuous Nuclear Hormone Receptors: LXR, FXR, VDR, PXR, and CAR. Mol Cell Endocrinol 2011, 334, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Newman, D. J.; Cragg, G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Koehn, F. E.; Carter, G. T. The Evolving Role of Natural Products in Drug Discovery. Nat Rev Drug Discov 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J. A. R.; Leal, A. S.; Valdeira, A. S.; Gonçalves, B. M. F.; Alho, D. P. S.; Figueiredo, S. A. C.; Silvestre, S. M.; Mendes, V. I. S. Oleanane-, Ursane-, and Quinone Methide Friedelane-Type Triterpenoid Derivatives: Recent Advances in Cancer Treatment. Eur J Med Chem 2017, 142, 95–130. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G. A.; Siordia-Reyes, A. G.; Meckes-Fischer, M.; Jiménez-Arellanes, A. Hepatoprotective Properties of Oleanolic and Ursolic Acids in Antitubercular Drug-Induced Liver Damage. Asian Pac J Trop Med 2016, 9, 644–651. [Google Scholar] [CrossRef]
- Ayeleso, T. B.; Matumba, M. G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Tsai, S. J.; Yin, M. C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. Journal of Food Science 2008. [Google Scholar] [CrossRef]
- Radwan, M. O.; Ismail, M. A. H.; El-Mekkawy, S.; Ismail, N. S. M.; Hanna, A. G. Synthesis and Biological Activity of New 18β-Glycyrrhetinic Acid Derivatives. Arabian Journal of Chemistry 2016, 9, 390–399. [Google Scholar] [CrossRef]
- Laszczyk, M. N. Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy. Planta Med 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Radwan, M. O.; Abd-Alla, H. I.; Alsaggaf, A. T.; El-Mezayen, H.; Abourehab, M. A. S.; El-Beeh, M. E.; Tateishi, H.; Otsuka, M.; Fujita, M. Gypsogenin Battling for a Front Position in the Pentacyclic Triterpenes Game of Thrones on Anti-Cancer Therapy: A Critical Review—Dedicated to the Memory of Professor Hanaa M. Rady. Molecules 2023, 28, 5677. [Google Scholar] [CrossRef] [PubMed]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S. F.; Bishayee, A.; Farooqi, A. A.; Sureda, A.; Nabavi, S. M. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. International Journal of Molecular Sciences 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Liese, J.; Abhari, B. A.; Fulda, S. Smac Mimetic and Oleanolic Acid Synergize to Induce Cell Death in Human Hepatocellular Carcinoma Cells. Cancer Lett 2015, 365, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory Effect of Oleanolic Acid on Hepatocellular Carcinoma via ERK-P53-Mediated Cell Cycle Arrest and Mitochondrial-Dependent Apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Li, D. Oleanolic Acid Suppresses the Proliferation of Lung Carcinoma Cells by MiR-122/Cyclin G1/MEF2D Axis. Mol Cell Biochem 2015, 400, 1–7. [Google Scholar] [CrossRef]
- Janakiram, N. B.; Indranie, C.; Malisetty, S. V.; Jagan, P.; Steele, V. E.; Rao, C. V. Chemoprevention of Colon Carcinogenesis by Oleanolic Acid and Its Analog in Male F344 Rats and Modulation of COX-2 and Apoptosis in Human Colon HT-29 Cancer Cells. Pharm Res 2008, 25, 2151–2157. [Google Scholar] [CrossRef]
- Mu, D.-W.; Guo, H.-Q.; Zhou, G.-B.; Li, J.-Y.; Su, B. Oleanolic Acid Suppresses the Proliferation of Human Bladder Cancer by Akt/MTOR/S6K and ERK1/2 Signaling. Int J Clin Exp Pathol 2015, 8, 13864–13870. [Google Scholar]
- I. Chakravarti, B.; Maurya, R.; Siddiqui, J. A.; Bid, H. K.; Rajendran, S. M.; Yadav, P. P.; Konwar, R. In Vitro Anti-Breast Cancer Activity of Ethanolic Extract of Wrightia Tomentosa: Role of pro-Apoptotic Effects of Oleanolic Acid and Urosolic Acid. J Ethnopharmacol 2012, 142, 72–79. [Google Scholar] [CrossRef]
- Ciftci, H.; O. Radwan, M.; E. Ozturk, S.; Ulusoy, N. G.; Sozer, E.; E. Ellakwa, D.; Ocak, Z.; Can, M.; F.S. Ali, T.; I. Abd-Alla, H.; Yayli, N.; Tateishi, H.; Otsuka, M.; Fujita, M. Design, Synthesis and Biological Evaluation of Pentacyclic Triterpene Derivatives: Optimization of Anti-ABL Kinase Activity. Molecules 2019, 24, 3535–3535. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer Activity of Oleanolic Acid and Its Derivatives: Recent Advances in Evidence, Target Profiling and Mechanisms of Action. Biomedicine & Pharmacotherapy 2022, 145, 112397. [Google Scholar] [CrossRef]
- Kinjo, J.; Okawa, M.; Udayama, M.; Sohno, Y.; Hirakawa, T.; Shii, Y.; Nohara, T. Hepatoprotective and Hepatotoxic Actions of Oleanolic Acid-Type Triterpenoidal Glucuronides on Rat Primary Hepatocyte Cultures. Chem Pharm Bull (Tokyo) 1999, 47, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y.-F.; Wu, Q.; Xu, S.-F.; Shi, F.-G.; Klaassen, C. D. Oleanolic Acid Reprograms the Liver to Protect against Hepatotoxicants, but Is Hepatotoxic at High Doses. Liver Int 2019, 39, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-F.; Liu, J.; Wu, K. C.; Klaassen, C. D. Protection against Phalloidin-Induced Liver Injury by Oleanolic Acid Involves Nrf2 Activation and Suppression of Oatp1b2. Toxicology Letters 2015, 232, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Du, X.; Chen, S.; Feng, X.; Cheng, Y.; Zhang, L.; Gao, Y.; Li, S.; He, X.; Wang, R.; Zhou, X.; Yang, Y.; Luo, W.; Chen, W. Oral Administration of Oleanolic Acid, Isolated from Swertia Mussotii Franch, Attenuates Liver Injury, Inflammation, and Cholestasis in Bile Duct-Ligated Rats. Int J Clin Exp Med 2015, 8, 1691–1702. [Google Scholar]
- Chen, P.; Zeng, H.; Wang, Y.; Fan, X.; Xu, C.; Deng, R.; Zhou, X.; Bi, H.; Huang, M. Low Dose of Oleanolic Acid Protects against Lithocholic Acid–Induced Cholestasis in Mice: Potential Involvement of Nuclear Factor-E2-Related Factor 2-Mediated Upregulation of Multidrug Resistance-Associated Proteins. Drug Metab Dispos 2014, 42, 844–852. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Stankevicius, E.; Herrera, M. D.; Østergaard, L.; Andersen, M. R.; Ruiz-Gutierrez, V.; Simonsen, U. Oleanolic Acid Induces Relaxation and Calcium-Independent Release of Endothelium-Derived Nitric Oxide. British Journal of Pharmacology 2008, 155, 535–546. [Google Scholar] [CrossRef]
- Buus, N. H.; Hansson, N. C.; Rodriguez-Rodriguez, R.; Stankevicius, E.; Andersen, M. R.; Simonsen, U. Antiatherogenic Effects of Oleanolic Acid in Apolipoprotein E Knockout Mice. European Journal of Pharmacology 2011, 670, 519–526. [Google Scholar] [CrossRef]
- Li, Y.-F.; Hu, L.-H.; Lou, F.-C.; Li, J.; Shen, Q. PTP1B Inhibitors from Ardisia Japonica. J Asian Nat Prod Res 2005, 7, 13–18. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-Hyperglycemic Activity of a TGR5 Agonist Isolated from Olea Europaea. Biochem Biophys Res Commun 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Guo, F.; Gao, Y.; Li, X.; Lei, X. Natural Product 2-Oxokolavenol Is a Novel FXR Agonist. Molecules 2022, 27, 8968. [Google Scholar] [CrossRef]
- Yamada, T.; Sugimoto, K. Guggulsterone and Its Role in Chronic Diseases. Adv Exp Med Biol 2016, 929, 329–361. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Feng, X.; Rong, H.; Pan, Z.; Inaba, Y.; Qiu, L.; Zheng, W.; Lin, S.; Wang, R.; Wang, Z.; Wang, S.; Liu, H.; Li, S.; Xie, W.; Li, Y. The Antiparasitic Drug Ivermectin Is a Novel FXR Ligand That Regulates Metabolism. Nat Commun 2013, 4, 1937. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Mihály-Bison, J.; Schuster, D.; Afonyushkin, T.; Binder, M.; Guan, S.; Cheng, C.; Wolber, G.; Stuppner, H.; Guo, D.; Bochkov, V. N.; Rollinger, J. M. Pharmacophore-Based Discovery of FXR-Agonists. Part II: Identification of Bioactive Triterpenes from Ganoderma Lucidum. Bioorganic & Medicinal Chemistry 2011, 19, 6779–6791. [Google Scholar] [CrossRef]
- Renga, B.; Mencarelli, A.; D’Amore, C.; Cipriani, S.; D’Auria, M. V.; Sepe, V.; Chini, M. G.; Monti, M. C.; Bifulco, G.; Zampella, A.; Fiorucci, S. Discovery That Theonellasterol a Marine Sponge Sterol Is a Highly Selective FXR Antagonist That Protects against Liver Injury in Cholestasis. PLoS One 2012, 7, e30443. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.; Zhong, B.; Nurieva, R. I.; Ding, S.; Dong, C. Ursolic Acid Suppresses Interleukin-17 (IL-17) Production by Selectively Antagonizing the Function of RORγt Protein*. Journal of Biological Chemistry 2011, 286, 22707–22710. [Google Scholar] [CrossRef]
- Lin, Y.-N.; Wang, C. C. N.; Chang, H.-Y.; Chu, F.-Y.; Hsu, Y.-A.; Cheng, W.-K.; Ma, W.-C.; Chen, C.-J.; Wan, L.; Lim, Y.-P. Ursolic Acid, a Novel Liver X Receptor α (LXRα) Antagonist Inhibiting Ligand-Induced Non-alcoholic Fatty Liver and Drug-Induced Lipogenesis. J Agric Food Chem 2018, 66, 11647–11662. [Google Scholar] [CrossRef]
- Gu, M.; Zhao, P.; Zhang, S.; Fan, S.; Yang, L.; Tong, Q.; Ji, G.; Huang, C. Betulinic Acid Alleviates Endoplasmic Reticulum Stress-Mediated Non-alcoholic Fatty Liver Disease through Activation of Farnesoid X Receptors in Mice. Br J Pharmacol 2019, 176, 847–863. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, W.; Lin, S.; Guo, F.; Zhu, Y.; Wei, Y.; Liu, X.; Jin, S.; Jin, L.; Li, Y. Identification of an Oleanane-Type Triterpene Hedragonic Acid as a Novel Farnesoid X Receptor Ligand with Liver Protective Effects and Anti-Inflammatory Activity. Mol Pharmacol 2018, 93, 63–72. [Google Scholar] [CrossRef]
- Fallon, C. M.; Smyth, J. S.; Quach, A.; Lajczak-McGinley, N.; O’Toole, A.; Barrett, K. E.; Sheridan, H.; Keely, S. J. Pentacyclic Triterpenes Modulate Farnesoid X Receptor Expression in Colonic Epithelial Cells: Implications for Colonic Secretory Function. Journal of Biological Chemistry 2022, 298. [Google Scholar] [CrossRef]
- Modica, S.; Murzilli, S.; Salvatore, L.; Schmidt, D. R.; Moschetta, A. Nuclear Bile Acid Receptor FXR Protects against Intestinal Tumorigenesis. Cancer Research 2008, 68, 9589–9594. [Google Scholar] [CrossRef]
- Hu, M.; Luo, Q.; Alitongbieke, G.; Chong, S.; Xu, C.; Xie, L.; Chen, X.; Zhang, D.; Zhou, Y.; Wang, Z.; Ye, X.; Cai, L.; Zhang, F.; Chen, H.; Jiang, F.; Fang, H.; Yang, S.; Liu, J.; Diaz-Meco, M. T.; Su, Y.; Zhou, H.; Moscat, J.; Lin, X.; Zhang, X. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Molecular Cell 2017, 66, 141–153.e6. [Google Scholar] [CrossRef]
- Shanmugam, M. K.; Dai, X.; Kumar, A. P.; Tan, B. K. H.; Sethi, G.; Bishayee, A. Oleanolic Acid and Its Synthetic Derivatives for the Prevention and Therapy of Cancer: Preclinical and Clinical Evidence. Cancer Lett 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.; Schubert-Zsilavecz, M.; Merk, D. Medicinal Chemistry and Pharmacological Effects of Farnesoid X Receptor (FXR) Antagonists. Curr Top Med Chem 2014, 14, 2188–2205. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cai, J.; Gonzalez, F. J. The Role of Farnesoid X Receptor in Metabolic Diseases, and Gastrointestinal and Liver Cancer. Nat Rev Gastroenterol Hepatol 2021, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X Receptor (FXR): Structures and Ligands. Comput Struct Biotechnol J 2021, 19, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Okamoto, A. Y.; Repa, J. J.; Tu, H.; Learned, R. M.; Luk, A.; Hull, M. V.; Lustig, K. D.; Mangelsdorf, D. J.; Shan, B. Identification of a Nuclear Receptor for Bile Acids. Science 1999, 284, 1362–1365. [Google Scholar] [CrossRef]
- Parks, D. J.; Blanchard, S. G.; Bledsoe, R. K.; Chandra, G.; Consler, T. G.; Kliewer, S. A.; Stimmel, J. B.; Willson, T. M.; Zavacki, A. M.; Moore, D. D.; Lehmann, J. M. Bile Acids: Natural Ligands for an Orphan Nuclear Receptor. Science 1999, 284, 1365–1368. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, F. Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F. J.; Willson, T. M.; Edwards, P. A. Activation of the Nuclear Receptor FXR Improves Hyperglycemia and Hyperlipidemia in Diabetic Mice. Proc Natl Acad Sci U S A 2006, 103, 1006–1011. [Google Scholar] [CrossRef]
- Pellicciari, R.; Costantino, G.; Fiorucci, S. Farnesoid X Receptor: From Structure to Potential Clinical Applications. J Med Chem 2005, 48, 5383–5403. [Google Scholar] [CrossRef]
- Xu, X.; Shi, X.; Chen, Y.; Zhou, T.; Wang, J.; Xu, X.; Chen, L.; Hu, L.; Shen, X. HS218 as an FXR Antagonist Suppresses Gluconeogenesis by Inhibiting FXR Binding to PGC-1α Promoter. Metabolism 2018, 85, 126–138. [Google Scholar] [CrossRef]
- Festa, C.; Finamore, C.; Marchianò, S.; Di Leva, F. S.; Carino, A.; Monti, M. C.; del Gaudio, F.; Ceccacci, S.; Limongelli, V.; Zampella, A.; Fiorucci, S.; De Marino, S. Investigation around the Oxadiazole Core in the Discovery of a New Chemotype of Potent and Selective FXR Antagonists. ACS Med. Chem. Lett. 2019, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Azu, N.; Rosenzweig, J. A.; Jackson, D. A. Guggulsterone for Chemoprevention of Cancer. Curr Pharm Des 2016, 22, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.; Henry, R. R.; Sanyal, A. J.; Morrow, L.; Marschall, H.-U.; Kipnes, M.; Adorini, L.; Sciacca, C. I.; Clopton, P.; Castelloe, E.; Dillon, P.; Pruzanski, M.; Shapiro, D. Efficacy and Safety of the Farnesoid X Receptor Agonist Obeticholic Acid in Patients with Type 2 Diabetes and Non-alcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, M.; Takada, I.; Makishima, M.; Sano, K. Farnesoid X Receptor Activation Enhances Transforming Growth Factor β-Induced Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Cells. International Journal of Molecular Sciences 2018, 19, 1898. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wong, C. Oleanolic Acid Is a Selective Farnesoid X Receptor Modulator. Phytother Res 2010, 24, 369–373. [Google Scholar] [CrossRef]
- Kanno, Y.; Tanuma, N.; Takahashi, A.; Inouye, Y. TO901317, a Potent LXR Agonist, Is an Inverse Agonist of CAR. J Toxicol Sci 2013, 38, 309–315. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Fan, X.; Zeng, H.; Deng, R.; Li, D.; Huang, M.; Bi, H. Oleanolic Acid Attenuates Obstructive Cholestasis in Bile Duct-Ligated Mice, Possibly via Activation of NRF2-MRPs and FXR Antagonism. Eur J Pharmacol 2015, 765, 131–139. [Google Scholar] [CrossRef]
- Wang, S.-R.; Xu, T.; Deng, K.; Wong, C.-W.; Liu, J.; Fang, W.-S. Discovery of Farnesoid X Receptor Antagonists Based on a Library of Oleanolic Acid 3-O-Esters through Diverse Substituent Design and Molecular Docking Methods. Molecules 2017, 22, 690. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, F.; Song, Z.; Huang, H.; Chen, Y.; Shen, Y.; Jia, Y.; Chen, J. Oleanolic Acid Protects against Pathogenesis of Atherosclerosis, Possibly via FXR-Mediated Angiotensin (Ang)-(1–7) Upregulation. Biomedicine & Pharmacotherapy 2018, 97, 1694–1700. [Google Scholar] [CrossRef]
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and Activity of Pentacyclic Triterpenes Codrugs. A Review. Mini-Reviews in Medicinal Chemistry 21 1509–1526. [CrossRef]
- Mariotti, V.; Strazzabosco, M.; Fabris, L.; Calvisi, D. F. Animal Models of Biliary Injury and Altered Bile Acid Metabolism. Biochim Biophys Acta Mol Basis Dis 2018, 1864 (4 Pt B), 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Meng, C.; Huang, C.; Liu, F.; Xia, C. Oleanolic Acid Alleviates ANIT-Induced Cholestatic Liver Injury by Activating Fxr and Nrf2 Pathways to Ameliorate Disordered Bile Acids Homeostasis. Phytomedicine 2022, 102, 154173. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Meng, C.; Gu, Q.; Huang, C.; Liu, F.; Xia, C. NRF2 and FXR Dual Signaling Pathways Cooperatively Regulate the Effects of Oleanolic Acid on Cholestatic Liver Injury. Phytomedicine 2023, 108, 154529. [Google Scholar] [CrossRef] [PubMed]
- S, W.; Y, H.; S, N.; H, C.; M, Y.; X, Z.; X, G.; X, W.; Z, S.; Ws, F. Discovery of 12β-Oxygenated Oleanolic Acid Alkyl Esters as Potent and Selective FXR Modulators Exhibiting Hyperglycemia Amelioration in Vivo. Bioorganic chemistry 2022, 129. [Google Scholar] [CrossRef]
- Ma, H.; Bao, Y.; Niu, S.; Wang, S.; Li, Y.; He, H.; Zhang, N.; Fang, W. Structure Optimization of 12β-O-γ-Glutamyl Oleanolic Acid Derivatives Resulting in Potent FXR Antagonist/Modulator for NASH Therapy. Pharmaceuticals (Basel) 2023, 16, 758. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.; He, A.; Wang, S.; Chen, Y.; Lu, J.; Lv, J.; Li, S.; Wang, J.; Qian, M.; Li, H.; Shen, X. Mebhydrolin Ameliorates Glucose Homeostasis in Type 2 Diabetic Mice by Functioning as a Selective FXR Antagonist. Metabolism 2021, 119, 154771. [Google Scholar] [CrossRef]
- Feng, H.; Hu, Y.; Zhou, S.; Lu, Y. Farnesoid X Receptor Contributes to Oleanolic Acid-Induced Cholestatic Liver Injury in Mice. J Appl Toxicol 2022, 42, 1323–1336. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Wan, X.-L.; Xu, Y.; Liu, J. Repeated Oral Administration of Oleanolic Acid Produces Cholestatic Liver Injury in Mice. Molecules 2013, 18, 3060–3071. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic Acid and Ursolic Acid: Research Perspectives. Journal of Ethnopharmacology 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D. E. The Mechanisms of Action of PPARs. Annu Rev Med 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Zhang, F.; Lavan, B. E.; Gregoire, F. M. Selective Modulators of PPAR-Gamma Activity: Molecular Aspects Related to Obesity and Side-Effects. PPAR Res 2007, 2007, 32696. [Google Scholar] [CrossRef] [PubMed]
- T, A.; F, Z.; Sa, M.; Le, C.; A, S.; F, G.; A, B.; Ge, M.; Ta, G. Halofenate Is a Selective Peroxisome Proliferator-Activated Receptor Gamma Modulator with Antidiabetic Activity. Diabetes 2006, 55. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, X.; Qiu, L.; Pan, Z.; Wang, R.; Lin, S.; Hou, D.; Jin, L.; Li, Y. Identification of the Antibiotic Ionomycin as an Unexpected Peroxisome Proliferator-Activated Receptor γ (PPARγ) Ligand with a Unique Binding Mode and Effective Glucose-Lowering Activity in a Mouse Model of Diabetes. Diabetologia 2013, 56, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, L. M.; Degenhardt, T.; Koppen, A.; Kalkhoven, E.; Desvergne, B.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor Beta/Delta (PPARbeta/Delta) but Not PPARalpha Serves as a Plasma Free Fatty Acid Sensor in Liver. Mol Cell Biol 2009, 29, 6257–6267. [Google Scholar] [CrossRef]
- Fajas, L.; Auboeuf, D.; Raspé, E.; Schoonjans, K.; Lefebvre, A.-M.; Saladin, R.; Najib, J.; Laville, M.; Fruchart, J.-C.; Deeb, S.; Vidal-Puig, A.; Flier, J.; Briggs, M. R.; Staels, B.; Vidal, H.; Auwerx, J. The Organization, Promoter Analysis, and Expression of the Human PPARγ Gene*. Journal of Biological Chemistry 1997, 272, 18779–18789. [Google Scholar] [CrossRef]
- Orasanu, G.; Ziouzenkova, O.; Devchand, P. R.; Nehra, V.; Hamdy, O.; Horton, E. S.; Plutzky, J. The PPARγ Agonist Pioglitazone Represses Inflammation In A PPARα-Dependent Manner In Vitro and In Vivo In Mice. J Am Coll Cardiol 2008, 52, 869–881. [Google Scholar] [CrossRef]
- Huang, T. H.-W.; Peng, G.; Kota, B. P.; Li, G. Q.; Yamahara, J.; Roufogalis, B. D.; Li, Y. Pomegranate Flower Improves Cardiac Lipid Metabolism in a Diabetic Rat Model: Role of Lowering Circulating Lipids. British Journal of Pharmacology 2005, 145, 767–774. [Google Scholar] [CrossRef]
- Christensen, K. B.; Jørgensen, M.; Kotowska, D.; Petersen, R. K.; Kristiansen, K.; Christensen, L. P. Activation of the Nuclear Receptor PPARγ by Metabolites Isolated from Sage (Salvia Officinalis L.). J Ethnopharmacol 2010, 132, 127–133. [Google Scholar] [CrossRef]
- Rau, O.; Wurglics, M.; Paulke, A.; Zitzkowski, J.; Meindl, N.; Bock, A.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Carnosic Acid and Carnosol, Phenolic Diterpene Compounds of the Labiate Herbs Rosemary and Sage, Are Activators of the Human Peroxisome Proliferator-Activated Receptor Gamma. Planta Med 2006, 72, 881–887. [Google Scholar] [CrossRef]
- Petersen, R. K.; Christensen, K. B.; Assimopoulou, A. N.; Fretté, X.; Papageorgiou, V. P.; Kristiansen, K.; Kouskoumvekaki, I. Pharmacophore-Driven Identification of PPARγ Agonists from Natural Sources. J Comput Aided Mol Des 2011, 25, 107–116. [Google Scholar] [CrossRef]
- Georgiadis, I.; Karatzas, T.; Korou, L.-M.; Katsilambros, N.; Perrea, D. Beneficial Health Effects of Chios Gum Mastic and Peroxisome Proliferator-Activated Receptors: Indications of Common Mechanisms. Journal of Medicinal Food 2015, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, T.; Smyrnioudis, I.; Konishi, M.; Takahashi, M.; Kim, H. K.; Nishimaki, M.; Xiang, M.; Sakamoto, S. Effects of Chios Mastic Gum and Exercise on Physical Characteristics, Blood Lipid Markers, Insulin Resistance, and Hepatic Function in Healthy Japanese Men. Food Sci Biotechnol 2018, 27, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Tzani, A.; Konstantopoulos, P.; Doulamis, I.; Liakea, A.; Minia, A.; Antoranz, A.; Korou, L.-M.; Kavantzas, N.; Alexopoulos, L.; Stamatelopoulos, K.; Iliopoulos, D.; Perrea, D. Chios Mastic Gum Inhibits Diet-Induced Non-Alcoholic Steatohepatitis in Mice via Activation of PPAR-α. Atherosclerosis 2020, 315, e47–e48. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; Lazaros, G. Overview of Chios Mastic Gum (Pistacia Lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Porter, W. W.; Suh, N.; Honda, T.; Gribble, G. W.; Leesnitzer, L. M.; Plunket, K. D.; Mangelsdorf, D. J.; Blanchard, S. G.; Willson, T. M.; Sporn, M. B. A Synthetic Triterpenoid, 2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic Acid (CDDO), Is a Ligand for the Peroxisome Proliferator-Activated Receptor Gamma. Mol Endocrinol 2000, 14, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P. A.; Kennedy, M. A.; Mak, P. A. LXRs; Oxysterol-Activated Nuclear Receptors That Regulate Genes Controlling Lipid Homeostasis. Vascul Pharmacol 2002, 38, 249–256. [Google Scholar] [CrossRef]
- Dixon, E. D.; Nardo, A. D.; Claudel, T.; Trauner, M. The Role of Lipid Sensing Nuclear Receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes 2021, 12, 645. [Google Scholar] [CrossRef]
- Higuchi, N.; Kato, M.; Shundo, Y.; Tajiri, H.; Tanaka, M.; Yamashita, N.; Kohjima, M.; Kotoh, K.; Nakamuta, M.; Takayanagi, R.; Enjoji, M. Liver X Receptor in Cooperation with SREBP-1c Is a Major Lipid Synthesis Regulator in Non-alcoholic Fatty Liver Disease. Hepatol Res 2008, 38, 1122–1129. [Google Scholar] [CrossRef]
- Griffett, K.; Burris, T. P. Development of LXR Inverse Agonists to Treat MAFLD, NASH, and Other Metabolic Diseases. Front Med (Lausanne) 2023, 10, 1102469. [Google Scholar] [CrossRef]
- Lin, Y.-N.; Chang, H.-Y.; Wang, C. C. N.; Chu, F.-Y.; Shen, H.-Y.; Chen, C.-J.; Lim, Y.-P. Oleanolic Acid Inhibits Liver X Receptor Alpha and Pregnane X Receptor to Attenuate Ligand-Induced Lipogenesis. J. Agric. Food Chem. 2018, 66, 10964–10976. [Google Scholar] [CrossRef]
- Leal, A. S.; Reich, L. A.; Moerland, J. A.; Zhang, D.; Liby, K. T. Chapter Four - Potential Therapeutic Uses of Rexinoids. In Advances in Pharmacology; Copple, B. L., Rockwell, C. E., Eds.; Advances in Immunopharmacology; Academic Press, 2021; Vol. 91, pp 141–183. [CrossRef]
- Liby, K. T.; Yore, M. M.; Sporn, M. B. Triterpenoids and Rexinoids as Multifunctional Agents for the Prevention and Treatment of Cancer. Nat Rev Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Stöckli, J.; Fazakerley, D. J.; James, D. E. GLUT4 Exocytosis. J Cell Sci 2011, 124, 4147–4159. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S. A.; Goodwin, B.; Willson, T. M. The Nuclear Pregnane X Receptor: A Key Regulator of Xenobiotic Metabolism. Endocr Rev 2002, 23, 687–702. [Google Scholar] [CrossRef]
- Moreau, A.; Vilarem, M. J.; Maurel, P.; Pascussi, J. M. Xenoreceptors CAR and PXR Activation and Consequences on Lipid Metabolism, Glucose Homeostasis, and Inflammatory Response. Mol Pharm 2008, 5, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, S.; Yamazaki, Y.; Takizawa, D.; Negishi, M. New Insights on the Xenobiotic-Sensing Nuclear Receptors in Liver Diseases--CAR and PXR--. Curr Drug Metab 2008, 9, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Chang, C.; Mani, S.; Krasowski, M. D.; Reschly, E. J.; Iyer, M.; Kholodovych, V.; Ai, N.; Welsh, W. J.; Sinz, M.; Swaan, P. W.; Patel, R.; Bachmann, K. Human Pregnane X Receptor Antagonists and Agonists Define Molecular Requirements for Different Binding Sites. Mol Pharmacol 2007, 72, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-N.; Chen, C.-J.; Chang, H.-Y.; Cheng, W.-K.; Lee, Y.-R.; Chen, J.-J.; Lim, Y.-P. Oleanolic Acid-Mediated Inhibition of Pregnane X Receptor and Constitutive Androstane Receptor Attenuates Rifampin-Isoniazid Cytotoxicity. J Agric Food Chem 2017, 65, 8606–8616. [Google Scholar] [CrossRef]
- Pastwińska, J.; Karaś, K.; Sałkowska, A.; Karwaciak, I.; Chałaśkiewicz, K.; Wojtczak, B. A.; Bachorz, R. A.; Ratajewski, M. Identification of Corosolic and Oleanolic Acids as Molecules Antagonizing the Human RORγT Nuclear Receptor Using the Calculated Fingerprints of the Molecular Similarity. Int J Mol Sci 2022, 23, 1906. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).