Submitted:
19 September 2023
Posted:
21 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell preparation
2.3. Mouse drug treatment
2.4. Flow cytometry
2.5. Hematoxylin and eosin staining
2.5. Statistical analysis
3. Results
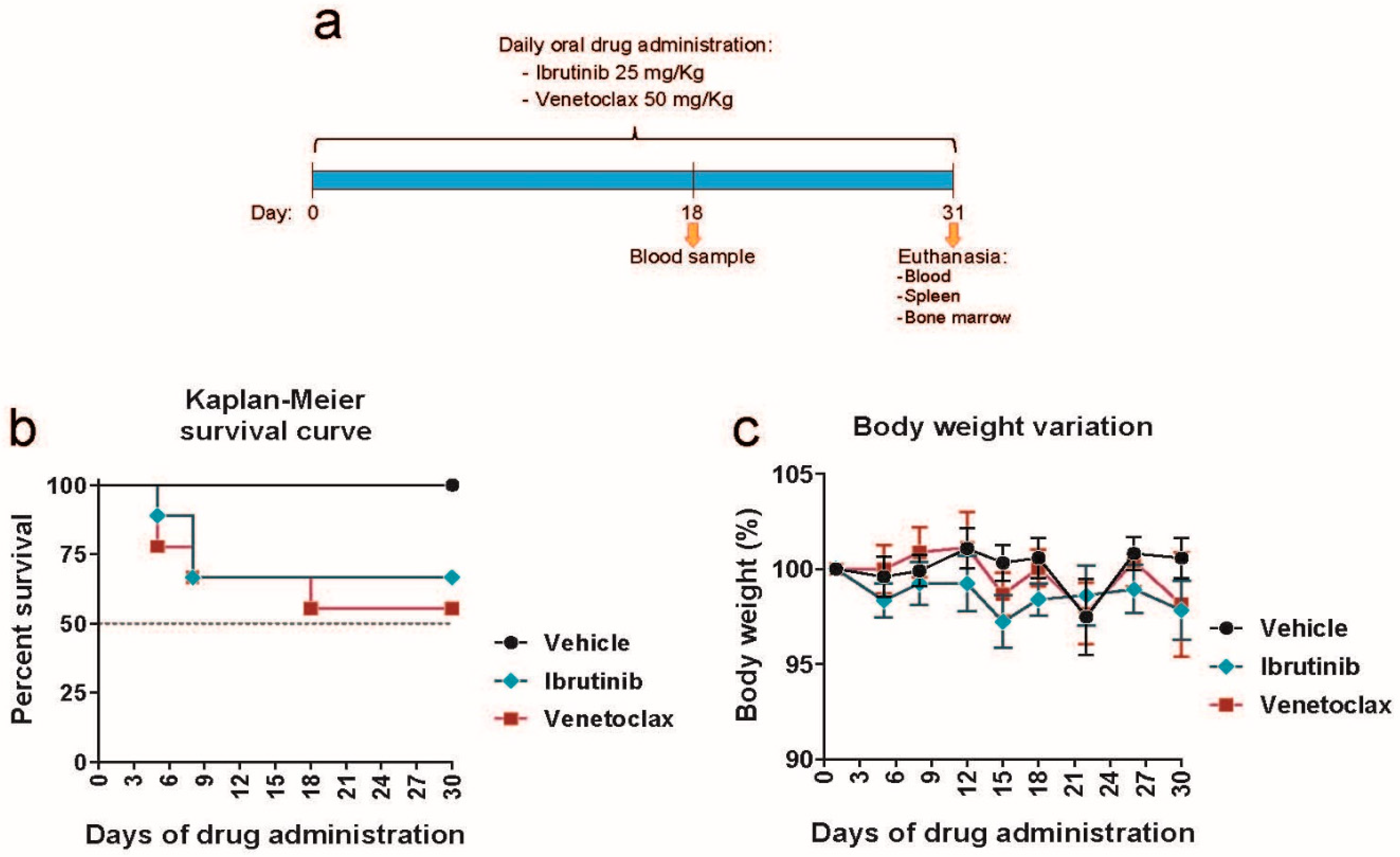
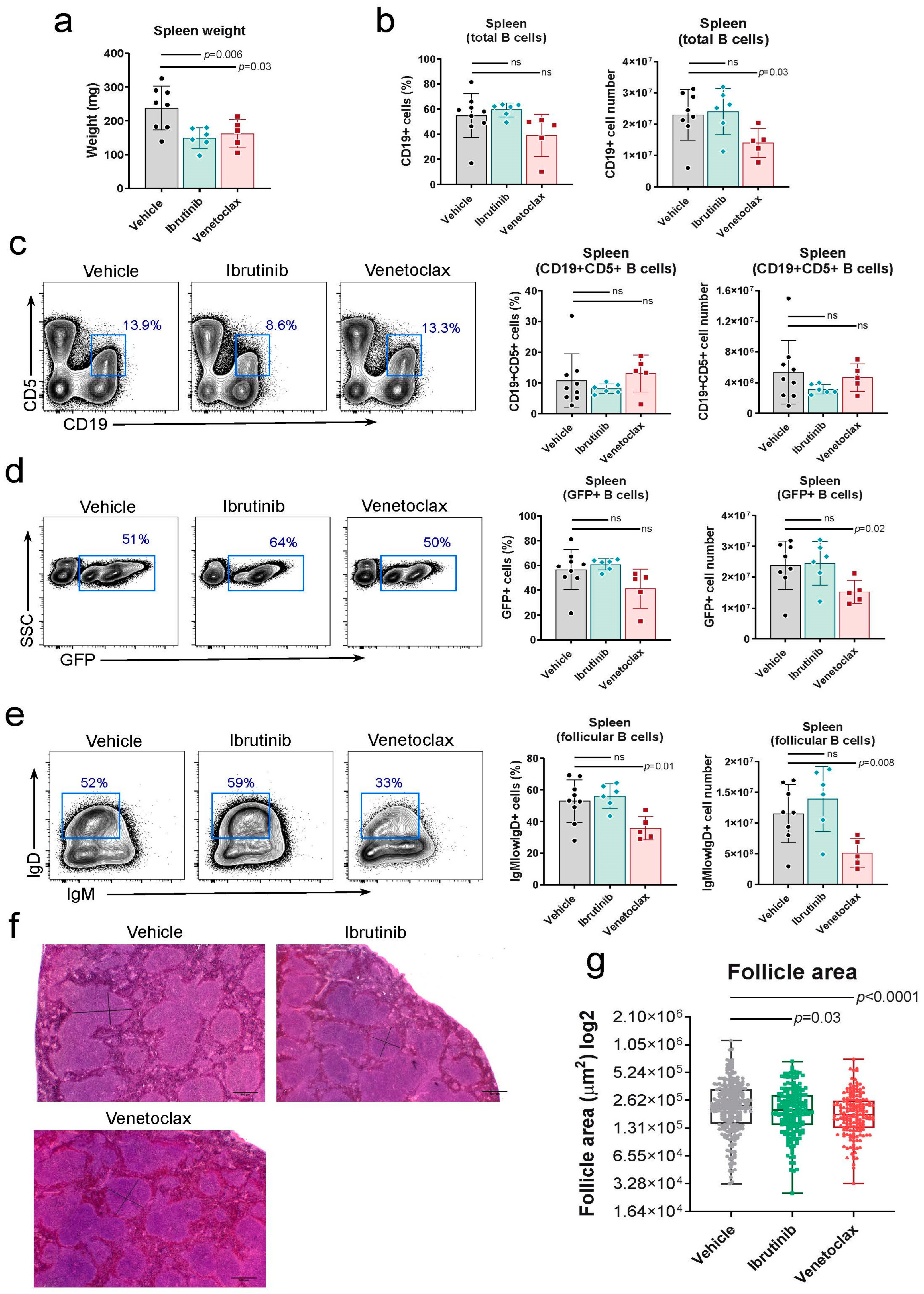
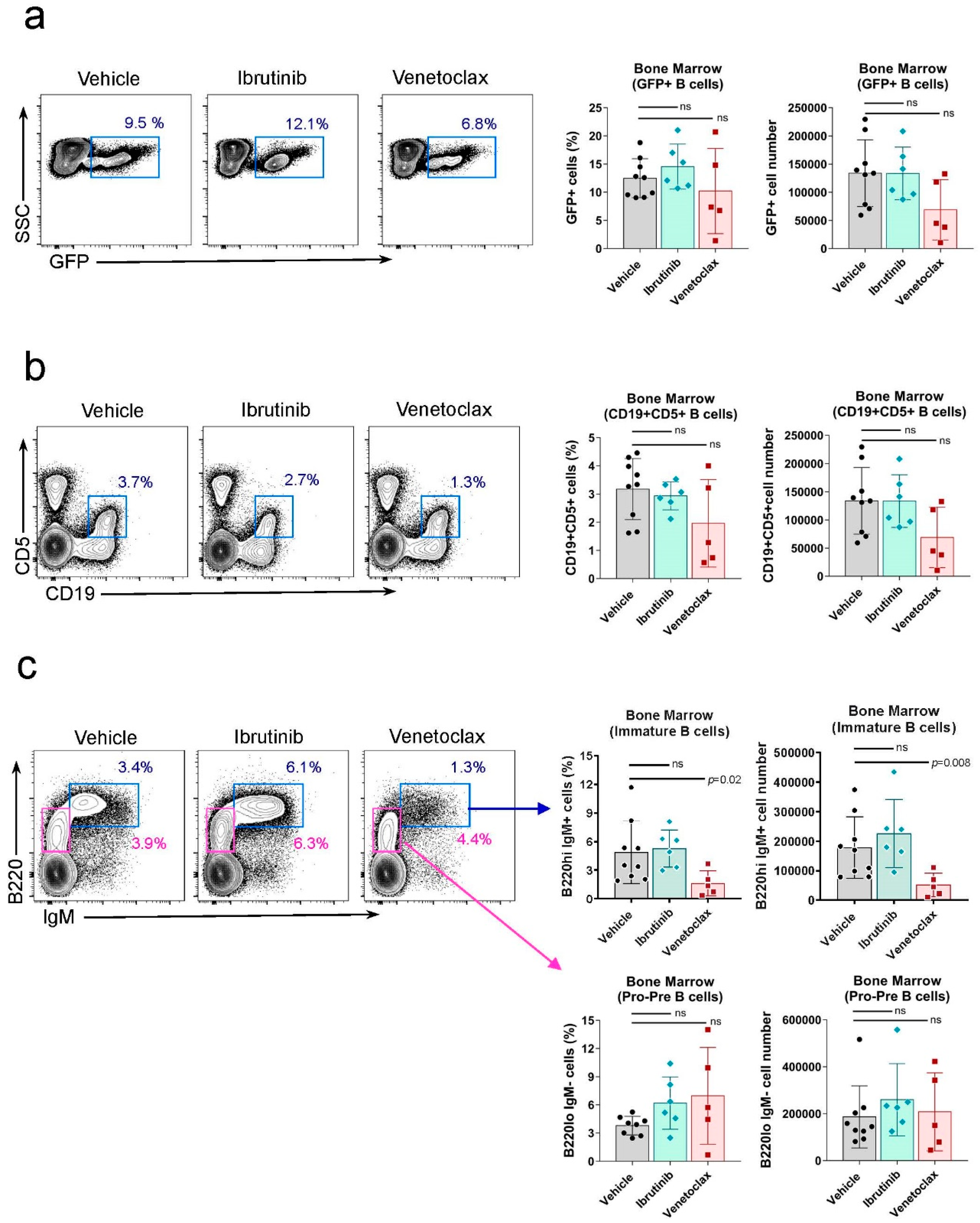
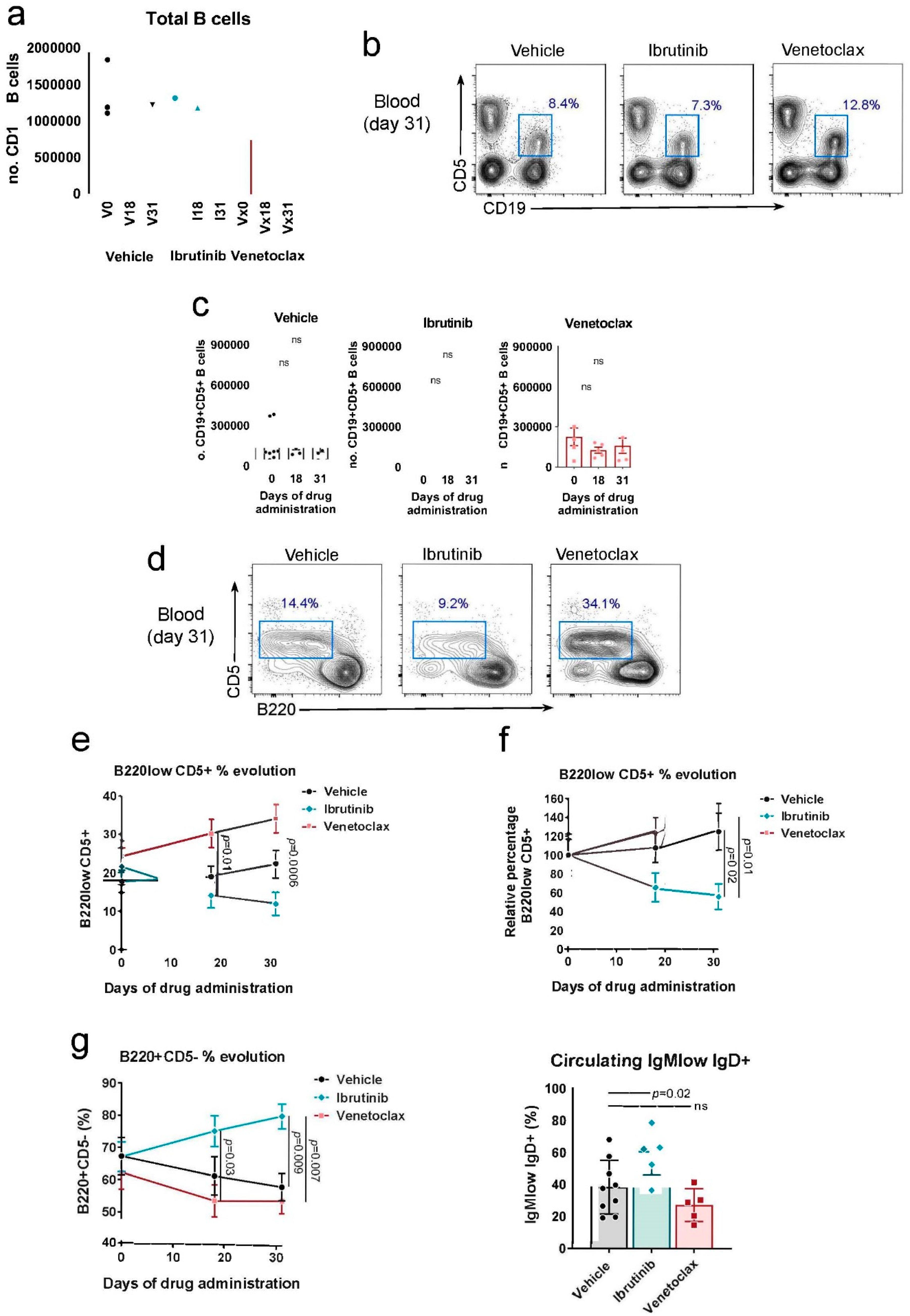
3.1. Testing the Rosa26-RRAS2fl/flxmb1-Cre Mouse Model of CLL in Response to Ibrutinib and Venetoclax
4. Discussion
5. Conclusions
References
- Bosch, F.; Dalla-Favera, R. Chronic Lymphocytic Leukaemia: From Genetics to Treatment. Nat. Rev. Clin. Oncol. 2019, 16, 684–701. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA. Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.; Richardson, L.C.; Allemani, C.; Bonaventure, A.; Harewood, R.; Moore, A.R.; Stewart, S.L.; Weir, H.K.; Coleman, M.P.; Members), C.W.G. (US Adult Leukemia Survival Trends in the United States by Subtype: A Population-Based Registry Study of 370,994 Patients Diagnosed during 1995-2009. Cancer 2018, 124, 3856–3867. [Google Scholar] [CrossRef] [PubMed]
- Chronic Lymphocytic Leukemia - Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/clyl.html (accessed on 16 September 2022).
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.U.; Kater, A.P.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic Lymphocytic Leukaemia: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic Lymphocytic Leukaemia. The Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Ferreri, A.M.; Caligaris-Cappio, F. Chronic Lymphocytic Leukemia. Crit. Rev. Oncol. Hematol. 2007, 64, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Gary-Gouy, H.; Sainz-Perez, A.; Marteau, J.-B.; Marfaing-Koka, A.; Delic, J.; Merle-Beral, H.; Galanaud, P.; Dalloul, A. Natural Phosphorylation of CD5 in Chronic Lymphocytic Leukemia B Cells and Analysis of CD5-Regulated Genes in a B Cell Line Suggest a Role for CD5 in Malignant Phenotype1. J. Immunol. 2007, 179, 4335–4344. [Google Scholar] [CrossRef]
- Landau, D.A.; Carter, S.L.; Stojanov, P.; McKenna, A.; Stevenson, K.; Lawrence, M.S.; Sougnez, C.; Stewart, C.; Sivachenko, A.; Wang, L.; et al. Evolution and Impact of Subclonal Mutations in Chronic Lymphocytic Leukemia. Cell 2013, 152, 714–726. [Google Scholar] [CrossRef]
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations Driving CLL and Their Evolution in Progression and Relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef]
- Knisbacher, B.A.; Lin, Z.; Hahn, C.K.; Nadeu, F.; Duran-Ferrer, M.; Stevenson, K.E.; Tausch, E.; Delgado, J.; Barbera-Mourelle, A.; Taylor-Weiner, A.; et al. Molecular Map of Chronic Lymphocytic Leukemia and Its Impact on Outcome. Nat. Genet. 2022, 1–11. [Google Scholar] [CrossRef]
- Hallek, M.; Wanders, L.; Ostwald, M.; Busch, R.; Senekowitsch, R.; Stern, S.; Schick, H.-D.; Kuhn-Hallek, I.; Emmerich, B. Serum Β2-Microglobulin and Serum Thymidine Kinase Are Independent Predictors of Progression-Free Survival in Chronic Lymphocytic Leukemia and Immunocytoma. Leuk. Lymphoma 1996, 22, 439–447. [Google Scholar] [CrossRef]
- Cohen, J.A.; Bomben, R.; Pozzo, F.; Tissino, E.; Härzschel, A.; Hartmann, T.N.; Zucchetto, A.; Gattei, V. An Updated Perspective on Current Prognostic and Predictive Biomarkers in Chronic Lymphocytic Leukemia in the Context of Chemoimmunotherapy and Novel Targeted Therapy. Cancers 2020, 12, 894. [Google Scholar] [CrossRef]
- Rassenti, L.Z.; Jain, S.; Keating, M.J.; Wierda, W.G.; Grever, M.R.; Byrd, J.C.; Kay, N.E.; Brown, J.R.; Gribben, J.G.; Neuberg, D.S.; et al. Relative Value of ZAP-70, CD38, and Immunoglobulin Mutation Status in Predicting Aggressive Disease in Chronic Lymphocytic Leukemia. Blood 2008, 112, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Chiorazzi, N. B Cell Receptor Signaling in Chronic Lymphocytic Leukemia. Trends Immunol. 2013, 34, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.T.; Al-Sawaf, O.; Fischer, K.; Woyach, J.A. Current Perspectives on Therapy for Chronic Lymphocytic Leukemia. Am. Soc. Clin. Oncol. Educ. Book 2020, 320–329. [Google Scholar] [CrossRef]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 Selective Inhibitor Venetoclax Induces Rapid Onset Apoptosis of CLL Cells in Patients via a TP53-Independent Mechanism. Blood 2016, 127, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.; Woyach, J.A. Inhibiting Bruton’s Tyrosine Kinase in CLL and Other B-Cell Malignancies. Target. Oncol. 2019, 14, 125–138. [Google Scholar] [CrossRef]
- Bichi, R.; Shinton, S.A.; Martin, E.S.; Koval, A.; Calin, G.A.; Cesari, R.; Russo, G.; Hardy, R.R.; Croce, C.M. Human Chronic Lymphocytic Leukemia Modeled in Mouse by Targeted TCL1 Expression. Proc. Natl. Acad. Sci. 2002, 99, 6955–6960. [Google Scholar] [CrossRef] [PubMed]
- Bertilaccio, M.T.S.; Scielzo, C.; Simonetti, G.; Ponzoni, M.; Apollonio, B.; Fazi, C.; Scarfò, L.; Rocchi, M.; Muzio, M.; Caligaris-Cappio, F.; et al. A Novel Rag2−/−γc−/−-Xenograft Model of Human CLL. Blood 2010, 115, 1605–1609. [Google Scholar] [CrossRef]
- Patten, P.E.M.; Ferrer, G.; Chen, S.-S.; Kolitz, J.E.; Rai, K.R.; Allen, S.L.; Barrientos, J.C.; Ioannou, N.; Ramsay, A.G.; Chiorazzi, N. A Detailed Analysis of Parameters Supporting the Engraftment and Growth of Chronic Lymphocytic Leukemia Cells in Immune-Deficient Mice. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Klein, U.; Lia, M.; Crespo, M.; Siegel, R.; Shen, Q.; Mo, T.; Ambesi-Impiombato, A.; Califano, A.; Migliazza, A.; Bhagat, G.; et al. The DLEU2/MiR-15a/16-1 Cluster Controls B Cell Proliferation and Its Deletion Leads to Chronic Lymphocytic Leukemia. Cancer Cell 2010, 17, 28–40. [Google Scholar] [CrossRef]
- ten Hacken, E.; Wu, C.J. Understanding CLL Biology through Mouse Models of Human Genetics. Blood 2021, 138, 2621–2631. [Google Scholar] [CrossRef]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. RAS Isoforms and Mutations in Cancer at a Glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. RAS Oncogenes: The First 30 Years. Nat. Rev. Cancer 2003, 3, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.N.; Yan, M.; Chan, A.M. A Thirty-Year Quest for a Role of R-Ras in Cancer: From an Oncogene to a Multitasking GTPase. Cancer Lett. 2017, 403, 59–65. [Google Scholar] [CrossRef]
- Graham, S.M.; Oldham, S.M.; Martin, C.B.; Drugan, J.K.; Zohn, I.E.; Campbell, S.; Der, C.J. TC21 and Ras Share Indistinguishable Transforming and Differentiating Activities. Oncogene 1999, 18, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Cox, A.D.; Drivas, G.; Rush, M.G.; D’Eustachio, P.; Der, C.J. Aberrant Function of the Ras-Related Protein TC21/R-Ras2 Triggers Malignant Transformation. Mol. Cell. Biol. 1994, 14, 4108–4115. [Google Scholar] [CrossRef]
- Movilla, N.; Crespo, P.; Bustelo, X.R. Signal Transduction Elements of TC21, an Oncogenic Member of the R-Ras Subfamily of GTP-Binding Proteins. Oncogene 1999, 18, 5860–5869. [Google Scholar] [CrossRef] [PubMed]
- Janapati, S.; Wurtzel, J.; Dangelmaier, C.; Manne, B.K.; Bhavanasi, D.; Kostyak, J.C.; Kim, S.; Holinstat, M.; Kunapuli, S.P.; Goldfinger, L.E. TC21/RRas2 Regulates Glycoprotein VI–FcRγ-Mediated Platelet Activation and Thrombus Stability. J. Thromb. Haemost. 2018, 16, 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rangwala, F.; Fulkerson, P.C.; Ling, B.; Reed, E.; Cox, A.D.; Kamholz, J.; Ratner, N. Role of TC21/R-Ras2 in Enhanced Migration of Neurofibromin-Deficient Schwann Cells. Oncogene 2004, 23, 368–378. [Google Scholar] [CrossRef]
- Larive, R.M.; Abad, A.; Cardaba, C.M.; Hernández, T.; Cañamero, M.; de Álava, E.; Santos, E.; Alarcón, B.; Bustelo, X.R. The Ras-like Protein R-Ras2/TC21 Is Important for Proper Mammary Gland Development. Mol. Biol. Cell 2012, 23, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Macha, M.A.; Matta, A.; Sriram, U.; Thakkar, A.; Shukla, N.K.; Datta Gupta, S.; Ralhan, R. Clinical Significance of TC21 Overexpression in Oral Cancer. J. Oral Pathol. Med. 2010, 39, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sud, N.; Chattopadhyay, T.K.; Ralhan, R. TC21/R-Ras2 Upregulation in Esophageal Tumorigenesis: Potential Diagnostic Implications. Oncology 2005, 69, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Hao, X.; Ge, C.; Zhao, F.; Zhu, M.; Chen, T.; Yao, M.; He, X.; Li, J. TC21 Promotes Cell Motility and Metastasis by Regulating the Expression of E-Cadherin and N-Cadherin in Hepatocellular Carcinoma. Int. J. Oncol. 2010, 37, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Pyon, J.-K.; Lee, S.H.; Lee, Y.J.; Kang, S.G.; Kim, C.H.; Kim, D.W.; Nam, H.S.; Park, Y.H.; Jeong, D.J.; et al. Greater Expression of TC21/R-Ras2 in Highly Aggressive Malignant Skin Cancer. Int. J. Dermatol. 2011, 50, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Larive, R.M.; Moriggi, G.; Menacho-Márquez, M.; Cañamero, M.; Álava, E. de; Alarcón, B.; Dosil, M.; Bustelo, X.R. Contribution of the R-Ras2 GTP-Binding Protein to Primary Breast Tumorigenesis and Late-Stage Metastatic Disease. Nat. Commun. 2014, 5, 3881. [Google Scholar] [CrossRef]
- Clark, G.J.; Kinch, M.S.; Gilmer, T.M.; Burridge, K.; Der, C.J. Overexpression of the Ras-Related TC21/R-Ras2 Protein May Contribute to the Development of Human Breast Cancers. Oncogene 1996, 12, 169–176. [Google Scholar]
- Capri, Y.; Flex, E.; Krumbach, O.H.F.; Carpentieri, G.; Cecchetti, S.; Lißewski, C.; Rezaei Adariani, S.; Schanze, D.; Brinkmann, J.; Piard, J.; et al. Activating Mutations of RRAS2 Are a Rare Cause of Noonan Syndrome. Am. J. Hum. Genet. 2019, 104, 1223–1232. [Google Scholar] [CrossRef]
- Niihori, T.; Nagai, K.; Fujita, A.; Ohashi, H.; Okamoto, N.; Okada, S.; Harada, A.; Kihara, H.; Arbogast, T.; Funayama, R.; et al. Germline-Activating RRAS2 Mutations Cause Noonan Syndrome. Am. J. Hum. Genet. 2019, 104, 1233–1240. [Google Scholar] [CrossRef]
- Fernández-Pisonero, I.; Clavaín, L.; Robles-Valero, J.; Lorenzo-Martín, L.F.; Caloto, R.; Nieto, B.; García-Macías, C.; Oeste, C.L.; Sánchez-Martín, M.; Abad, A.; et al. A Hotspot Mutation Targeting the R-RAS2 GTPase Acts as a Potent Oncogenic Driver in a Wide Spectrum of Tumors. Cell Rep. 2022, 38, 110522. [Google Scholar] [CrossRef]
- Clavaín, L.; Fernández-Pisonero, I.; Movilla, N.; Lorenzo-Martín, L.F.; Nieto, B.; Abad, A.; García-Navas, R.; Llorente-González, C.; Sánchez-Martín, M.; Vicente-Manzanares, M.; et al. Characterization of Mutant Versions of the R-RAS2/TC21 GTPase Found in Tumors. Oncogene 2022, 1–17. [Google Scholar] [CrossRef]
- Delgado, P.; Cubelos, B.; Calleja, E.; Martínez-Martín, N.; Ciprés, A.; Mérida, I.; Bellas, C.; Bustelo, X.R.; Alarcón, B. Essential Function for the GTPase TC21 in Homeostatic Antigen Receptor Signaling. Nat. Immunol. 2009, 10, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martín, N.; Fernández-Arenas, E.; Cemerski, S.; Delgado, P.; Turner, M.; Heuser, J.; Irvine, D.J.; Huang, B.; Bustelo, X.R.; Shaw, A.; et al. T Cell Receptor Internalization from the Immunological Synapse Is Mediated by TC21 and RhoG GTPase-Dependent Phagocytosis. Immunity 2011, 35, 208–222. [Google Scholar] [CrossRef]
- Mendoza, P.; Martínez-Martín, N.; Bovolenta, E.R.; Reyes-Garau, D.; Hernansanz-Agustín, P.; Delgado, P.; Diaz-Muñoz, M.D.; Oeste, C.L.; Fernández-Pisonero, I.; Castellano, E.; et al. R-Ras2 Is Required for Germinal Center Formation to Aid B Cells during Energetically Demanding Processes. Sci. Signal. 2018, 11, eaal1506. [Google Scholar] [CrossRef] [PubMed]
- Hortal, A.M.; Oeste, C.L.; Cifuentes, C.; Alcoceba, M.; Fernández-Pisonero, I.; Clavaín, L.; Tercero, R.; Mendoza, P.; Domínguez, V.; García-Flores, M.; et al. Overexpression of Wild Type RRAS2, without Oncogenic Mutations, Drives Chronic Lymphocytic Leukemia. Mol. Cancer 2022, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.-H.; Calado, D.P.; Casola, S.; Ansel, K.M.; Xiao, C.; Xue, Y.; Murphy, A.; Frendewey, D.; Valenzuela, D.; Kutok, J.L.; et al. Regulation of the Germinal Center Response by MicroRNA-155. Science 2007, 316, 604–608. [Google Scholar] [CrossRef]
- Hobeika, E.; Thiemann, S.; Storch, B.; Jumaa, H.; Nielsen, P.J.; Pelanda, R.; Reth, M. Testing Gene Function Early in the B Cell Lineage in Mb1-Cre Mice. Proc. Natl. Acad. Sci. 2006, 103, 13789–13794. [Google Scholar] [CrossRef]
- Hacken, E. ten; Sivina, M.; Kim, E.; O’Brien, S.; Wierda, W.G.; Ferrajoli, A.; Estrov, Z.; Keating, M.J.; Oellerich, T.; Scielzo, C.; et al. Functional Differences Between IgM and IgD Signaling in Chronic Lymphocytic Leukemia. J. Immunol. Baltim. Md 1950 2016, 197, 2522–2531. [Google Scholar] [CrossRef]
- Damle, R.N.; Ghiotto, F.; Valetto, A.; Albesiano, E.; Fais, F.; Yan, X.-J.; Sison, C.P.; Allen, S.L.; Kolitz, J.; Schulman, P.; et al. B-Cell Chronic Lymphocytic Leukemia Cells Express a Surface Membrane Phenotype of Activated, Antigen-Experienced B Lymphocytes: Presented in Part at the 42nd Annual Meeting of the American Society of Hematology, December 1-5, 2000, San Francisco, CA. Blood 2002, 99, 4087–4093. [Google Scholar] [CrossRef]
- Kutsch, N.; Fink, A.M.; Fischer, K. Management of Front Line Chronic Lymphocytic Leukemia. Am. J. Hematol. 2022, 97, S3–S10. [Google Scholar] [CrossRef]
- Hallek, M.; Al-Sawaf, O. Chronic Lymphocytic Leukemia: 2022 Update on Diagnostic and Therapeutic Procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef]
- Barr, P.M.; Robak, T.; Owen, C.; Tedeschi, A.; Bairey, O.; Bartlett, N.L.; Burger, J.A.; Hillmen, P.; Coutre, S.; Devereux, S.; et al. Sustained Efficacy and Detailed Clinical Follow-up of First-Line Ibrutinib Treatment in Older Patients with Chronic Lymphocytic Leukemia: Extended Phase 3 Results from RESONATE-2. Haematologica 2018, 103, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Haug, A.; Jäger, U.; Pichler, V.; Pfaff, S.; Wester, H.-J.; Hacker, M.; Kazianka, L.; Staber, P.B. In Human Visualization of Ibrutinib-Induced CLL Compartment Shift. Cancer Immunol. Res. 2020, 8, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Coutre, S.; Choi, M.; Furman, R.R.; Eradat, H.; Heffner, L.; Jones, J.A.; Chyla, B.; Zhou, L.; Agarwal, S.; Waskiewicz, T.; et al. Venetoclax for Patients with Chronic Lymphocytic Leukemia Who Progressed during or after Idelalisib Therapy. Blood 2018, 131, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.P.; Slinger, E.; Cretenet, G.; Martens, A.W.; Balasubramanian, S.; Leverson, J.D.; Eldering, E. Combined Ibrutinib and Venetoclax Treatment vs Single Agents in the TCL1 Mouse Model of Chronic Lymphocytic Leukemia. Blood Adv. 2021, 5, 5410–5414. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Landau, D.A.; Taylor-Weiner, A.; Bozic, I.; Zhang, H.; Sarosiek, K.; Wang, L.; Stewart, C.; Fan, J.; Hoellenriegel, J.; et al. Clonal Evolution in Patients with Chronic Lymphocytic Leukaemia Developing Resistance to BTK Inhibition. Nat. Commun. 2016, 7, 11589. [Google Scholar] [CrossRef]
- Woyach, J.A.; Furman, R.R.; Liu, T.-M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.-H.; Steggerda, S.M.; Versele, M.; et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




