1. Introduction
Kaolinite and halloysite are clay minerals that are frequently used to formulate a large variety of materials ranging from low-added to high-added values products. Raw clays containing kaolinite and halloysite are used for applications ranging from domestic uses through the biomedical and energy fields. The advantage of these clay minerals is their availability and environmentally friendly impact. Besides, some drawbacks are related to the occurrence of secondary phases in their main deposits, which lead to the requirement of different physical and chemical treatments in order to control the purity of their respective raw materials. Both kaolinite and halloysite are TO dioctahedric phyllosilicate, but kaolinite is characterized by platelet-like particles while halloysite exhibits tubular and /or cylindrical shape [
1]. Halloysite has the same general chemical composition as kaolinite with additional water content. Two types of halloysite are usually considered, accordingly to the general formula Al
2Si
2O
5(OH)
4.nH
2O, where the (001) d-spacing is equal to 7 Å or 10 Å when n = 0 and 2 respectively [
2,
3]. Consequently, the two types of halloysite are labelled “halloysite 7 Å” and “halloysite 10 Å”. These differences in shape and water content may influence the microstructure and thermal transformations behavior of materials containing these halloysite and/or kaolinite. In the case of ceramics, the shaping process can enhance the initial texture, and during the sintering, the clay minerals are significantly modified [
4,
5,
6,
7,
8], thus leading to the evolution of final structure and properties. For kaolinite and halloysite, the literature indicate that upon heating, these clay minerals will undergo dehydration, dehydroxylation and recrystallization transformations. It has also been shown that these transformations are influenced by the initial crystallinity degree, the particle size and the amount of local defects of the kaolinite and halloysite raw materials. Following former studies that were performed regarding the correlation between the formulation and sintering behavior of some ceramics [
8,
9,
10,
11,
12,
13], the present study aims at characterizing the texture evolutions of kaolinite and halloysite based-samples from room temperature until the complete dehydroxylation. The
in situ pole figures of the appropriate (
hkl) reflections were recorded from 25 up to 1000°C on the DiffAbs beamline at SOLEIL synchrotron.
2. Materials and methods
2.1. Clay materials and sample preparation
The raw materials used in this study were two kaolins and a halloysite (NZCC halloysite), labeled KRG, KCS and H respectively. All these raw materials were supplied by Imerys Limoges, France. Their main characteristics were already presented in a former paper [
14] and are listed in
Table 1,
Table 2 and
Table 3. Kaolins KRG and KCS contained kaolinite clay mineral (99%), while the halloysite (noted H) mainly included halloysite clay mineral and a few amount of secondary minerals (quartz and cristobalite).
Shaping of samples was performed using the tape casting method in order to promote the preferential alignment of clay particles along the casting support. Optimized amounts of dolaflusB11® (CERADEL), Polyvinyl alcohol 22000® (VMR) and Polyethylene glycol 300® (ALDRICH) were derived from preliminary studies [
8,
14]. They were used as dispersant, binder and plasticizer respectively in the kaolinite and halloysite-based slurries. The slurry preparation started with the mixture of kaolinite and or halloysite–rich clays (52.5 wt% of sold content) with deionized water containing 0.2g of dolaflux B11. After the homogenization of the previous mixture overnight onto roll-mixer, a first grinding step was conducted in a planetary mill (Fritsch) for 6 hours at 180 rpm. Then the optimized binder and plasticizer content was added (binder to plasticizer ratio = 1) prior to the second milling/mixing step for 16 hours at 100 rpm in the planetary miller. Finally, the as-obtained slurry was set on the roll-mixer in order to remove the entrapped bubbles and sieved at 125 µm before the tape casting operation to eliminate the non-solubilized binder and plasticizer. Five slurries were formulated using each clay material and then a mixture of equivalent mass ratio of kaolin and halloysite. The related samples were named: KRG100, KCS100, H100, KRG50H50 and KCS50H50 accordingly to the relative mass content of the clay material.
All slurries were cast with a non-continuous equipment, using a casting rate of 0.42 mm/min and the casting gap that defined the thickness of the green tapes (ranging between 200 µm to 300 µm) could be precisely monitored. The green tapes were air-dried at room temperature for 24h before collecting disk-like (10 and 30 mm in diameter) and square-like samples for the in situ experiments.
2.2. Methods
The evolution as a function of the temperature of the texture within samples containing different amounts of halloysite and kaolinite and shaped by tape casting was studied by X-ray diffraction at the DiffAbs beamline located in SOLEIL Synchrotron. In addition, the typical dehydroxylation onset and offset temperatures were determined as well as the earlier structural reorganization. Pole figures were derived upon
in situ measurements performed up to 800°C. Furthermore, the furnace allowed heating samples up to 1100°C and using a peculiar configuration to extract pair distribution functions.). We used the DHS 1100 furnace supplied by Anton Paar Company. The confinement of the atmosphere around the sample is realized using a hemispherical PEEK (PolyEther Ether Ketone) dome. The measurements were done using the Kappa diffractometer available on the beamline and an XPAD S140 hybrid pixel detector [
15] located on the 2 arm. All the pole figures were extracted according to the methodology described in [
16]
X-ray diffraction (XRD) patterns were recorded on dried samples shaped by tape casting. The samples were previously cut into small squares of 5 mm in edge, and then fixed onto silicon wafers using metallic grafting. According to the energy of the X-ray beam that was fixed at 17.9 keV, and the incident angle was fixed at 5.54° in order to match the reflection conditions of the (002) planes of kaolinite and halloysite. The heating rate was fixed at 20 °C/min with an acquisition every 25°C. These conditions were defined during previous measurements at the ESRF D2AM beamline [
14]. The recording duration was a couple of min for each poles figure.
3. Results and discussion
3.1. Texture characterization using (002) and (111) reflections poles figures
The materials texture plays a significant role on their final properties such as mechanical resistance, thermal conductivity, and electrical resistance, etc. Since in the case of phyllosilicate-based ceramics, the layered structure of clay minerals may induce preferential orientation, it is of great interest to understand how the texture can be influenced by such particles alignment. Moreover, the structural changes like dehydroxylation could be highlighted in relation with the starting phyllosilicate(s). In the present study, tape casting, which is a liquid route shaping process, could favor the texture within samples containing layered silicates. SEM images of the starting sample are presented on
Figure 1
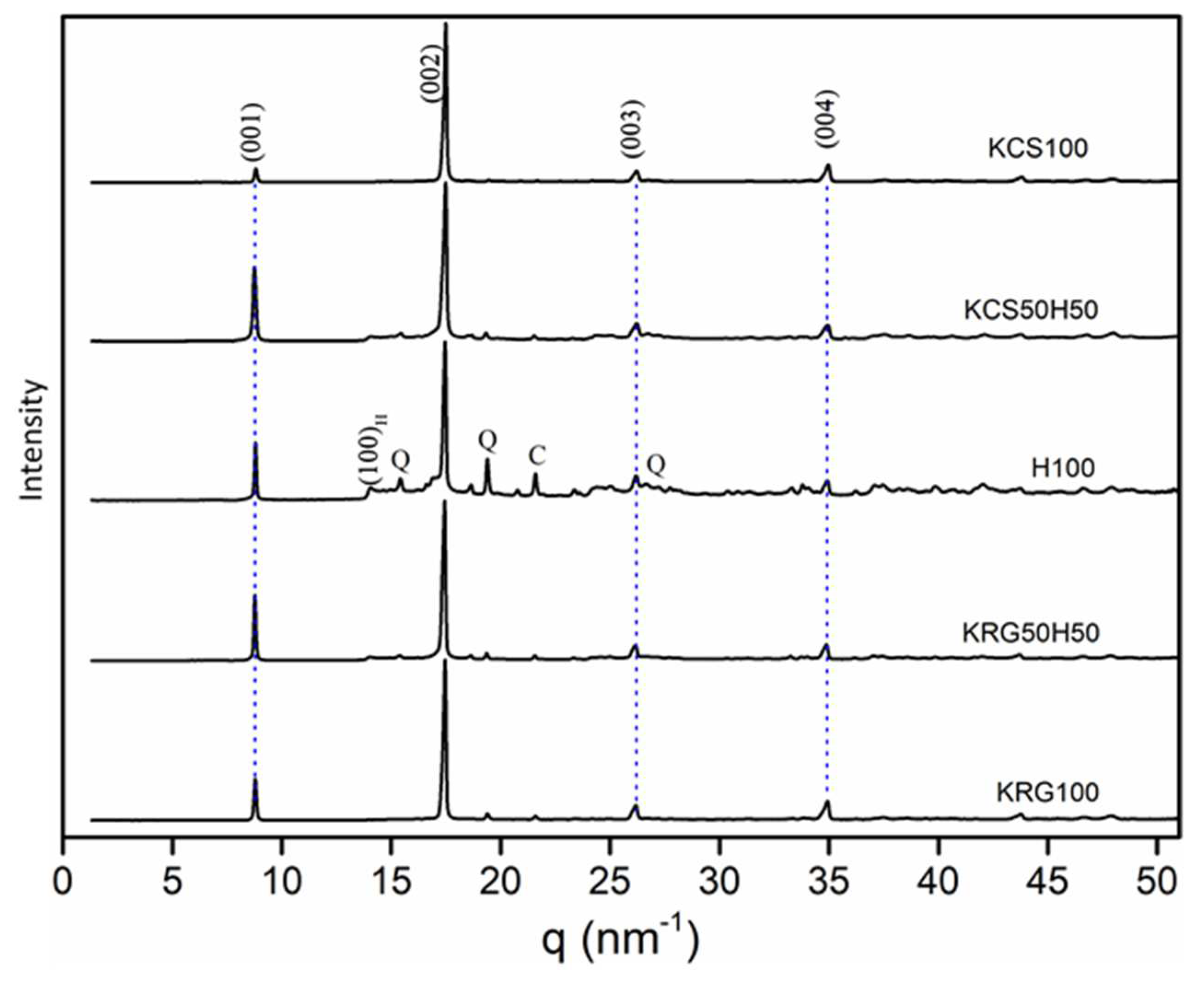
The (002) and (111) crystallographic planes were considered for kaolinite and halloysite in order pointed out the initial texture and the differences occurring due to the phyllosilicates characteristics and the heat treatment in the range 300 to 800°C. In a first set of experiments, samples constituted with only one type of raw clay were analyzed: H100, KCS100 and KRG100. Thereafter, mixtures having 50% halloysite and 50% kaolin were analyzed, namely: KCS50H50 and KRG50H50. The XRD patterns of the studied samples are provided on
Figure 2. It is obvious that for most samples, the shaping through tape casting enhanced the intensity of the (00ℓ) reflections due to in-plane alignment of platelets.
3.1.1. Texture characterization of the kaolin and halloysite samples
The influence of the temperature on the crystal preferential orientation was investigated through
in situ high temperature measurements on samples KRG100, KCS100 and H100 until the end of the dehydroxylation of kaolinite and halloysite (R.1).
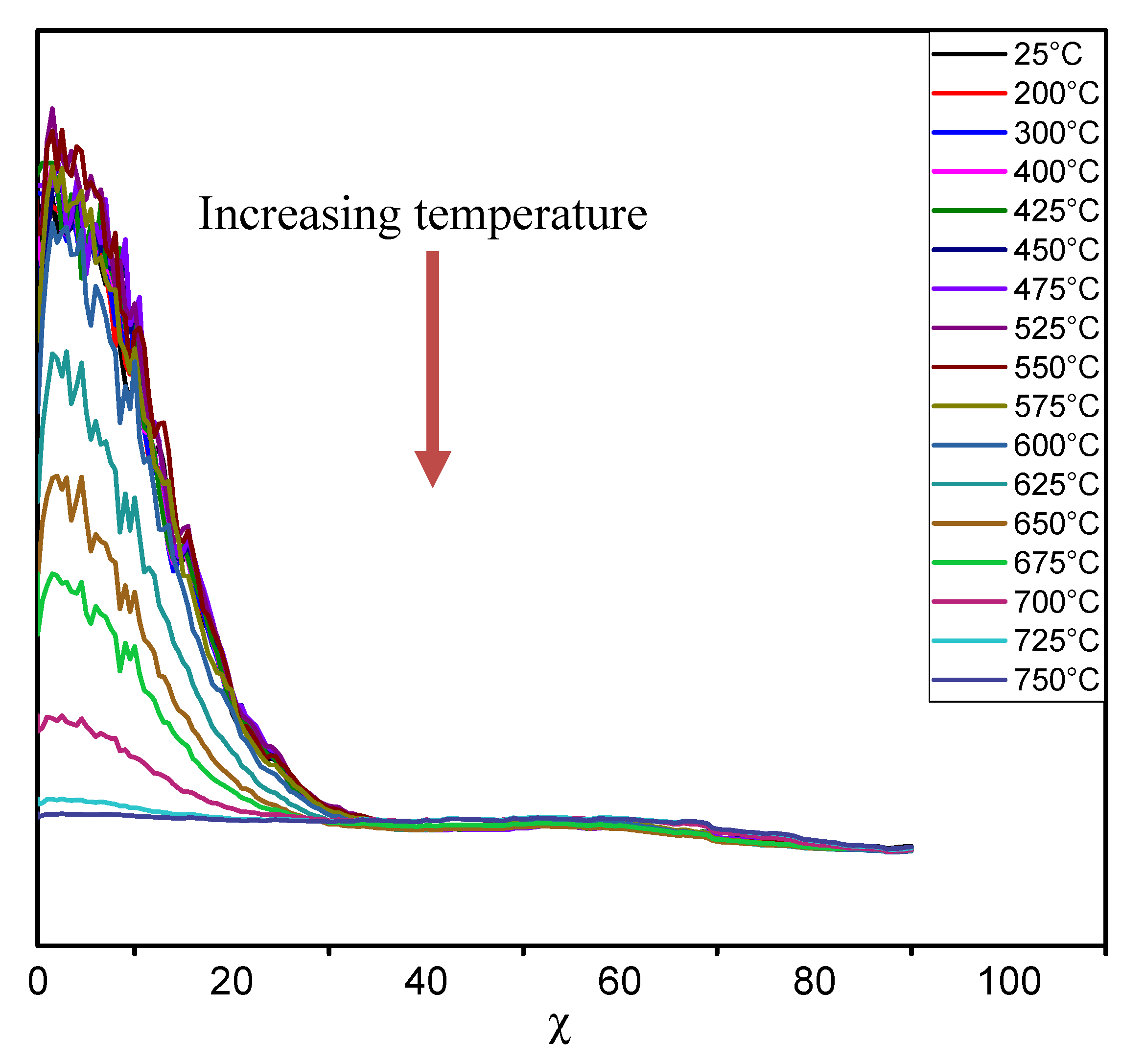
Figure 3 shows the evolution of the diffracted intensity of the (002) kaolinite reflection as a function of the χ tilt angle and the temperature. The local arrangement of the hydroxyl groups that will be removed during the dehydroxylation reaction is illustrated on
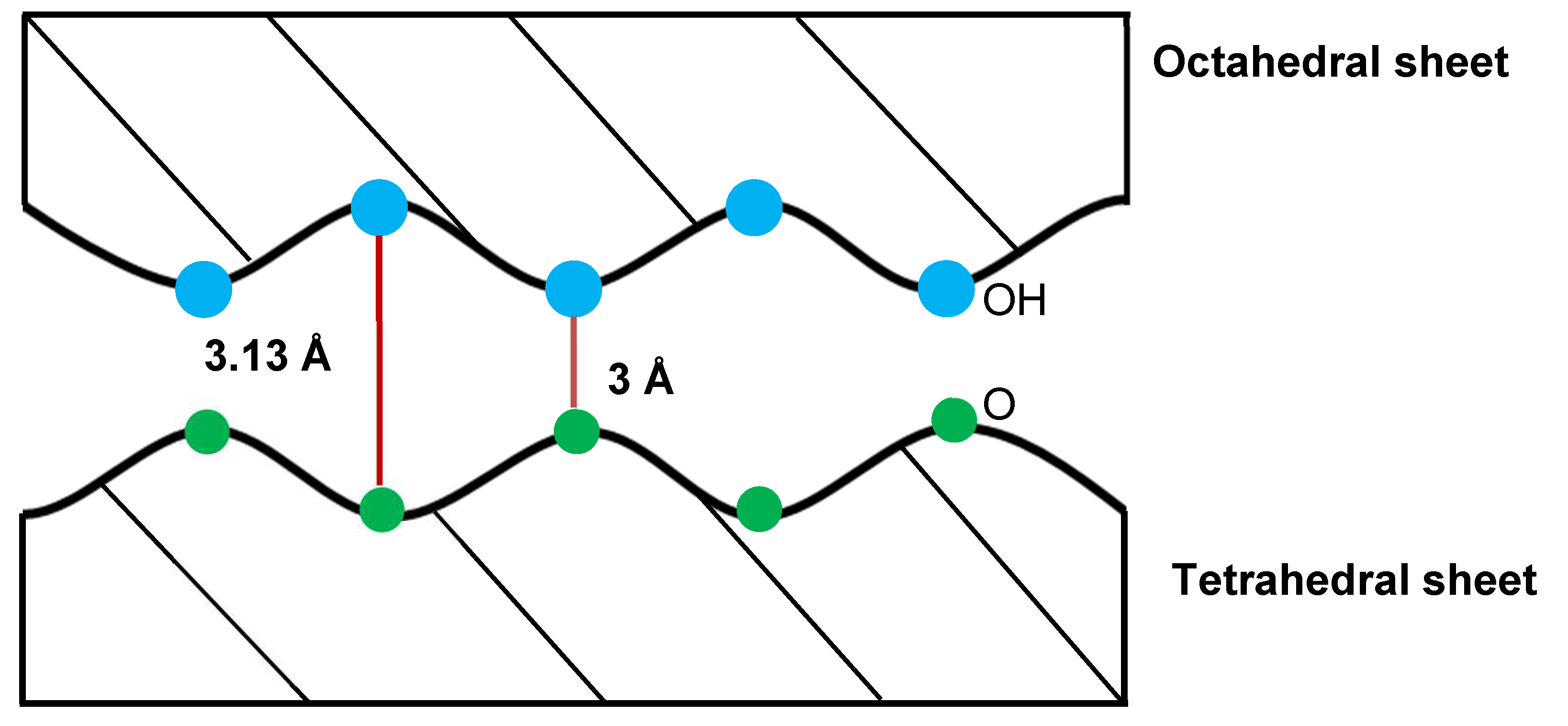
Figure 4.
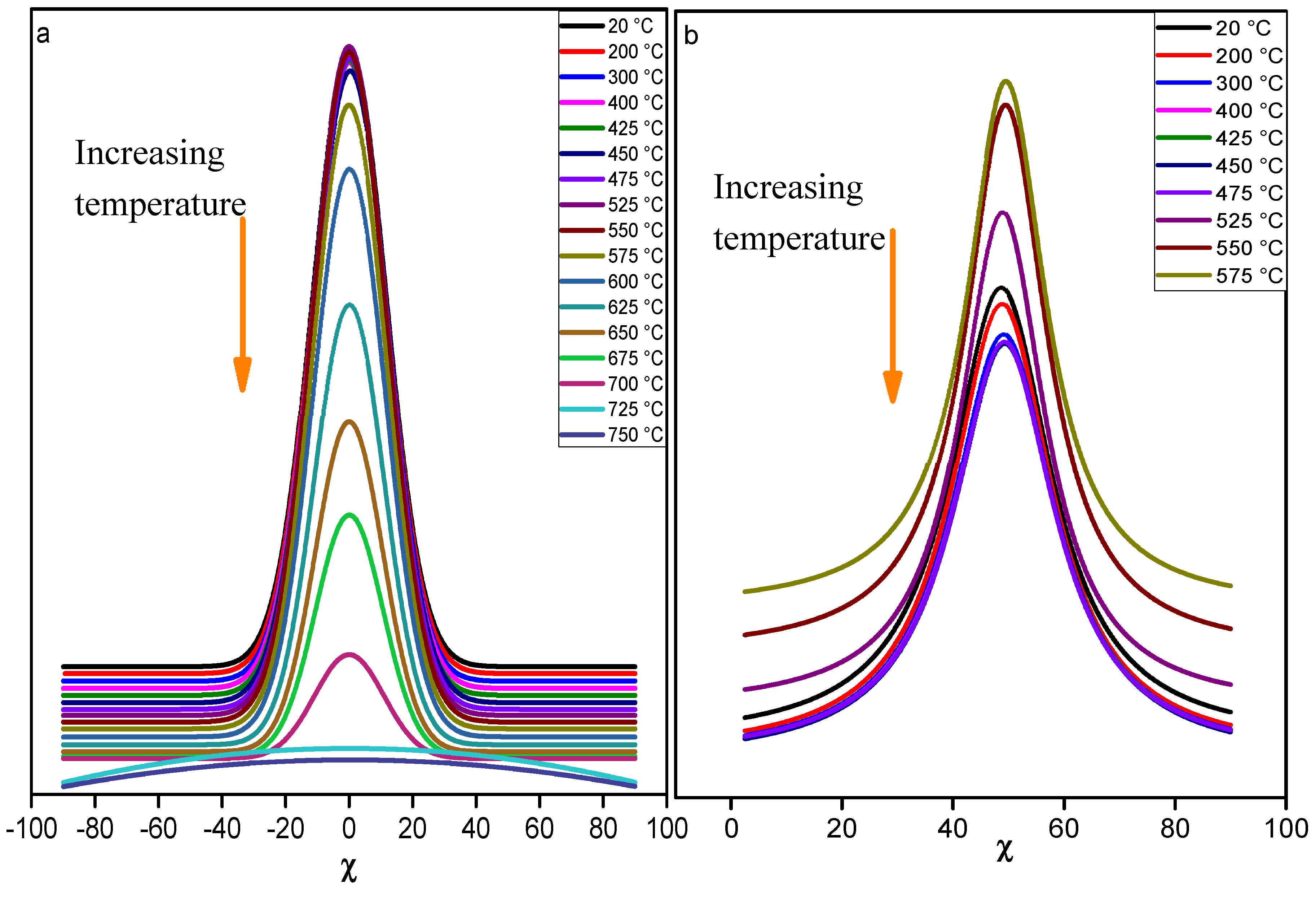
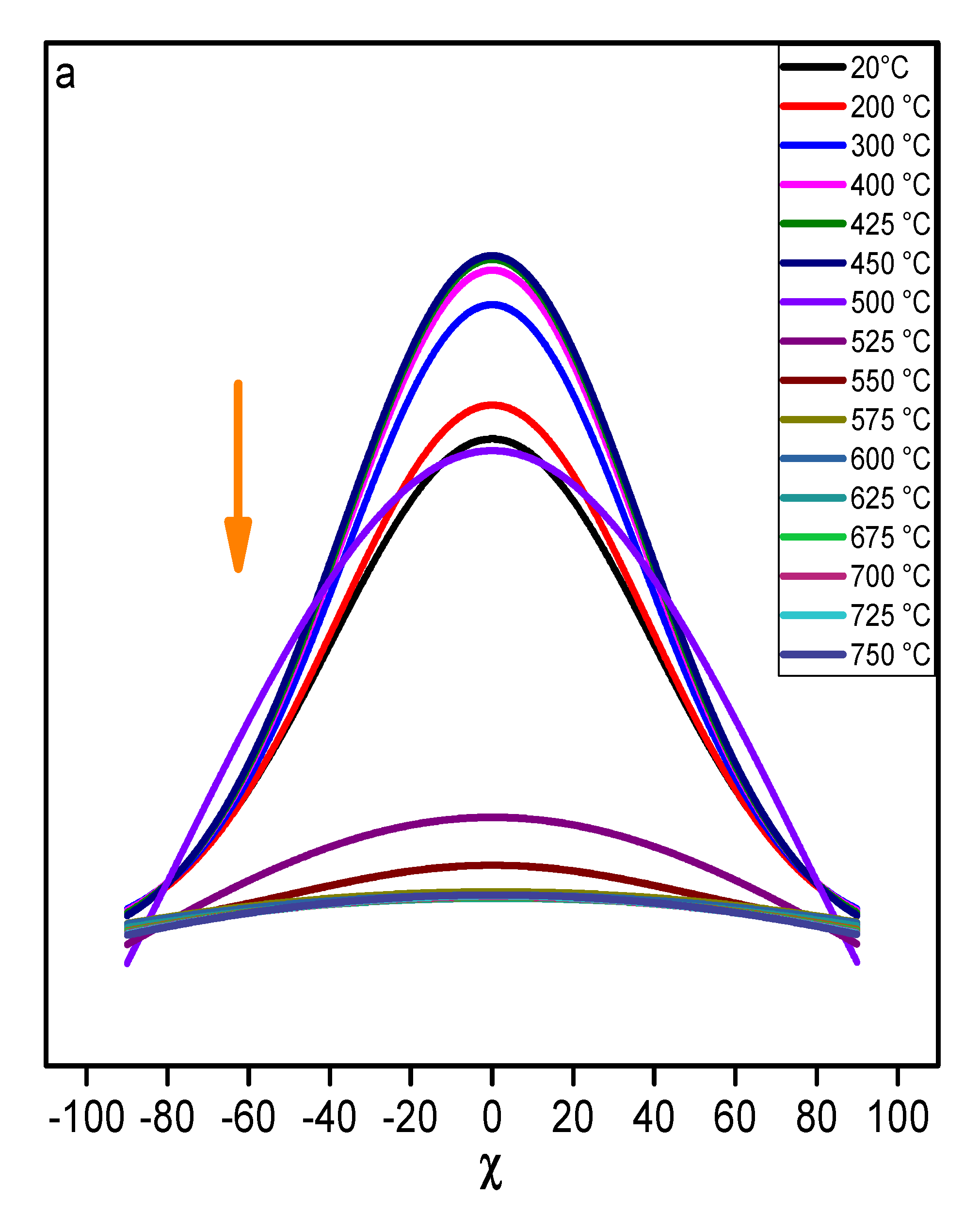
The main trend observed for the distribution of (002) and (111) diffracted intensities with respect of tilt angle χ during the
in situ heat treatment of KGR100 is presented on
Figure 5. From room temperature to 550 °C, the intensity of the (002) and (111) reflections remain quite constant. Above 575 °C, the intensity of (002) reflection decreases progressively and finally vanishes close to 750 °C. In the case of (111) reflection, no intensity was collected above 575 °C. These behaviors for the (002) and (111) reflections are in line with a strong texture of kaolinite platelets along the
c-axis, and the preservation of this preferred orientation until the complete transformation of kaolinite into metakaolinite.
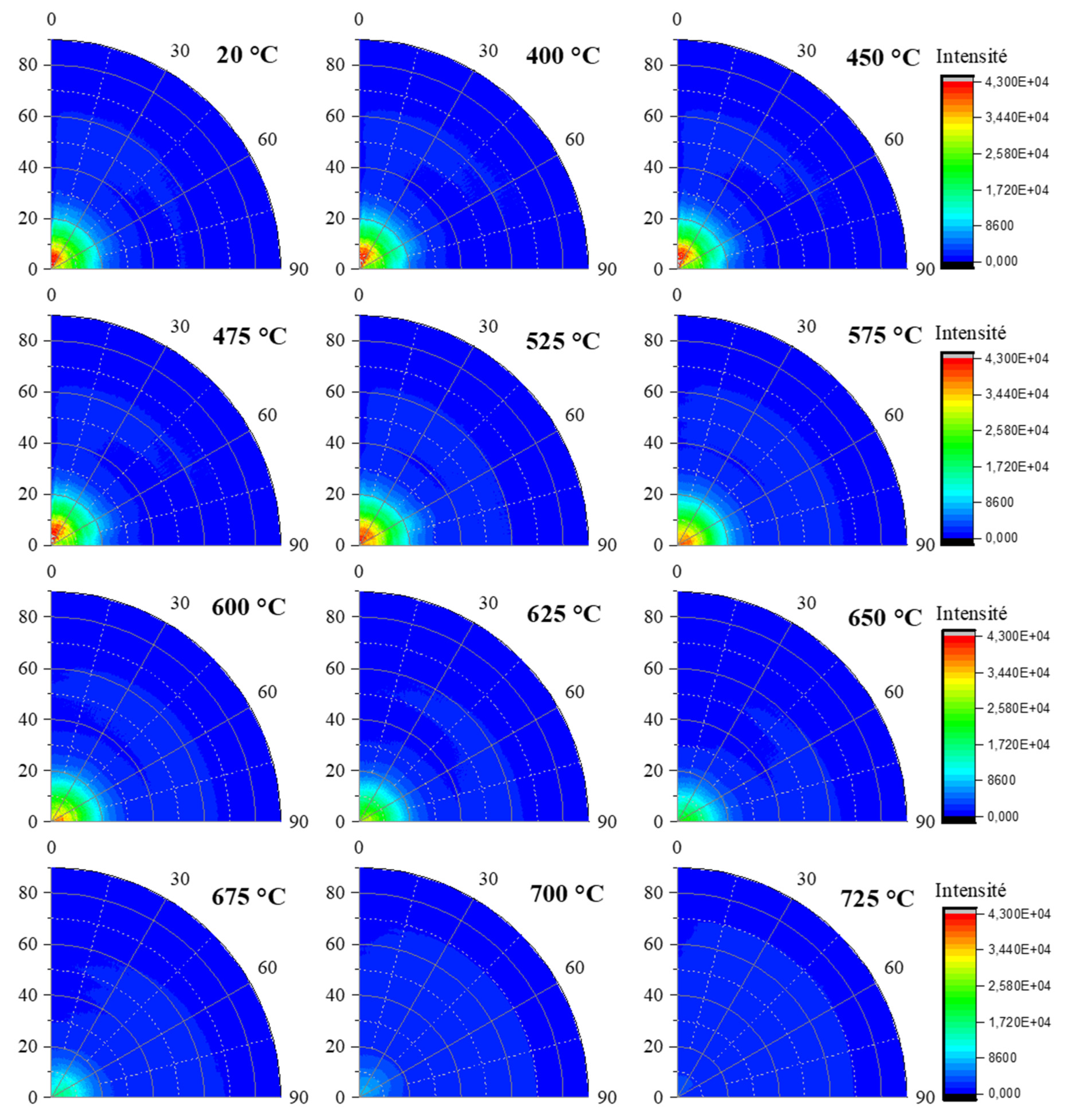
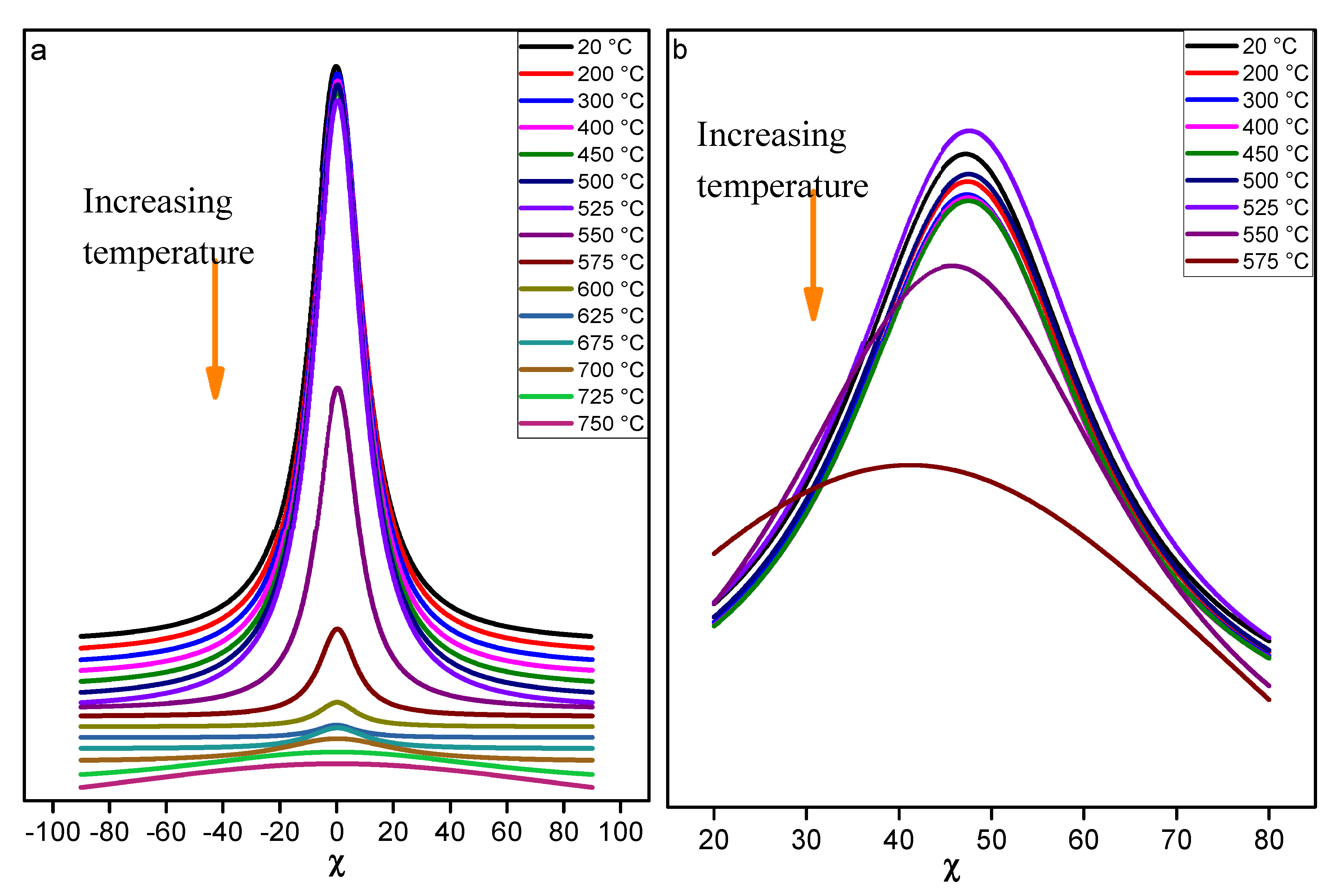
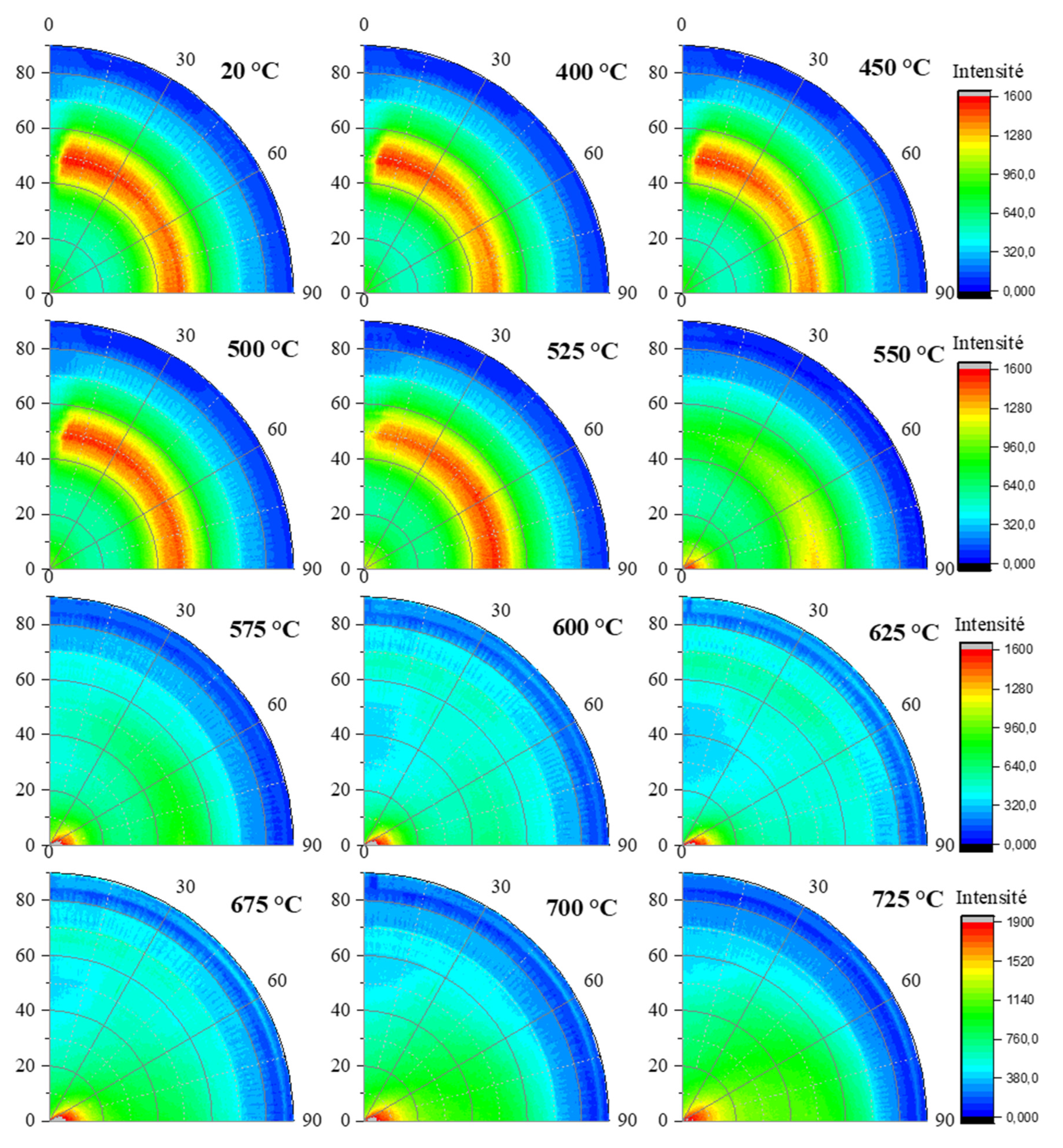
In order to highlight the differences between the two reflections (002) and (111), the poles figures were represented for specific temperatures as shown on
Figure 6 and
Figure 7. For both the azimuthal (φ) and tilt (χ) angles, the range selected was between 0° and 90 °. As expected, a centered and intense maximum appeared in the range 0 to 20° of angle χ onto the poles figures related to (002) reflection (
Figure 6). A maximum of intensity is noted in the temperature range [25 - 575 °C], followed by a continuous decrease until 725 °C. The (111) reflection (
Figure 7) exhibited a ring-like intensity distribution located around χ = 50° onto the poles figures in the temperature range [25 - 575 °C]. Above this temperature, the ring-like intensity vanishes while a point (higher intensity region) appeared close to the center of poles figures (χ = 0°) for the (111) reflection (
Figure 8). It is noted that the ring intensity is not uniform. This is due to the wiggling of the flat sample / foil (edges lifting from the flat surface) with the temperature, most probably creating shadows.
Considering the aforementioned results, three main points can be cited:
The studied samples exhibited a fiber type texture with the c-axis aligned preferentially perpendicular to the sample surface;
The dehydroxylation of kaolinite started around 575 °C for sample KRG100;
The initial preferred alignment along the c-axis is kept unchanged until the total dehydroxylation of kaolinite observed at 725 °C within KRG100, in agreement with the disappearance of the reflections (002) and (111).
A transitory phase seemed to occur during the dehydroxylation, since a new intensity is detected in the center of poles figure, meaning that this transitory phase had a lattice parameter corresponding to the (111) reflection. Indeed, it is such oriented that these crystalline planes are // surface, which makes intensity appears at chi = 0 and possibly perpendicular to the c axis.
In our previous study performed on the D2AM beamline at the ESRF Grenoble [
14], we noted that the dehydroxylation for KRG sample occurred in the range425 to 650°C. Indeed, we considered the change in slope from the intensity ratio. While considering the FWHM change, we noted a good correlation with the change in texture regarding the results obtained using poles figures measurements. It is likely that before the effective detachment of the hydroxyl groups. Moreover, the delamination concept [
14] could be assumed in this very early step. Since the shape index and the particle size values are higher for kaolin KRG compared to kaolin KCS, a higher and larger dehydroxylation temperature range is expected for KRG100 samples.
In the case of sample KCS100, the distribution of (002) and (111) diffracted intensities with respect of tilt angle χ during the
in situ heat treatment, is presented in
Figure 8. It appears that the intensities of both reflections are constant up to 525 °C. Above this temperature, the (111) reflection disappeared, while the (002) reflection intensity tended to decrease progressively before disappearing at 625 °C. The intensity profile seemed unchanged until total dehydroxylation of the kaolinite within KCS100 sample. Consequently, it can be concluded that and KCS100 sample exhibited a strong texture along the
c-axis as for sample KRG100. Moreover, in both samples, the crystals orientation is not modified during the dehydroxylation process. It was noted that the effect of dehydroxylation on the texture occurred at higher temperature for CS kaolin regarding the dehydroxylation range determined during former experiments at the ESRF. The same comment regarding the occurrence of several mechanisms could explain this trend. Indeed the FWHM of the kaolinite reflection (002) in KCS samples clearly indicate a change in slope around 525°C. This may lead to conclude that, as in the case of KRG100 samples, a relaxation seemed to predominate prior to the effective detachment of the first hydroxyl groups. Since the particle size and the shape index values for kaolin KCS are lower compared to those of kaolin KRG, the determined dehydroxylation temperature range for KCS100 sample is lower.
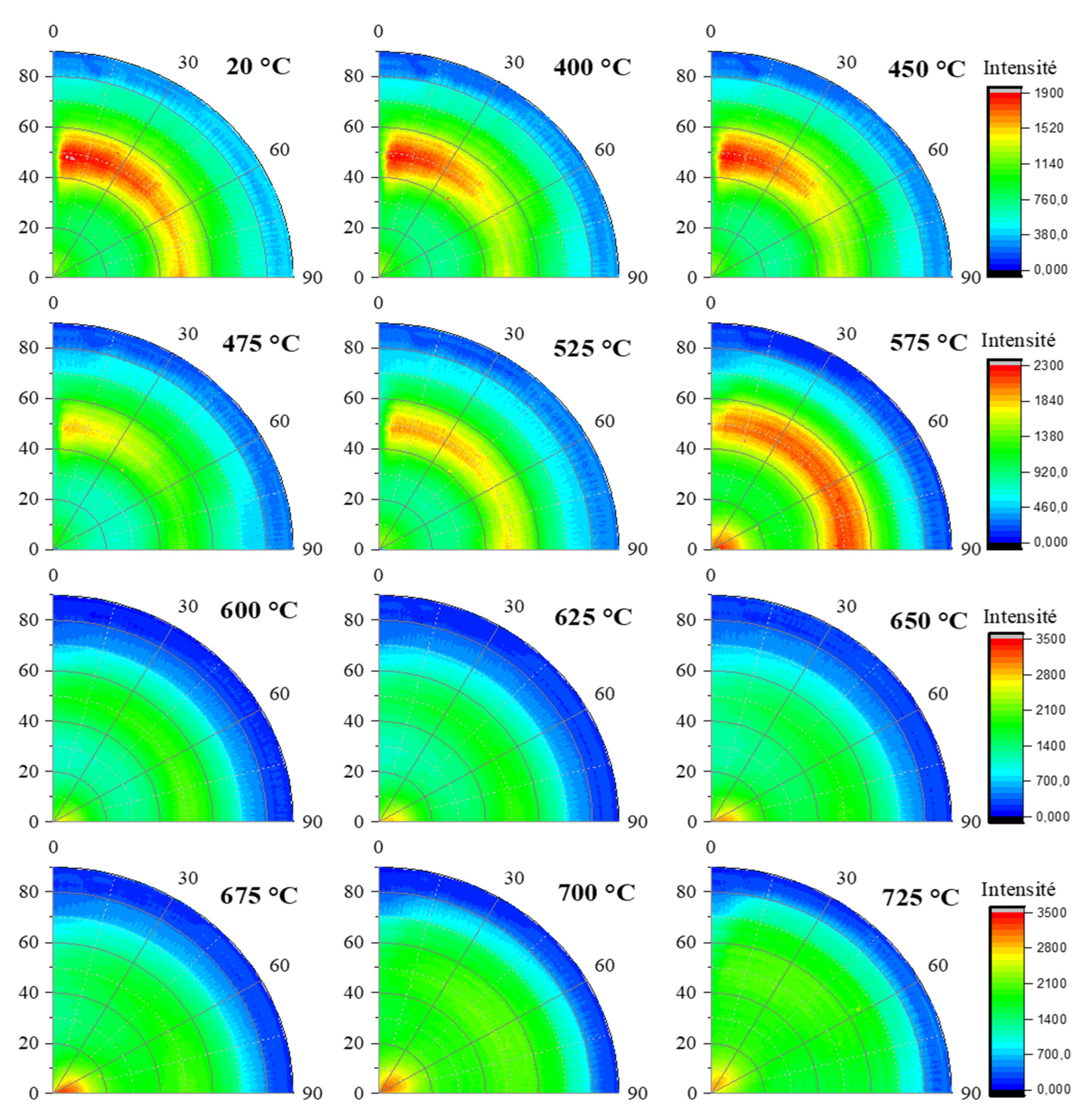
Figure 9 and
Figure 10 present the (002) and (111) poles figures obtained for sample KCS100 at different temperatures. The same trend observed for with KRG100 is observed since the (002) diffraction intensity is located close to the center, while the (111) diffraction signal appeared as a ring portion located at χ = 50 ° onto the poles figures. The sample KCS100 exhibited a fiber type texture along the
c- axis. Furthermore, the disappearance of the (111) diffraction signal at 550 °C lead to the occurrence of a transitory phase during the kaolinite dehydroxylation (same situation as for KRG100) related to a centered point on the corresponding poles figures. According to our previous work on these samples, we have demonstrated that the dehydroxylation mechanisms involved a delamination stage [
14] in the range 475-525°C. Consequently, the observed transitory phase could be related to this phenomenon, leading to a partial modification of the initial kaolinite structure while maintaining the (00l) reflections unchanged. Indeed, some authors [
17,
18,
19] assumed that during this dehydroxylation, the XRD peaks of kaolinite disappeared abruptly at 550°C, and this is much in agreement with the behavior that we observed for the (111) reflection.
Since the particles of H100 sample are tubular, they may show a different trend compared to samples KRG100 and KCS100.
Figure 11 illustrates the
in situ evolution of the intensities of (002) reflections of halloysite regarding the tilt angle χ. Due to the rolling of the TO layers, the diffracted intensity of the (111) reflection is quasi-independent of the χ angle and it is not provided. It means that no texture effect is observed with respect to this reflection. The diffracted intensity of the (002) reflection of halloysite (
Figure 11) tended to increase in the temperature range [20 - 450 °C], and then it decreased and disappeared at 550 °C. The preferred alignment is less significant within H100 sample compared to KRG1000 and KCS100 samples. Besides, the heat treatment up to 450 °C tended to increase the texture within H100 sample, due to the roll out of some halloysite tubes [
20]. Above 450 °C, no significant texture is noted.
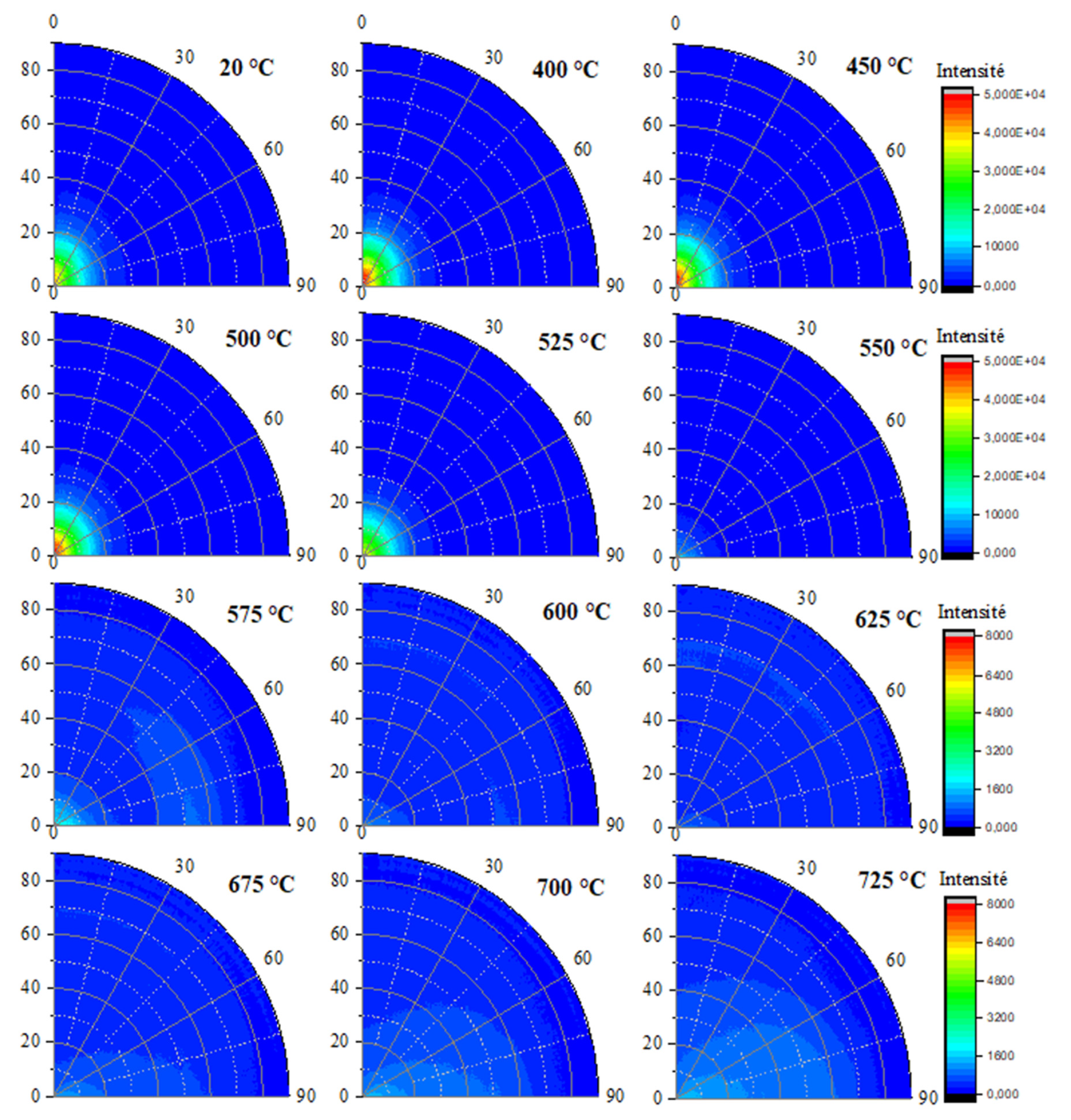
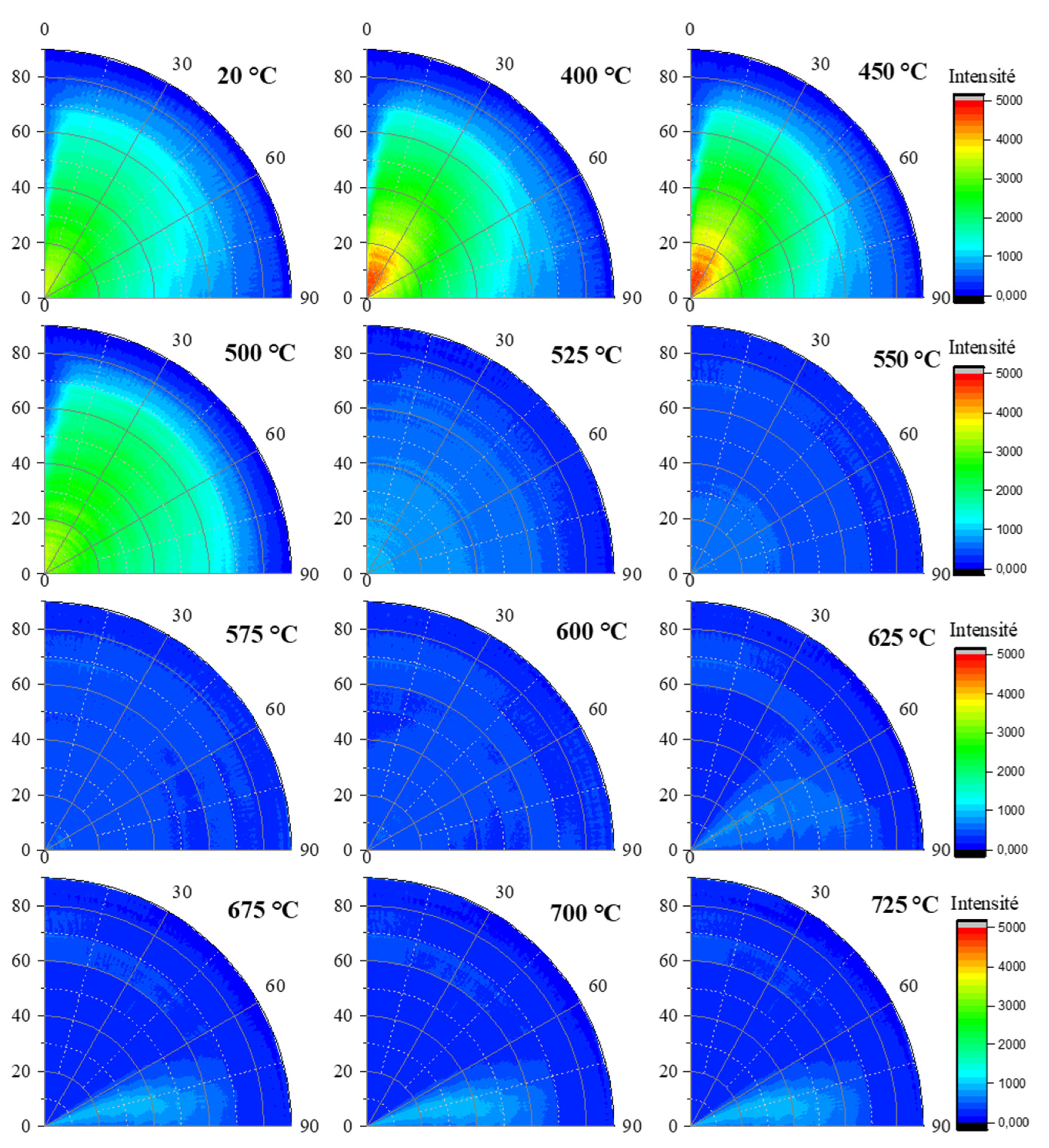
The (002) halloysite reflection poles figures presented in
Figure 12 confirm the lack of preferential orientation at room temperature, followed by a slight texture occurrence when temperature is increased up to 450 °C for H100 sample. The observed texture disappeared before the complete dehydroxylation of halloysite. The (111) halloysite reflection poles figures were not of great importance since it was already clearly assessed that no texture was associated with this reflection (
Figure 11 (b)). Due to the polydispersity particle size distribution of halloysite powder, the dehydroxylation of sample H100 tended to start quite 100°C lower than in the case the studied kaolins. Therefore, an overlapping may occur between the staking defects relaxation and the submicron-sized halloysite particles dehydroxylation. The current measurement mode allowed the accurate detection of these concomitant mechanisms within sample H100.
3.1.2. Texture behavior for the kaolin-halloysite mixtures
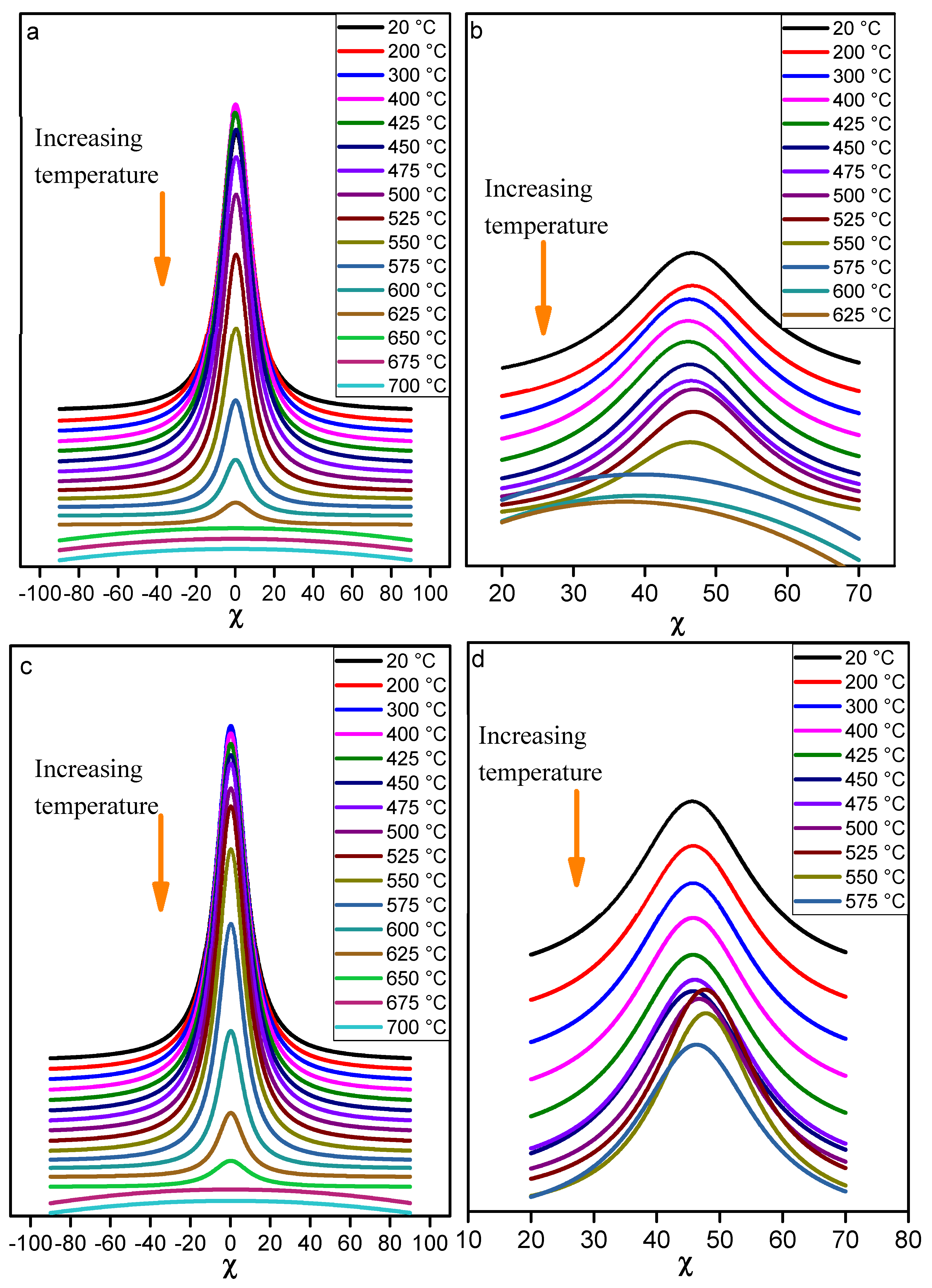
The mixtures containing 50 mass% of kaolin and 50 mass% of halloysite in each case were submitted to
in situ analyses. The diffracted intensity of (002) and (111) reflections of kaolinite are presented on
Figure 13, as a function of the tilt angle χfor different temperature for samples KRG50H50 and KCS50H50. A very significant texture is noted for both samples despite the presence of a great amount of halloysite. The (002) kaolinite reflection which is very closed to the (002) reflection of halloysite undergoes a slight intensity increase while the temperature increased from 20 to 400 °C. This trend is in agreement with the roll out of halloysite tubes, which interfered with the neighboring kaolinites crystallites signal. The intensity of the (002) reflection was stable in the temperature range 400 - 525 °C, then progressively decreased and disappeared around 675 °C. The (111) reflection exhibited a constant intensity in the range 25 °C - 550 °C for KCS50H50 sample, and then it disappeared above 550 °C. For KCS50H50 sample, the (111) reflection exhibited a constant intensity in the range 25 °C - 575 °C, but a shift of the maximum of the intensity distribution is observed (5° in χ) before its disappearance after 575 °C. This specific trend suggested a significant change into the crystals orientation with respect to the sample surface (supplemental tilting). The (111) reflection was loss when the diffracted intensity of the (002) reflection started to decrease in both samples, which confirmed the onset of dehydroxylation of kaolinite and halloysite as already indicated in the case of KCS100 and KRG100 samples. The (002) and (111) poles figures were determined (not shown here) and were in agreement with the major trends described above. It arise that each TO mineral undergoes decomposition with less interaction with other phases.
3.2. Exploring the dehydroxylation end point using X-ray scattering for samples KRG100, KCS100 and H100
During the dehydroxylation of kaolinite-based samples, we were able to clearly achieve the temperature range of kaolinite dehydroxylation and the changes in texture. Nevertheless, it was quite difficult to achieve such analysis with halloysite-based sample due to its weak texture. In addition, the presence of scattering signal coming from PEEK dome did not allow obtaining interesting responses on the occurrence of a transitory spinel-like phase upon dehydroxylation during the
in situ X-ray diffraction experiments. Furthermore, we intended to clarify the end-temperature of dehydroxylation without focusing on the classical (002) reflection. Therefore, we performed the
in situ wide angle X-ray scattering measurements, using the curved two-dimensional detector CirPAD (angular span in 2θ ~135°) [
15]. The operating conditions consisted in a heating rate of 20 °C/min from room temperature to 1100 °C, with data recording every 10s.
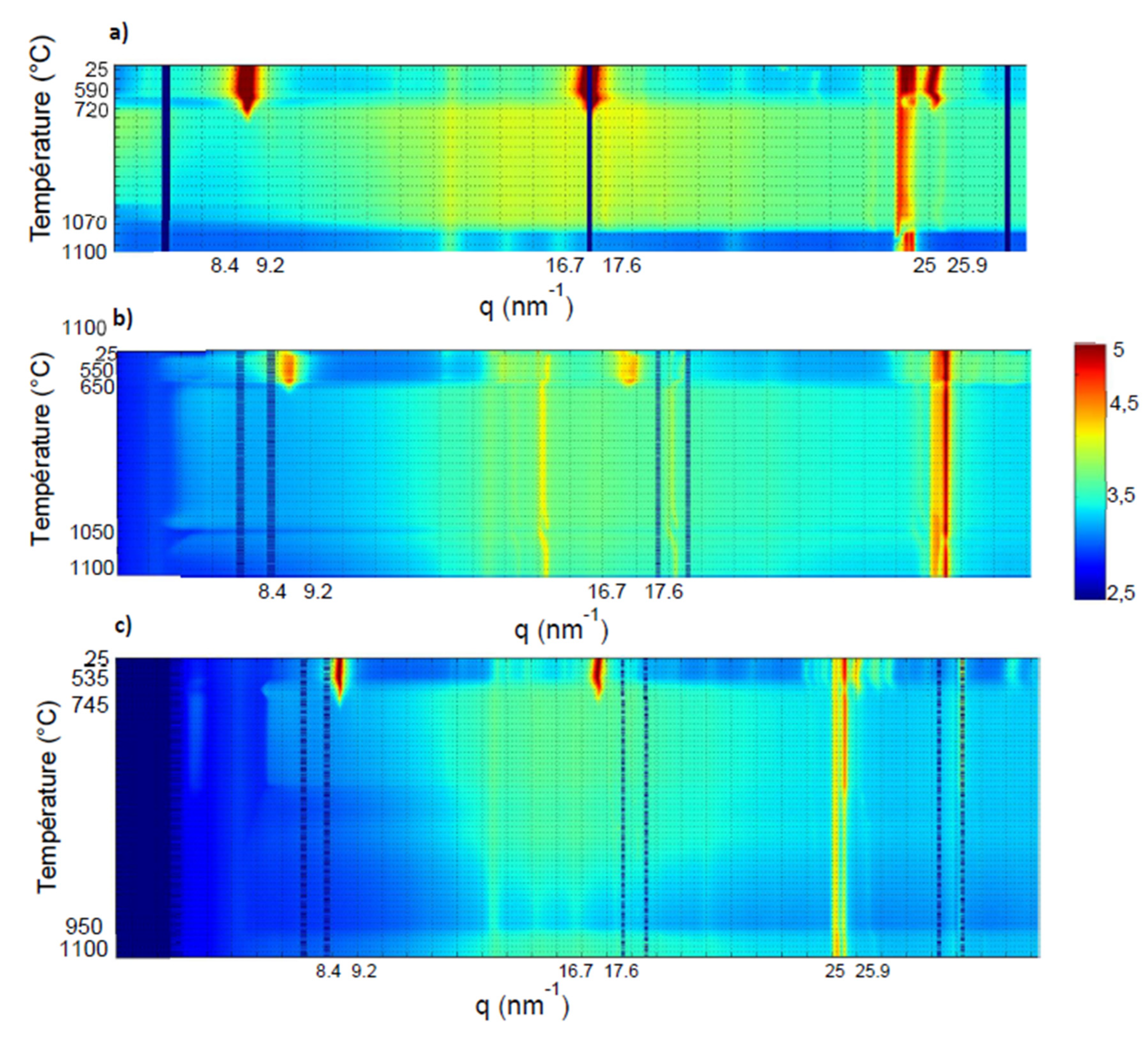
Figure 14 shows the evolution of the X-ray scattering patterns collected for the studied samples (KCS100, KRG100 and H100) in the temperature range [25 - 1100 °C]. The patterns are plot as a function of the norm of the Q-vector,
i.e. .Three main temperature ranges with specific behaviors were identified:
- -
25 °C - 725 °C where the characteristics peaks of kaolinite are observed, namely: (001), (002) and (003) located at 9 nm-1, 17.5 9 nm-1 and 25.5 9 nm-1 in Q respectively. These reflections disappeared around 720 °C, indicating the end of kaolinite dehydroxylation (complete transformation into amorphous metakaolinite).
- -
725 °C - 1000 °C, with a homogeneous intensity within the whole angular range, and no significant peak. This may be considered as the stability domain of metakaolinite, amorphous phase.
- -
1000 °C - 1100°C where the background intensity undergoes a significant change. This trend seems consistent with a specific structural change in the studied samples.
From literature, the kaolinite or halloysite transformation into metakaolinite, called dehydroxylation, consisted in the removal of hydroxyl groups located in the octahedral sheets, which combined, and released as water vapor. Due to these reactions, the octahedral sheets exhibit a significant deformation that lead to the distortion of the global kaolinite or halloysite structure. At the end of the process, the long-range order is lost and the amorphous phase obtained, metakaolinite [
21] will then gradually transform above a specific temperature (between 950 and 1100 °C) to a new crystalline phase (Al-Si spinel or mullite) [
20,
21,
22,
23,
24].
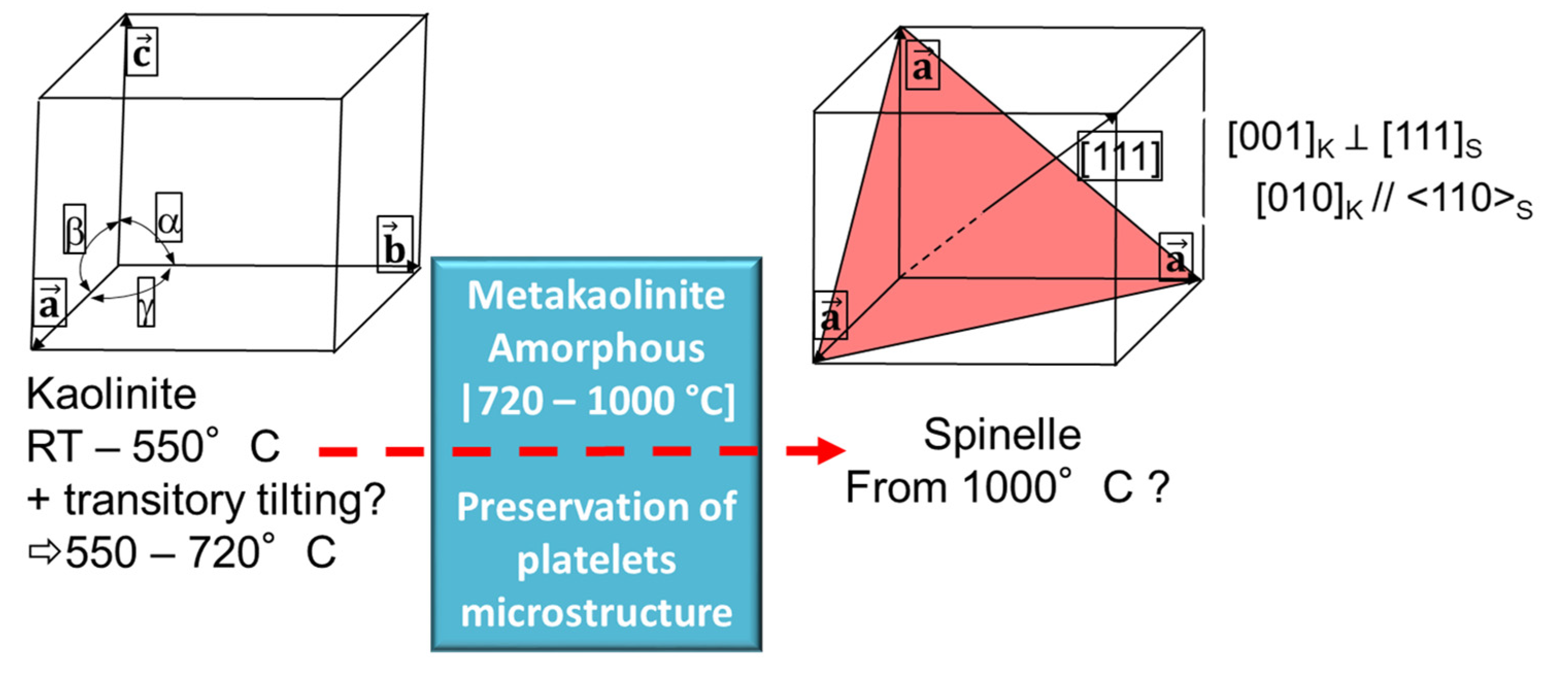
Accordingly, to our results, the new crystalline phase occurred at 1000°C. Nevertheless, we are not able to provide the effective nature of this phase yet. We can summarize a simplified representation of the sequence based on the kaolinite samples as illustrated on
Figure 15.
4. Conclusions
The present study aimed at understanding the texture changes in kaolinite and halloysite materials shaped by tape casting. The evolution of the texture as function of the temperature were followed to in situ high temperature x-ray diffraction measurements and determination of the pole figure evolutions. In kaolinite-based samples KCS100 and KRG100, a strong texture was observed with respect to the kaolinite c-axis (which is preferentially oriented along the sample surface normal). This was maintained until the total dehydroxylation of kaolinite, which transforms to metakaolinite. In the case of halloysite-based sample H100, a weak texture along the c-axis was developed upon heating from room temperature to 400 °C, thanks to the opening of some halloysite tubes. Nevertheless, this texture disappeared before the complete dehydroxylation of halloysite.
The dehydroxylation temperature range was determined for all the materials of interest: 575 - 725 °C for KRG100; 525 and 625 °C for KCS100. Therefore, the onset point of dehydroxylation is 550 °C ± 25 °C for these kaolinites. The main differences observed for these kaolins may be related to the variation of the stacking faults density, the higher shape index (25 for KCS100 and 40 for KRG100) and the grain size distribution of kaolinite platelets in KRG100 sample in comparison to KCS100 sample. It was also noted that during the dehydroxylation of kaolinite, the characteristic portion of ring related to the diffracted intensity of its (111) reflection located at χ= 45 ° tended to disappear above 550 °C and led to the formation of a new transitory phase with the appearance of a central peak (chi = 0) for the 111 poles figure. Indeed, this reflection is parallel to the sample flat surface (a direction perpendicular to the kaolinite c-axis). For H100 sample, the dehydroxylation starts at lower temperature 475°C, but it was difficult to assess the end of dehydroxylation due to the loss of texture.
When considering the kaolinite-halloysite mixtures, samples KRG50H50 and KCS50H50, a significant texture was observed along the c-axis. Indeed, the presence of kaolinite platelets predominated onto the alignment of halloysites tubes. Furthermore, it was noted that the halloysite influenced the (002) diffracted intensity into the temperature range 20 °C and 400 °C. Above 400°C, the behavior obtained for the (002) reflection in samples KRG50H50 and KCS50H50 was similar to the behavior noticed for pure kaolins KRG100 and KCS100 respectively. Again, the onset dehydroxylation temperature is identified as 550 °C ± 25 °C. The same trend observed for the (111) kaolinite reflection for samples KRG100 and KCS100 was noted in the samples KRG50H50 and KCS50H50. This transitory phase required further analysis to highlight its structural characteristics.
Further X-ray scattering experiments allowed highlighting the effective offset temperature of the dehydroxylation, which was identified as close to 720 °C. The metakaolinite obtained undergone structural transformation to another transitory phase at 1000°C. The latter phase identification through pair distribution functions analysis may bring new clues for the understanding of the metakaolinite to mullite transition sequence, which is out of the scope of the present study.
Author Contributions
Conceptualization, G.L.L.-N., I.D. and R.G.; Methodology, G.L.L.-N. and R.G. and I.D.; Software, C.M., R.G. and I.D.; Validation, C.M., G.L.L.-N. and R.G.; Formal Analysis, I.D., C.M., G.L.L.-N. and R.G.; Investigation, C.M., I.D., G.L.L.-N., R.G. and N.T.-D.; Resources, C.P., I.D., G.L.L.-N., C.M., R.G., N.T.-D and D.T.; Data Curation, C.M., I.D., G.L.L.-N. and R.G.; CM : data acq, data curation + xpad/cispad data conversion, generate pole figs. Writing-Original Draft Preparation G.L.L.-N. and I.D.; Writing–Review & Editing, G.L.L.-N., C.M. and R.G.; Visualization, G.L.L.-N., C.M. and R.G.; Supervision, G.L.L.-N., C.M., N.T.-D., R.G., D.T. and C.P.; Project Administration, G.L.L.-N., N.T.-D., C.P. and R.G.; Funding Acquisition, G.L.L.-N., C.M., D.T., N.T.-D., C.P. and R.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to SOLEIL synchrotron for providing the access to DiffAbs beamline and they thank the respective staff for support during experiments. We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities and we would like to thank xyz for assistance in using beamline BM02 (D2AM, CRG, ESRF).
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Mitra, G.B. Spiral Structure of 7 Å Halloysite: Mathematical Models. Clays Clay Miner. 2013, 61, 499–507. [Google Scholar] [CrossRef]
- Bailey, S.W. Halloysite—A critical assessment. In Volume II: Surface Chemistry. Structure and Mixed Layering of Clays, Proceedings of the 9th international Clay Conference, Strasbourg, France, 28 August–2 September 1989; Institut de Géologie—Université Louis-Pasteur: Strasbourg, France, 1990; pp. 89–98. [Google Scholar]
- Johnson, S.L. Thermal Stability of Halloysite byHigh-Pressure Differential Thermal Analysis. Clays Clay Miner. 1990, 38, 477–484. [Google Scholar] [CrossRef]
- Duran, C.; Kemal Tür, Y. Templated grain growth of textured mullite/zirconia composites. Mater. Lett. 2005, 59, 245–249. [Google Scholar] [CrossRef]
- Štubna, I.; Trník, A.; Vozár, L. Thermomechanical and thermodilatometric analysis of green alumina porcelain. Ceram. Int. 2009, 35, 1181–1185. [Google Scholar] [CrossRef]
- Boussois, K.; Deniel, S.; Tessier-Doyen, N.; Chateigner, D.; Dublanche-Tixier, C.; Blanchart, P. Characterization of textured ceramics containing mullite from phyllosilicates. Ceram. Int. 2013, 39, 5327–5333. [Google Scholar] [CrossRef]
- Deniel, S.; Tessier-Doyen,N; Dublanche-Tixier,C.; Chateigner,D.; Blanchart, P. Processing and characterization of textured mullite ceramics from phyllosilicates. J. Eur. Ceram. Soc. 2010, 30, 2427–2434.
- Lecomte-Nana, G.; Mokrani, A.; Tessier-Doyen, N.; Boussois, K.; Goure-Doubi, H. Texturation of model clay materials using tape casting and freezing. Ceram. Int. 2013, 39, 9047–9053. [Google Scholar] [CrossRef]
- Castelein, O.; Guinebretière, R.; Bonnet, J.P.; Blanchart, P. Shape, size and composition of mullite nanocrystals from a rapidly sintered kaolin. J. Eur. Ceram. Soc. 2001, 21, 2369–2376. [Google Scholar] [CrossRef]
- Maqueda, C.; Partal, P.; Villaverde, J.; Perez-Rodriguez, J.L. Characterization of sepiolite-gel-based formulations for controlled release of pesticides. Appl. Clay Sci. 2009, 46, 289–295. [Google Scholar] [CrossRef]
- Houta, N.; Lecomte-Nana, G.-L.; Tessier-Doyen, N.; Peyratout, C. Dispersion of phyllosilicates in aqueous suspensions: Role of the nature and amount of surfactant. J. Colloid Interface Sci. 2014, 425, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Chartier, T.; Streicher, E.; Boch, P. Preparation and characterization of tape cast aluminum nitride substrates. J. Eur. Ceram. Soc. 1992, 9, 231–242. [Google Scholar] [CrossRef]
- Chartier, T.; Badev, A.; Abouliatim, Y.; Lebaudy, P.; Lecamp, L. Stereolithography process: Influence of the rheology of silica suspensions and of the medium on polymerization kinetics—Cured depth and width. J. Eur. Ceram. Soc. 2012, 32, 1625–1634. [Google Scholar] [CrossRef]
- Daou I., Lecomte-Nana G.L., Tessier-Doyen N., Peyratout C., Gonon M.F., Guinebretiere R., Probing the dehydroxylation of kaolinite and halloysite by in situ high temperature x-ray diffraction. Minerals 2020, 10, 480, 1–19.
- Desjardins, K., Mocuta C., Dawiec A., Réguer S., Joly P., Dubuisson J.-M., Alves F., Noureddine A., Bompard F., Thiaudière D., “The CirPAD, a circular 1.4 M hybrid pixel detector dedicated to X-ray diffraction measurements at Synchrotron SOLEIL”. J. Synchr. Rad 2022, 29, 180–193. [CrossRef] [PubMed]
- Mocuta, C., Richard M.-I., Fouet J., Stanescu S., Barbier A., Guichet C., Thomas O., Hustache S., Zozulya A. V., Thiaudière D., Fast pole figure acquisition using area detectors at the DiffAbs beamline – Synchrotron SOLEIL. J. Appl. Crystallogr. 46,6, 1842–1853.
- Brindley, G.W.; Nakahira, M. The Kaolinite-Mullite Reaction Series: II, Metakaolin. J. Am. Ceram. Soc. 1959, 42, 314–318. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, M.-C.; Hon, M.-H. Phase transformation and growth of mullite in kaolin ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- Ptácek, P.; Frajkorová, F.; Šoukal, F.; Opravil, T. Kinetics and mechanism of three stages of thermal transformation of kaolinite to metakaolinite. Powder Technol. 2014, 264, 439–445. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Pampuch, R. Le mécanisme de la déshydratation des hydroxydes et des silicates phylliteux, Bull Groupe Fr. Argiles 1971, 23, 1, 107-118. [Google Scholar] [CrossRef]
- Brindley, G.W.; Nakahira, M. The Kaolinite-Mullite Reaction Series: II, Metakaolin. J. Am. Ceram. Soc. 1959, 42, 314–318. [Google Scholar] [CrossRef]
- Lecomte-Nana, G.-L. Transformations Thermiques, Organisation Structurale et Frittage des Composés Kaolinite-Muscovite; Université de Limoges: Limoges, France, 2004. [Google Scholar]
- Roy, R.; Roy, D.M.; Francis , E. E. New Data on Thermal Decomposition of Kaolinite and Halloysite. J. Am. Ceram. Soc. 2006, 38, 198–205. [Google Scholar]
Figure 1.
SEM images of surface and cross-sections of the kaolinite and halloysite-based samples.
Figure 1.
SEM images of surface and cross-sections of the kaolinite and halloysite-based samples.
Figure 2.
XRD patterns of the studied samples after shaping by tape casting and drying. The main reflections correspond to Kaolinite (JCPDS 00-005-0143) and/or halloysite (JCPDS 29-1487); while Q and C are respectively for quartz and cristobalite.
Figure 2.
XRD patterns of the studied samples after shaping by tape casting and drying. The main reflections correspond to Kaolinite (JCPDS 00-005-0143) and/or halloysite (JCPDS 29-1487); while Q and C are respectively for quartz and cristobalite.
Figure 3.
Evolution of the diffracted intensity of the kaolinite (002) reflection with respect to the χ tilt angle and the temperature.
Figure 3.
Evolution of the diffracted intensity of the kaolinite (002) reflection with respect to the χ tilt angle and the temperature.
Figure 4.
Illustration of hydrogen bonging and localization of hydroxyl groups within the structure of kaolinite.
Figure 4.
Illustration of hydrogen bonging and localization of hydroxyl groups within the structure of kaolinite.
Figure 5.
variation of the diffracted intensity regarding the temperature and the tilt angle χ for sample KRG100; a) (002) and b) (111) reflections.
Figure 5.
variation of the diffracted intensity regarding the temperature and the tilt angle χ for sample KRG100; a) (002) and b) (111) reflections.
Figure 6.
Evolution of in situ poles figures for the (002) reflection of kaolinite within sample KRG100 for different temperatures.
Figure 6.
Evolution of in situ poles figures for the (002) reflection of kaolinite within sample KRG100 for different temperatures.
Figure 7.
Evolution of in situ poles figures for the (111) reflection of kaolinite within sample KRG100 for different temperatures.
Figure 7.
Evolution of in situ poles figures for the (111) reflection of kaolinite within sample KRG100 for different temperatures.
Figure 8.
variation of the diffracted intensity regarding the temperature and the tilt angle χfor sample KCS100; a) (002) and b) (111) reflections.
Figure 8.
variation of the diffracted intensity regarding the temperature and the tilt angle χfor sample KCS100; a) (002) and b) (111) reflections.
Figure 9.
Evolution of in situ poles figures for the (002) reflection of kaolinite within sample KCS100 for different temperatures.
Figure 9.
Evolution of in situ poles figures for the (002) reflection of kaolinite within sample KCS100 for different temperatures.
Figure 10.
Evolution of in situ poles figures for the (111) reflection of kaolinite within sample KCS100 for different temperatures.
Figure 10.
Evolution of in situ poles figures for the (111) reflection of kaolinite within sample KCS100 for different temperatures.
Figure 11.
variation of the diffracted intensity regarding the temperature and the tilt angle χ for sample H100; (002) reflections.
Figure 11.
variation of the diffracted intensity regarding the temperature and the tilt angle χ for sample H100; (002) reflections.
Figure 12.
Evolution of in situ poles figures for the (002) reflection of halloysite within sample H100 for different temperatures.
Figure 12.
Evolution of in situ poles figures for the (002) reflection of halloysite within sample H100 for different temperatures.
Figure 13.
variation of the diffracted intensity regarding the temperature and the tilt angle χ for: a) and b) (002) and (111) reflections of KRG50H50 sample; c) and d) (002) and (111) reflections of KCS50H50 samples.
Figure 13.
variation of the diffracted intensity regarding the temperature and the tilt angle χ for: a) and b) (002) and (111) reflections of KRG50H50 sample; c) and d) (002) and (111) reflections of KCS50H50 samples.
Figure 14.
results of in situ X-ray diffusion analyses for samples a) KCS100, b) H100 et c) KRG100.
Figure 14.
results of in situ X-ray diffusion analyses for samples a) KCS100, b) H100 et c) KRG100.
Figure 15.
simplified illustration of the transformation sequence of kaolinite up to 1000°C.
Figure 15.
simplified illustration of the transformation sequence of kaolinite up to 1000°C.
Table 1.
Chemical and physical characteristics of raw materials halloysite H, kaolins KCS and KRG (Tr.: < 0.2 mass %).
Table 1.
Chemical and physical characteristics of raw materials halloysite H, kaolins KCS and KRG (Tr.: < 0.2 mass %).
| |
H |
KCS |
KRG |
| Oxides (mass %) |
Al2O3 |
37.15 |
39.54 |
39.98 |
| SiO2 |
47.47 |
44.06 |
44.77 |
| P2O5 |
0.15 |
0.05 |
0.03 |
| SO3 |
0.04 |
0.11 |
0.06 |
| Fe2O3 |
0.39 |
0.63 |
0.62 |
| ZrO2 |
0.02 |
0.02 |
0.03 |
| K2O |
|
0.04 |
0.05 |
| TiO2 |
|
0.58 |
0.41 |
| Na2O |
|
0.18 |
|
| SiO2/ Al2O3 (mass ratio) |
1.28 |
1.11 |
1.12 |
| Loss on ignition at 1050°C (mass %) |
14.76 |
14.78 |
14 |
Table 2.
density, particle size distribution and specific surface area of starting clay powders.
Table 2.
density, particle size distribution and specific surface area of starting clay powders.
| |
H |
KCS |
KRG |
| Density (g/cm3) |
2.54 |
2.61 |
2.59 |
| Grain size (µm) |
D10
D50
D90 |
0.30
2.00
12.54 |
2.36
4.73
8.09 |
5.66
8.57
12.80 |
| Type of distribution |
trimodal |
bimodal |
monomodal |
| Specific BET surface area (m2/g) |
26.28 |
9.29 |
6.61 |
Table 3.
crystallinity indexes HI, R2 (± 0.1) and L of kaolins KCS and KRG compared to reference kaolins.
Table 3.
crystallinity indexes HI, R2 (± 0.1) and L of kaolins KCS and KRG compared to reference kaolins.
| |
KGa-1b[116] [117] |
KGa-2[116] [117] |
KCS |
KRG |
| HI |
1,03 |
0,37 |
1,28 |
1,22 |
| R2 |
1,00 |
0,50 |
1,09 |
1,13 |
| L |
127 |
40 |
70 |
73 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).