1. Introduction
Head and Neck Sarcoma (HNS) are rare malignant tumors derived from mesenchymal cells characterized by rapid growth, local aggressive behavior, and multiple relapses. Several subtypes are recognized based on type of differentiation and clinicopathological features. For example, while in rhabdomyosarcoma (RMS) the cells show skeletal muscle differentiation and higher incidence in the childhood, osteosarcoma (OS) is characterized by tumor cells with osteoblastic features and main incidence in the third decade of life (1).
Based on the histological subtype, primary anatomical location, extent of surgical margins status, extracapsular extension in regional lymph nodes and metastatic lesions, multimodal treatment may be established and might include neoadjuvant or adjuvant chemotherapy (CTX), surgery and even adjuvant radiotherapy (RT). Regarding RMS, the role of RT remains uncertain, but retrospective studies have shown there seems to be bene-fit in local control, especially for embryonal or alveolar subtypes, besides in the scenario of advanced and recurrence disease (2)(3)(4).
The differences between RMS and OS are that the last one is more prevalent in the adult age (fourth to fifth decade of life) than the childhood, and are more common in the jawbones, basically, in the mandible (7% of all OSs described in the maxillofacial region) and represents 1% of malignant neoplasm in the head and neck (HN) region (5).
In the HN region, the factors associated with OS have not yet been established, but previous RT in the region could be considered as a trigger factor for radiation-induced sarcomas (RIS)(5). On the other hand, the differential diagnosis must be performed with other lesions of similar clinical behavior such as fibro-osseous lesions, odontogenic infections represented by osteomyelitis of the jaw bones among others.
OSs are associated with moderate to high rates of local recurrence, mainly associated with positive surgical margins, and with distant metastasis in up to 30% of cases (5)(6). For this reason, they are treated in a multimodal approach that may increase OSS rates when the combination of therapies such as neoadjuvant CTX, surgery and RT are per-formed (4)(5).
The role of RT in the treatment of these tumors is still uncertain. Some studies referred to an improvement in OSS and DFS rates with the addition of RT after surgery (5)(7). However, other studies have shown that RT performed exclusively has no advantages observed in the scenario of surgical margins microscopically compromised after surgery (5)(7)(8).
The clinical impact of RT in the context of multimodal treatment in OS is still controversial. For this reason, the present study aims to evaluate the clinical impact of adjuvant radiotherapy by survival rates analysis in patients diagnosed with HNS submitted to multimodal treatment at A.C. Camargo Cancer Center, Sao Paulo, Brazil between 2006 and 2020.
2. Material and Methods
2.1. Population, Samples, and Ethical Approval
This is an observational, descriptive, and retrospective analysis of electronic medical records of 39 patients diagnosed with HNS and submitted to multimodal treatment. Four groups were formed: a) patients who underwent surgery and chemotherapy CTX; b) patients who had surgery, CTX, and RT; c) patients who had surgery and RT; d) patients who had CTX and RT exclusively. Regarding RT performed, total dose, technique, field of irradiation and RT duration were described. This study was submitted for ethical approval by the Research Ethics Committee (REC) of the A.C. Camargo Cancer Center, Sao Paulo, Brazil, protocol number 5.693.810 and ethics code: 3002/20.
2.2. Demographic and Clinical Data Collected
Demographic data of patients of each group were collected, such as: age, gender, date of primary diagnosis, local tumor, recurrence date and the type of treatment performed (monotherapy or multimodal approach). Moreover, regarding histopathological subtype and other tumor-associated morphologic criteria were retrieved such as: clinical staging, depth of invasiveness, perineural and angiolymphatic invasion, number of positive lymph nodes, presence of distant metastases, surgical margins status and extranodal extension of the tumor with node metastasis.
2.3. Statistical Analysis
Data were described as absolute and relative frequency for categorical (qualitative) and by mean, standard deviation (SD), and range for numerical (quantitative) categories. To evaluate the association between qualitative variables, the independence test (Chi-square test with continuity correction or Fisher's exact test) was performed. Moreover, OSS, DFS and PFS rates were assessed using Kaplan-Meier and Log-Rank tests
3. Results
3.1. Descriptive Analysis Regarding Demographic, Clinical and Therapeutic Data
Among 39 patients, 64.1% were male and 35.9% female; 36.8% were young adults, 27.8% under 18 years and 10.2% older than 60 years. Moreover, 23.1% were diagnosed with RMS, 66.7% with OS, and 10.3% others histological subtypes. Local primary tumor site was mandible for 61.5%, 33.3% in maxilla and 5.1% in other areas. On the other hand, 59% showed early clinical stages (T1/T2) and 41% advanced stages (T3/T4) at the primary diagnosis. Surgery was performed in 87.2%, RT in 35.9% and CTX in 84.7% of population analyzed. RT was performed with non-IMRT techniques in 10 patients (71.4%) and IMRT in 4 patients (28.5%). These data are shown in
Table 1.
Table 2 shows the histological variants for each sarcoma above described to identify histopathological characteristics as prognostic factor and therapeutic planning:
Table 3 shows CTX regime as neoadjuvant and adjuvant therapy in multimodal treatment for patients diagnosed with HNS. Moreover, it was included data regarding patients submitted to salvage CTX:
3.2. Chi-Square Test
Table 4 shows the analysis of association and independence performed by Chi-square and Fischer’s exact tests, between demographic data, histological subtype, morphologic tumor criteria and therapeutic performed. A statistically significant p value of <=0.05 was considered. Statistical association was established between age, extranodal extension (ENE), RT performed and histological subtype with p values of 0.055, 0.03, 0.05, respectively.
3.3. OSS, DFS and PFS Rates
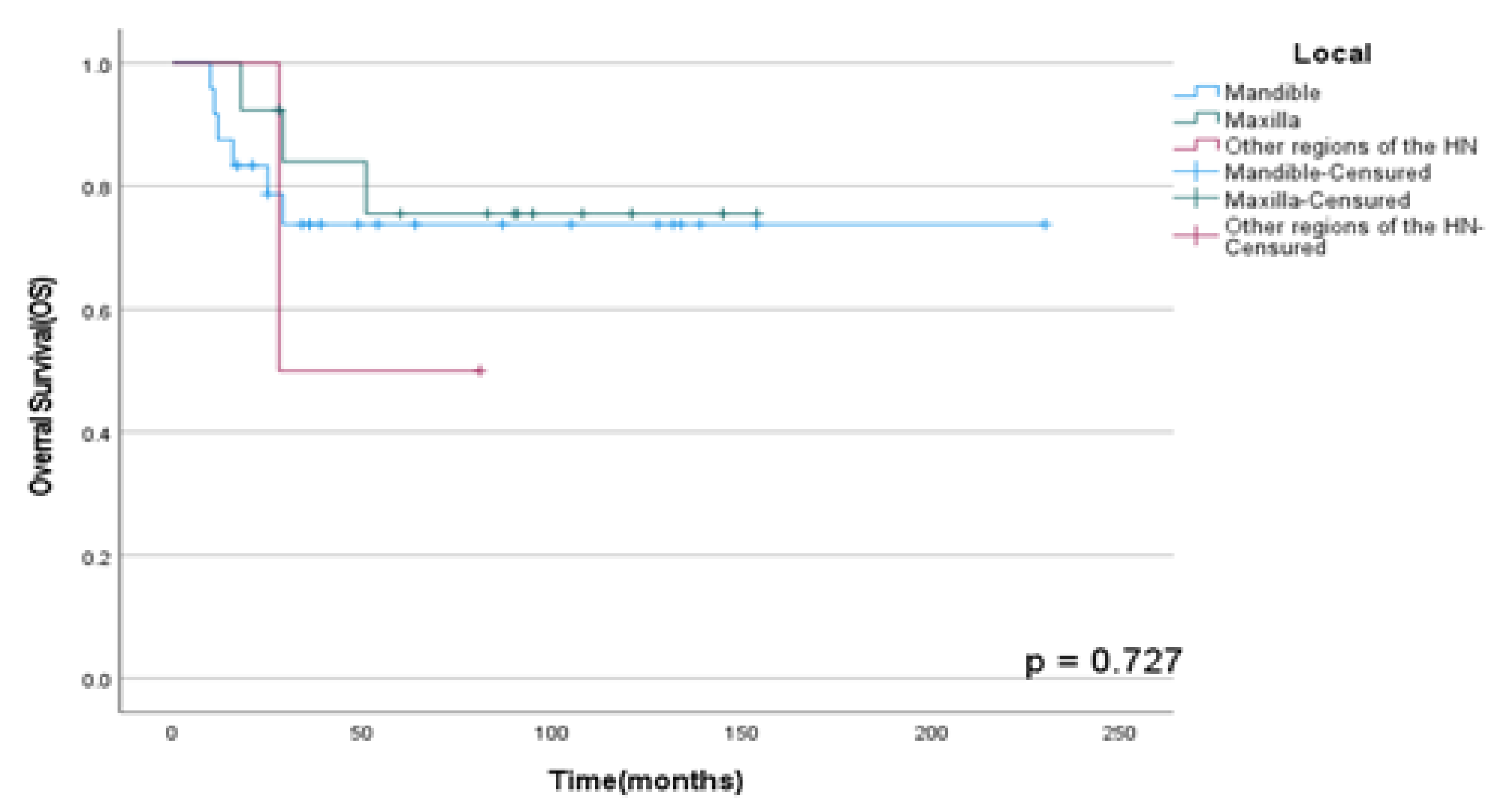
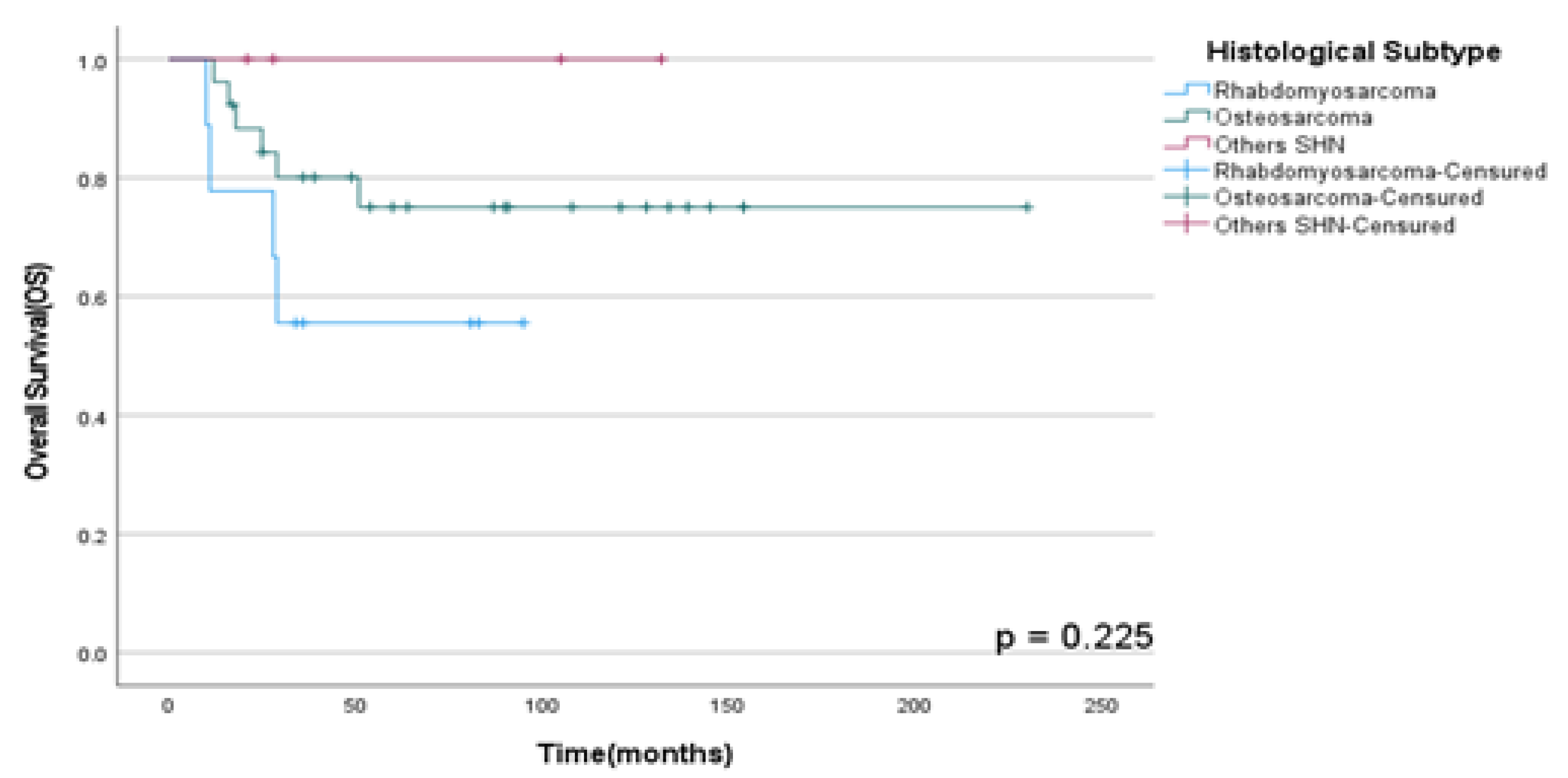
OSS rates in HNS according to primary local site and histological subtype (RMS, OS, and other HNS) were not statistically significant with p=0.73 and p =0.23 respectively as shown in
Figure 1 and
Figure 2.
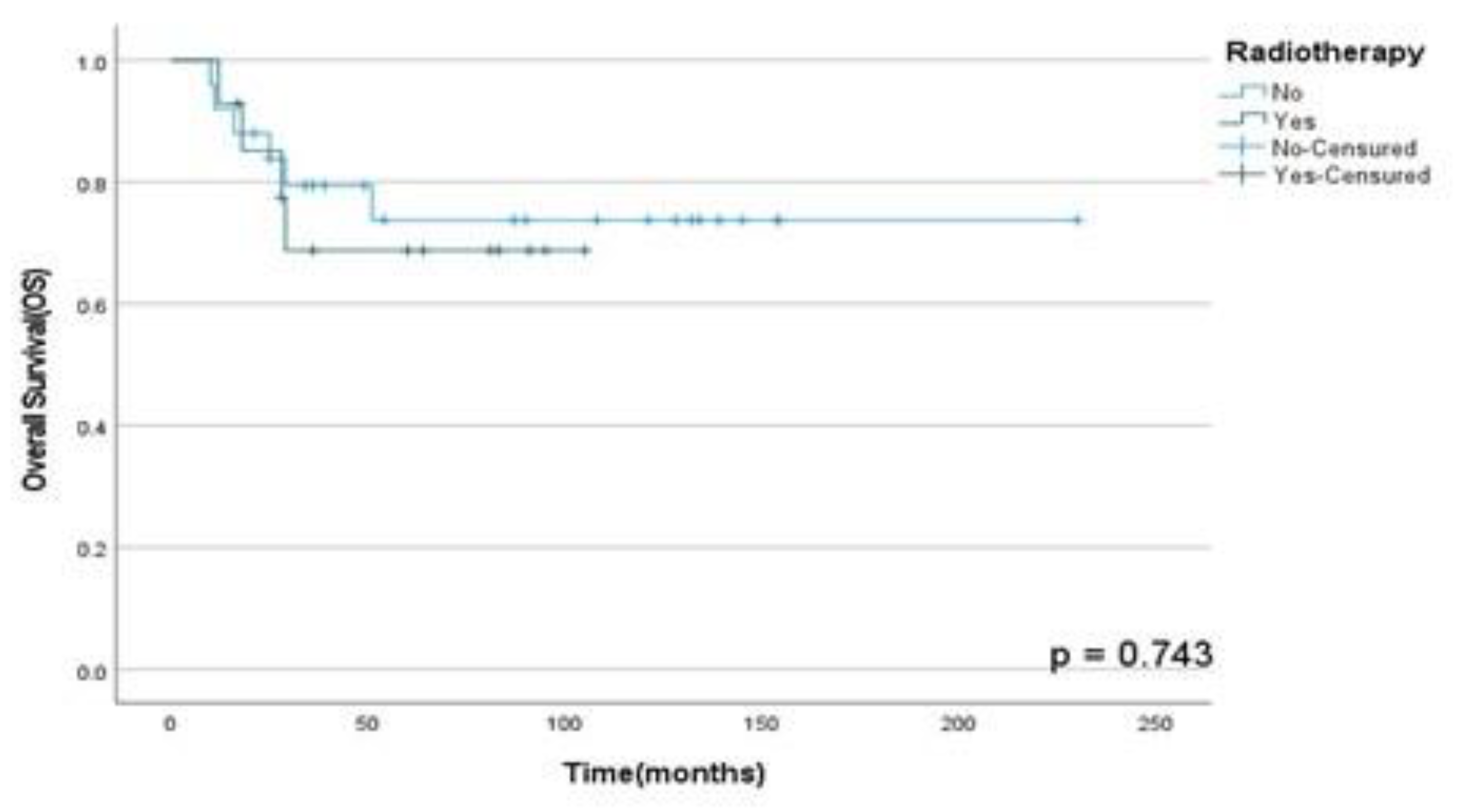
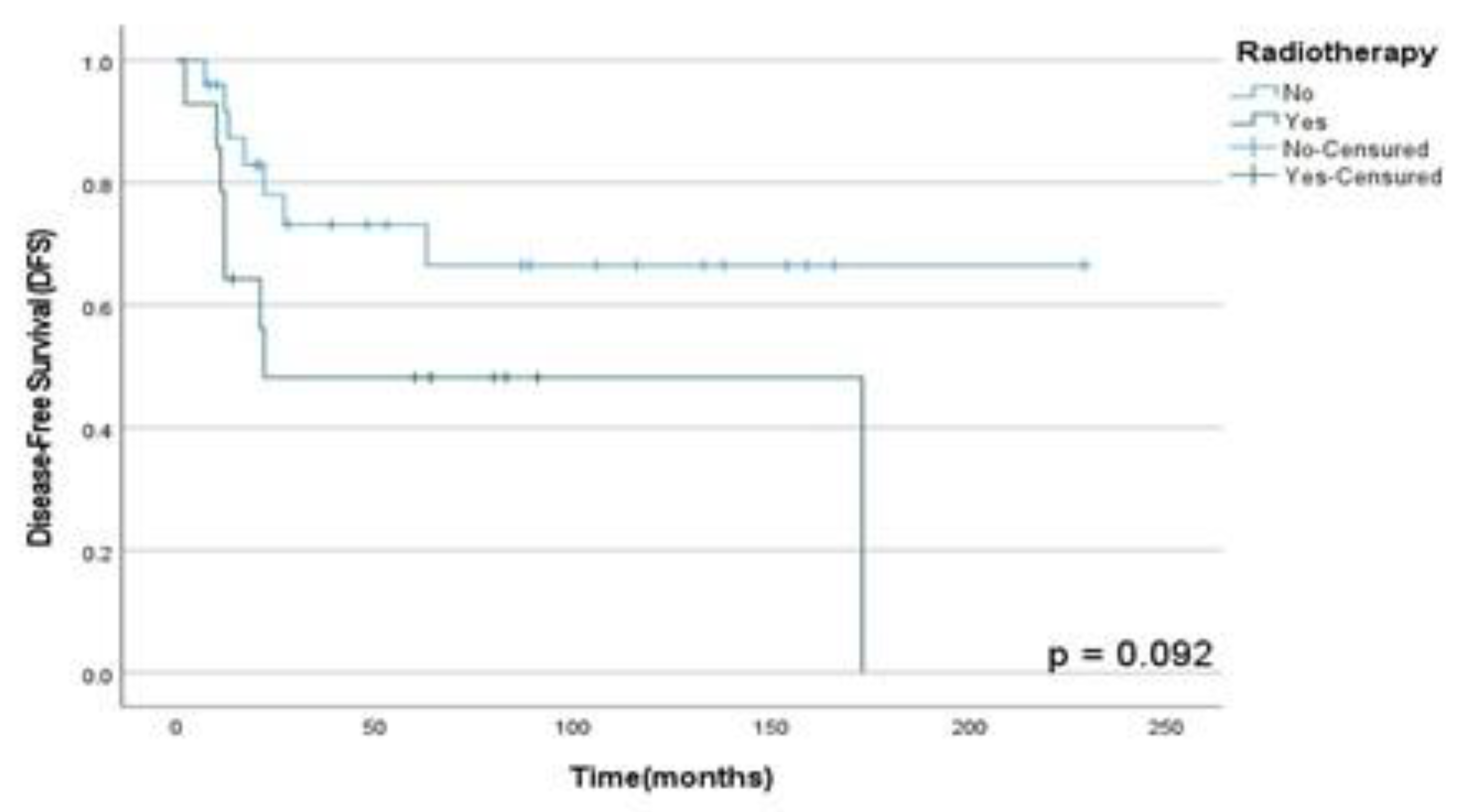
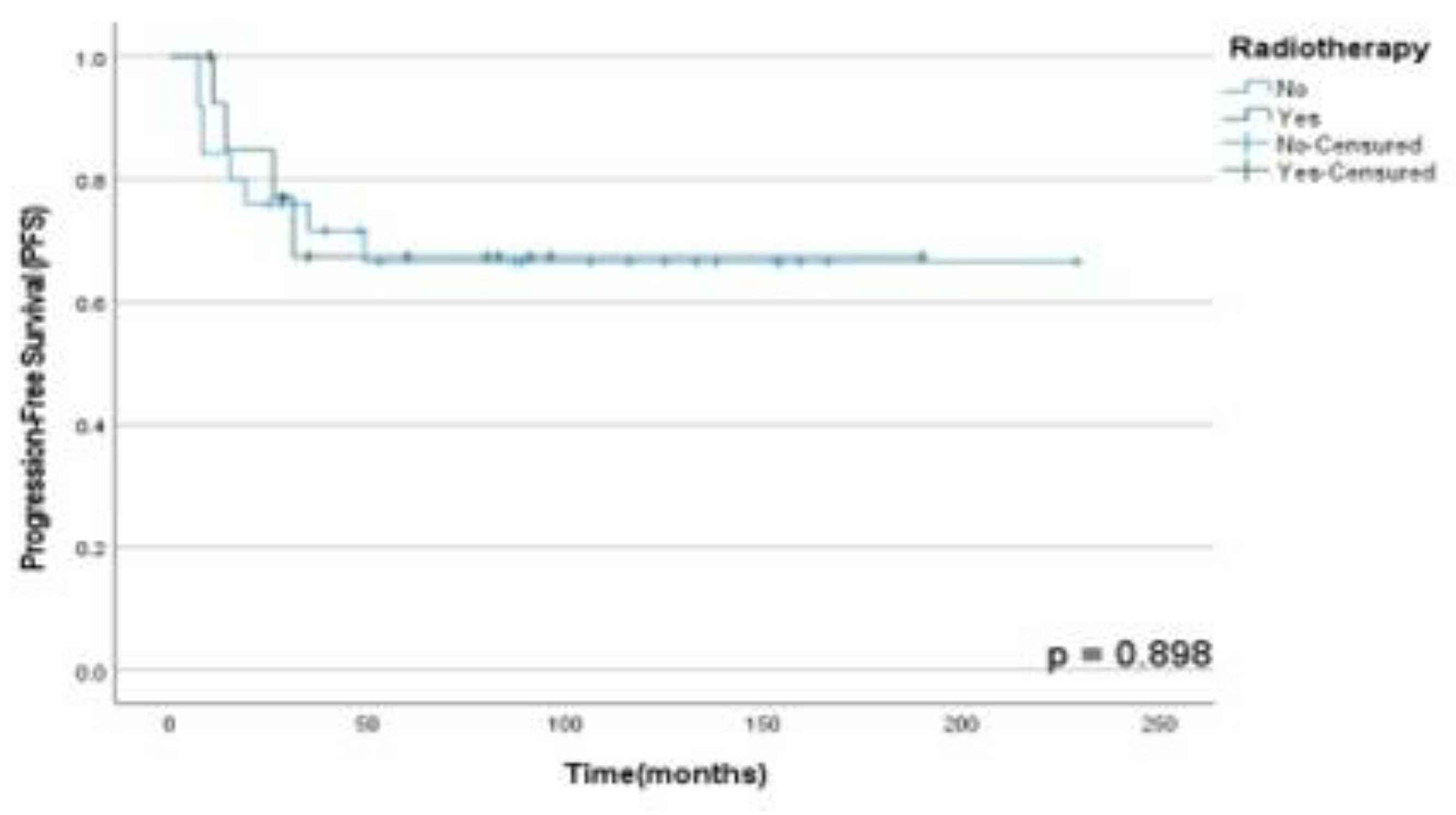
Moreover, it was found that RT in multimodal treatment has not a statistically significant role in OSS (p=0.74), DFS (p= 0.09) and PFS (p=0.90) rates in our population analyzed as shown in
Figure 3,
Figure 4 and
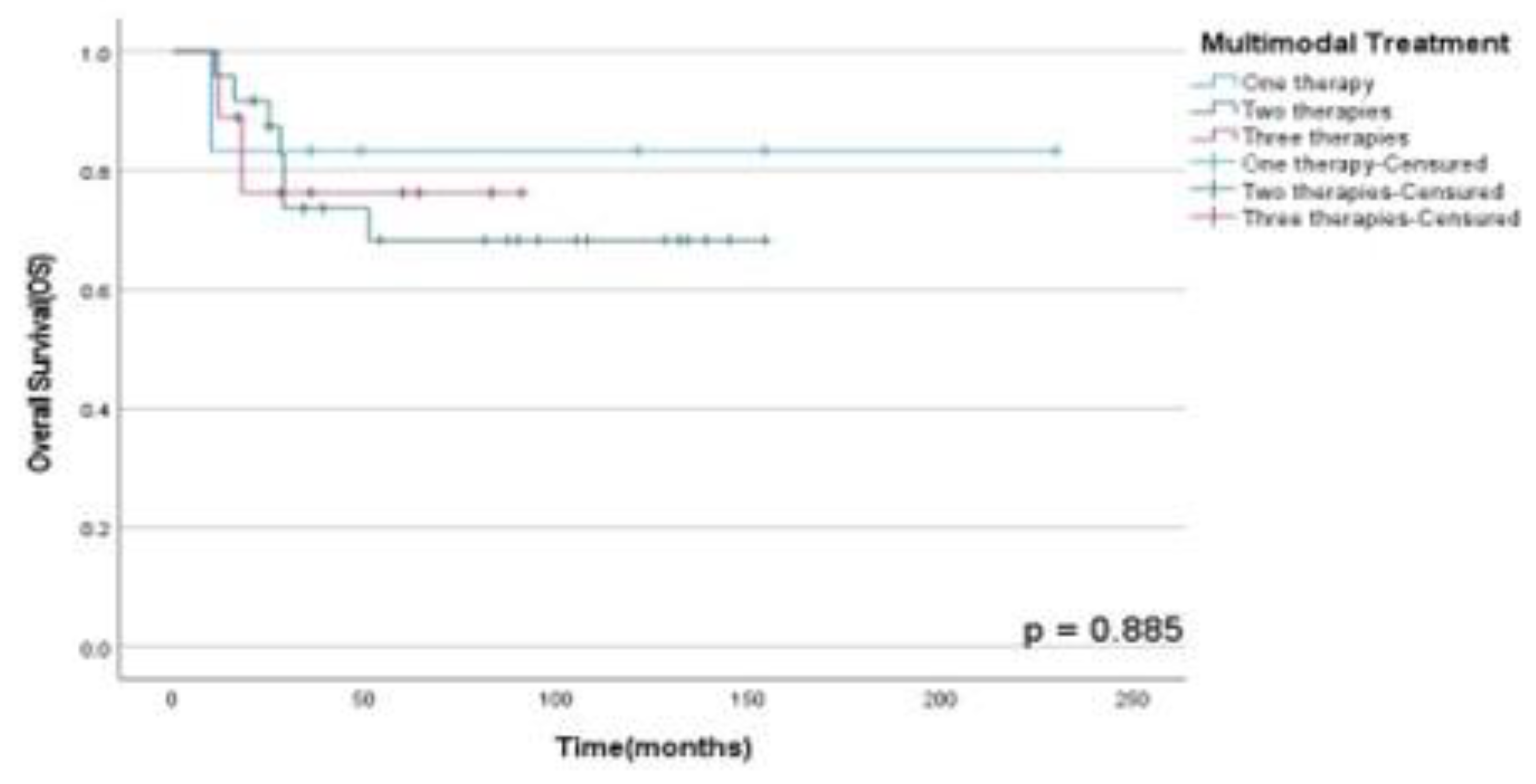
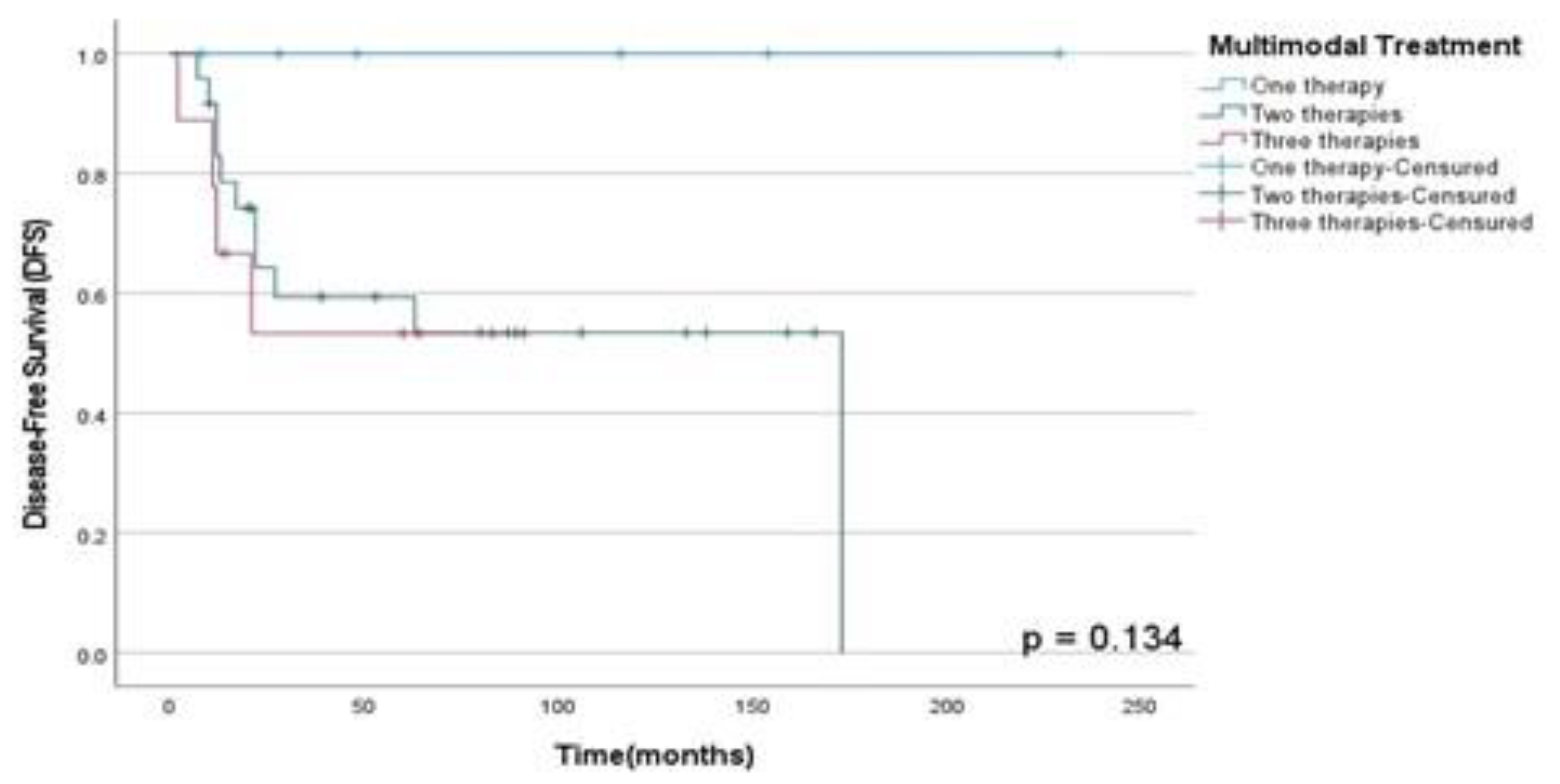
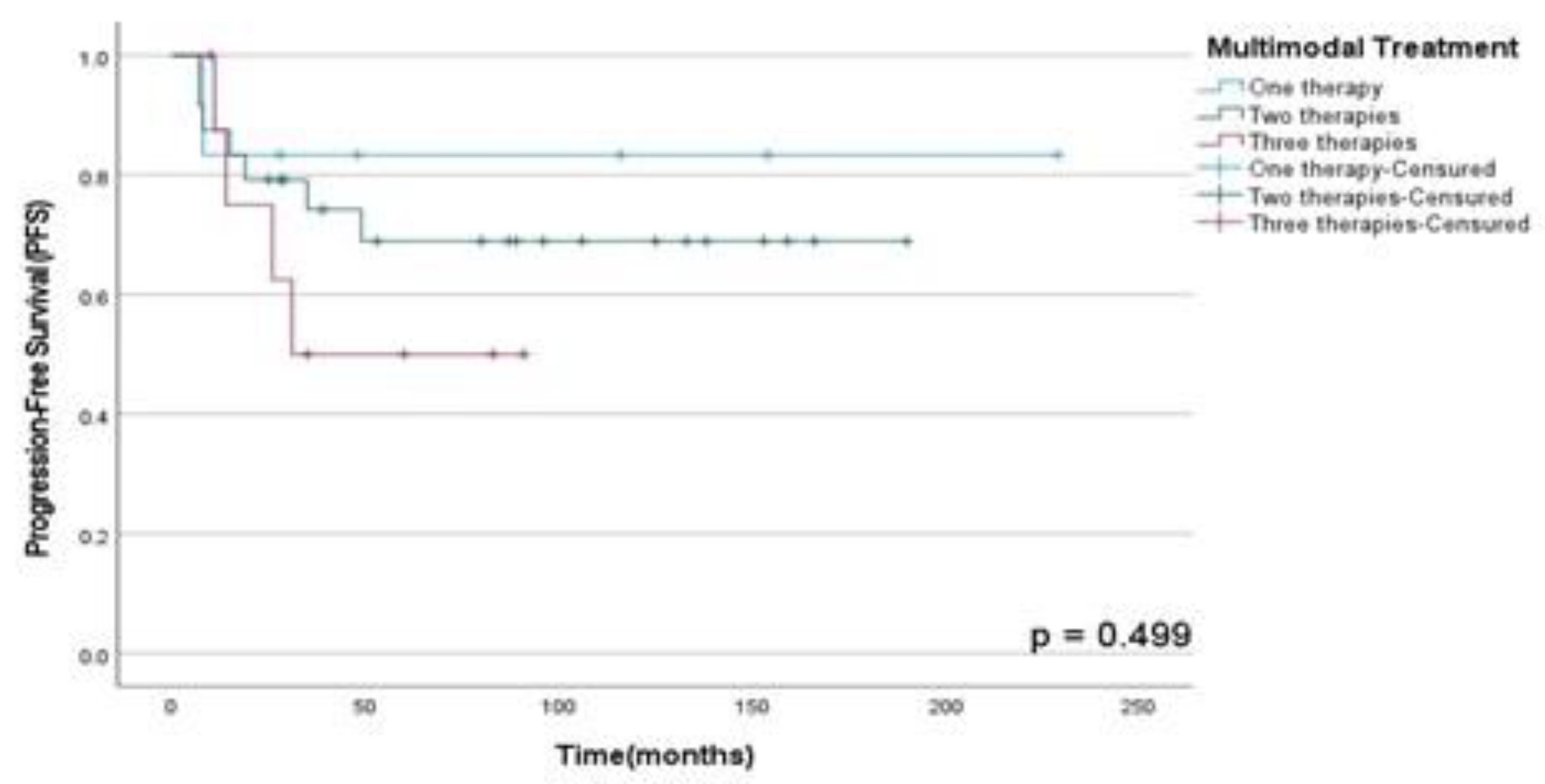
Figure 5. Finally, there were no statistically significant difference between OSS (p=0.89), DFS (p=0.13) and PFS (p=0.50) rates between patients submitted to monotherapy and multimodal therapy as observed in
Figure 6,
Figure 7 and
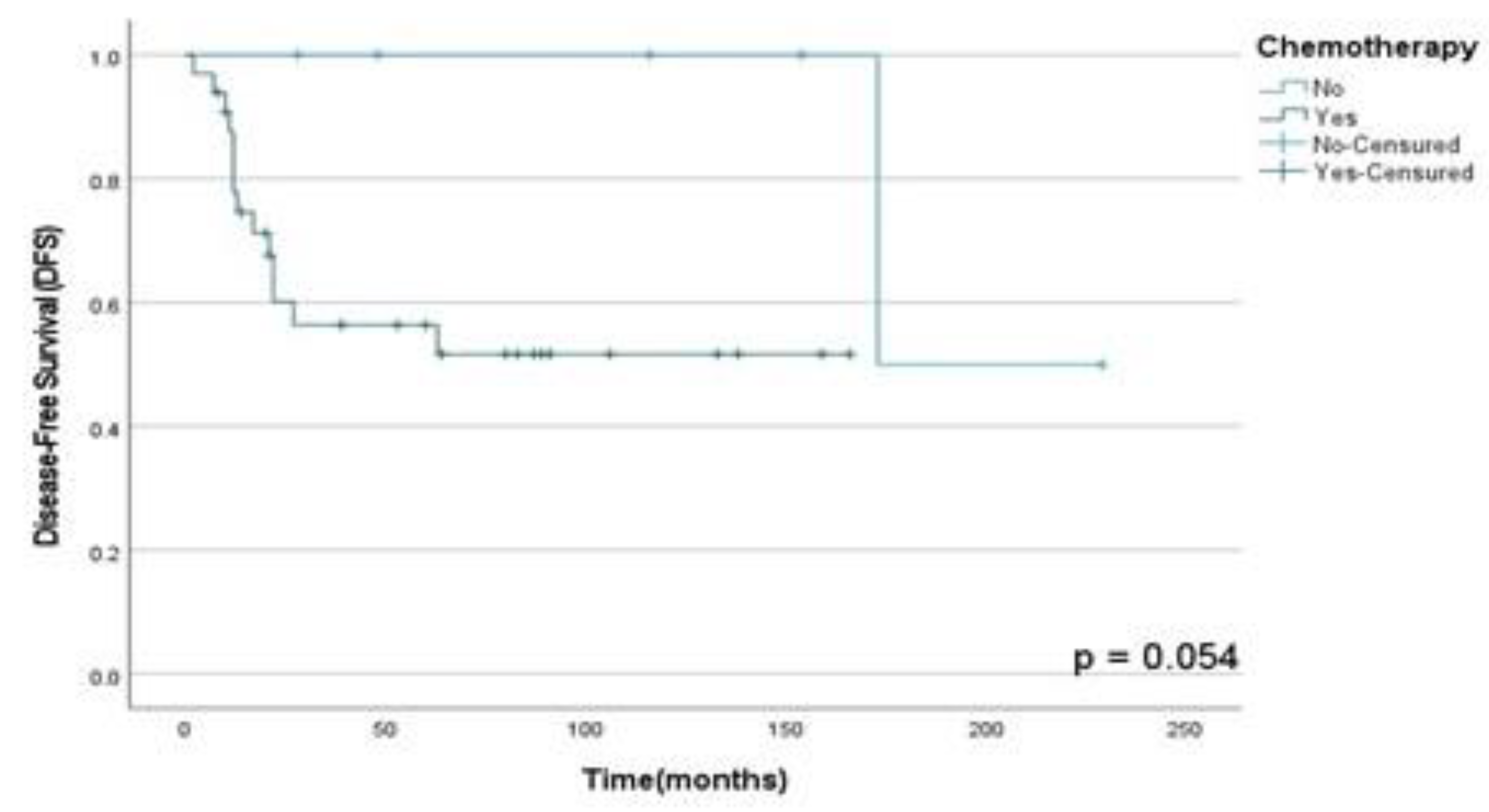
Figure 8; however, those who did CTX showed a decrease in the DFS rate than those who did not (p=0.05) in this population assessed as shown in
Figure 9.
4. Discussion
In our population the predominant histological subtype observed was OS followed by RMS, which was more frequent in younger patients, conversely to described in the current literature (9), who mentions that both are common in children. Furthermore, in our study, most patients (64.1%) were aged between 19 and 60 years, which is like reported in another study conducted by Frankart et al. (10), who describes the peak age incidence of RMS is between 3 and 5 years in 70% of these patients. Currently, the treatment is based on a multimodal approach that includes surgical removal followed by other adjuvant therapies (11).
From our results, 87.2% of the patients underwent surgical treatment, 46.2% adjuvant chemotherapy, and only 35.9% adjuvant RT. Thus, patients who underwent multimodal therapy survived longer with a 4-years OSS rate of 82% than patients who did only one therapy exclusively.
In our cohort, 61.5% of the patients had the primary tumor in the mandible and 33.3% in the maxilla. Moreover, patients who were diagnosed HNS in the mandible showed an increased rate of disease progression and recurrent lesions than in others local onset, in accordance with the contemporary literature (12)(13). The complex anatomic topography of the HN region and, consequently, the proximity to surrounding critical organs that cause difficulties for surgical removal without free margins could explain what mentioned above.
Among the use of multimodal approach in advanced or recurrent disease, neoadjuvant or adjuvant CTX, surgery and RT showed increased DFS rates, about 60-70% after 5-years, depending on the specific histological subtype or presence of metastatic disease, as described in the literature (9)(14)(15). Thus, according to the DFS rates in our study for patients who underwent multimodal therapy with 2 therapies showed a three-years DFS of 56% and those underwent 3 therapies a three-years DFS rate of 60%, like results found in the contemporary literature.
A recent study described that surgery is the gold standard treatment for patients diagnosed with OS (14); however, for the pediatric population, the combination of CTX + RT is commonly indicated. In our study, patients who underwent surgery showed increased survival rates for all HNS, with a 4-years actuarial OSS rate of 79% and 40% for non-surgical patients. .
CTX for RMS treatment as neoadjuvant or adjuvant therapy remains the gold standard in advanced lesions as described by Youteng et. al. (4); however, there are some limitations for the use in the childhood caused by side effects onset in these patients such as mucositis lesions in the oral cavity associated with lower immunity. On the other hand, in our results patients who underwent CTX as part of multimodal approach showed a 4-years actuarial OSS of 58% when compared with those who did not.
Moreover, in our study patients who underwent RT showed improved OSS, DFS and PFS rates than those who did not. Although a recent study showed lower survival rates in patients diagnosed with HNS and undergoing adjuvant RT in the first 3-years after treatment; our results were opposed to them. Thus, DFS rates in 3 years were reduced in 33% in all histological subtypes (HNS) assessed in this study; however, the RMS group showed less recurrence rates than other HNS that did not RT for primary tumor treatment. Therefore, RT as part of multimodal approach did not show a higher statistically survival rates in this population, so it could be associated with a low number of patients enrolled in our study, so a greater cohort of patients and longitudinal studies must be performed to clarify the role of therapy in the multimodal approach in this heterogeneous population including soft and hard tissues HNS.
5. Conclusions
According to our results, RT performed for primary HNS was not associated with improved OSS, DFS and PFS rates regardless of the age, gender, local primary tumor onset and histological subtype; however, multimodal approach might increase survival rates and locoregional control in the scenario of advanced and recurrent disease. Longitudinal studies, such as randomized or non-randomized clinical trials, must be conducted to validate our results.
Author Contributions
Conceptualization, W.E. B-P.; Data curation, W.E. B-P., E.P.F., C.H.A.; K.M.T., K.A.V-R., J.A.M-Z; Formal analysis, WW.E. B-P.; Funding acquisition, W.E. B-P.; Investigation, W.E. B-P.; Methodology, W.E. B-P., E.P.F. and A.C. d. A.P.; Resources, W.E. B-P., E.P.F. and A.C.A.P; Validation, W.E. B-P.; E.P.F.; J.G.V.; M.C.C; K.M.T.; K.A.V-R.; J.A. M-Z.; F.D.C. and A.C.A.P.; Writing – original draft, W.E. B-P.; Writing – review & editing, W.E. B-P., E.P.F., C.H.A., J.G.V., M.C.C.; K.M.T; K.A.V-R.; J.A.M-Z.; F.D.C., and A.C.A.P.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq) of Brazil (140071/2019-9).
Institutional Review Board Statement
Regarding the ethical approval, the number protocol was 5.693.810 / 3002/20 by our Ethics Institutional Committee from A.C. Camargo Cancer Center, Sao Paulo, Brazil.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper and it was forward to Editors.
Data Availability Statement
Acknowledgments
The authors thank the full Oncological Departments(Radiation Oncology, Head and Neck Surgery & Otorhinolaryngology and Anatomic Pathology) from A.C. Camargo Cancer Center, Sao Paulo, Brazil, and their teams who supported this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, P.; Surya, V.; Urs, A.B.; Augustine, J.; Mohanty, S.; Gupta, S. Sarcomas of the oral and maxillofacial region: analysis of 26 cases with emphasis on diagnostic challenges. Pathology & Oncology Research. 2019, 25, 593–601. [Google Scholar]
- Iatrou, I.; Theologie-Lygidakis, N.; Schoinohoriti, O.; Tzermpos, F.; Vessala, A.M. Rhabdomyosarcoma of the maxillofacial region in children and adolescents: report of 9 cases and literature review. Journal of Cranio-Maxillofacial Surgery. 2017, 45, 831–8. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yin, X.; Xu, W.; Wang, Y.; Han, W. The management of head and neck sarcoma. Journal of Craniofacial Surgery. 2020, 31, e189–92. [Google Scholar] [CrossRef] [PubMed]
- Yunteng, W.; Xuhui, M.; Guoxin, R.; Wei, G. Radical surgery for head and neck rhabdomyosarcoma failed primary chemotherapy. Journal of Craniofacial Surgery. 2019, 30, e113–6. [Google Scholar] [CrossRef] [PubMed]
- Ferrari D, Codecà C, Battisti N, Broggio F, Crepaldi F, Violati M, et al. Multimodality treatment of osteosarcoma of the jaw: a single institution experience. Medical Oncology. 2014, 31, 1–7. [Google Scholar]
- Kämmerer, P.W.; Shabazfar, N.; Makoie, N.V.; Moergel, M.; Al-Nawas, B. Clinical, therapeutic and prognostic features of osteosarcoma of the jaws–experience of 36 cases. Journal of Cranio-Maxillofacial Surgery. 2012, 40, 541–8. [Google Scholar] [CrossRef] [PubMed]
- Krasin MJ, Wiese KM, Spunt SL, Hua C ho, Daw N, Navid F, et al. Jaw dysfunction related to pterygoid and masseter muscle dosimetry after radiation therapy in children and young adults with head-and-neck sarcomas. International Journal of Radiation Oncology* Biology* Physics. 2012, 82, 355–60. [Google Scholar] [CrossRef] [PubMed]
- Goosens, V.; van den Berghe, I.; de Clercq, C.; Casselman, J. Radiation-induced mandibular adult spindle cell rhabdomyosarcoma. Int J Oral Maxillofac Surg. 2008, 37, 395–7. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Tejada, F.N.; Zamudio, A.; Marques-Piubelli, M.L.; Cuglievan, B.; Harrison, D. Advances in the management of pediatric sarcomas. Curr Oncol Rep. 2021, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Frankart, A.J.; Breneman, J.C.; Pater, L.E. Radiation Therapy in the Treatment of Head and Neck Rhabdomyosarcoma. Cancers (Basel). 2021, 13, 3567. [Google Scholar] [CrossRef] [PubMed]
- Mazic, M.C.; Bonevski, A.; Mikeskova, M.; Mihut, E.; Bisogno, G.; Jazbec, J. Treatment of rhabdomyosarcoma in children and adolescent from four low health expenditures average rates countries. Radiology and Oncology. 2020, 54, 455–60. [Google Scholar] [CrossRef] [PubMed]
- Yang J, Gao J, Qiu X, Hu J, Hu W, Wu X, et al. Intensity-Modulated Proton and Carbon-Ion Radiation Therapy in the Management of Head and Neck Sarcomas. Cancer Medicine. 2019, 8, 4574–86. [Google Scholar] [CrossRef] [PubMed]
- Akagündüz B, Telli TA, Goksu SS, Yildirim HC, Ozer M, Aydin SG, et al. Assessment of prognostic factors and adjuvant treatment modalities in adult head and neck soft tissue sarcoma patients treated with upfront surgery. Cureus. 2021, 13. [Google Scholar]
- Owosho AA, Xu B, Kadempour A, Yom SK, Randazzo J, Ghossein RA, et al. Metastatic solid tumors to the jaw and oral soft tissue: a retrospective clinical analysis of 44 patients from a single institution. Journal of Cranio-Maxillofacial Surgery. 2016, 44, 1047–53. [Google Scholar] [CrossRef] [PubMed]
- Owosho AA, Brady P, Wolden SL, Wexler LH, Antonescu CR, Huryn JM, et al. Long-term effect of chemotherapy–intensity-modulated radiation therapy (chemo-IMRT) on dentofacial development in head and neck rhabdomyosarcoma patients. Pediatr Hematol Oncol. 2016, 33, 383–92. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
OSS rates according to local tumor onset in patients diagnosed with HNS and submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2019.
Figure 1.
OSS rates according to local tumor onset in patients diagnosed with HNS and submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2019.
Figure 2.
OSS rates according to histological subtype in patients diagnosed with HNS and submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2019.
Figure 2.
OSS rates according to histological subtype in patients diagnosed with HNS and submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2019.
Figure 3.
OSS rates according to RT performed in patients diagnosed with HNS and submitted to monotherapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 3.
OSS rates according to RT performed in patients diagnosed with HNS and submitted to monotherapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 4.
DFS rates according to the RT performed for primary tumor treatment in patients diagnosed with HNS and submitted to multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 4.
DFS rates according to the RT performed for primary tumor treatment in patients diagnosed with HNS and submitted to multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 5.
PFS rates according to RT performed in patients diagnosed with HNS and submitted to unique therapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 5.
PFS rates according to RT performed in patients diagnosed with HNS and submitted to unique therapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 6.
OSS rates according to treatment performed in patients diagnosed with HNS and submitted to monotherapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 6.
OSS rates according to treatment performed in patients diagnosed with HNS and submitted to monotherapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 7.
DFS rates according to treatment performed in patients diagnosed with HNS and submitted to unique therapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 7.
DFS rates according to treatment performed in patients diagnosed with HNS and submitted to unique therapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 8.
PFS rates according to treatment performed in patients diagnosed with HNS and submitted to monotherapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 8.
PFS rates according to treatment performed in patients diagnosed with HNS and submitted to monotherapy or multimodal approach at A.C. Camargo Cancer Center between 2006-2019.
Figure 9.
DFS rates according to CTX performed in patients diagnosed with HNS and submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2019.
Figure 9.
DFS rates according to CTX performed in patients diagnosed with HNS and submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2019.
Table 1.
Demographic, clinical, morphologic, and therapeutic data of patients with HNS submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2020.
Table 1.
Demographic, clinical, morphologic, and therapeutic data of patients with HNS submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2020.
| Demographic, clinical, morphologic, and therapeutic data |
Number of patients (n) |
Percentage (%) |
Gender
Female
Male
Total |
14
25
39 |
35,9%
64,1%
100% |
Age
0-18 years
19 to 60 years
More than 60 years
Total |
10
25
4
39 |
25,7%
64,1%
10,2%
100% |
Primary tumor site
Mandible
Maxilla
Others
Total |
24
13
2
39 |
61,5%
33,3%
5,1%
100% |
Histological subtype
RMS
OS
Others
Total |
9
26
4
39 |
23,1%
66,7%
10,3%
100% |
Clinical staging (cT)
Tx/T1/T2
T3/T4
Total |
23
16
39 |
59,0%
41,0%
100% |
Perineural invasion (PI)
Yes
No
Total |
3
32
35 |
8,5%
91.5%
100% |
Lymph nodes (LNDs)
Yes
No
Total |
1
34
35 |
2.9%
97.1%
100% |
Surgery
Surgery
Amplified surgery
Surgery + Microsurgical reconstruction
No surgery
Total |
3
4
27
5
39 |
7.7%
10.3%
69.2%
12.8%
100% |
Chemotherapy (CTX)
Neoadjuvant
Adjuvant cc with RT
Adjuvant
No CTX
Total |
13
2
18
6
39 |
33.3%
5.2%
46.2%
15.4%
100% |
Radiotherapy (RT)
Yes
No
Total |
14
25
39 |
35.9%
64.1%
100% |
RT technique
IMRT
Non-IMRT
Total |
4
10
14 |
28.5%
71.4%
100% |
Table 2.
Descriptive analysis of histological variants for each HNS in patients submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2020.
Table 2.
Descriptive analysis of histological variants for each HNS in patients submitted to multimodal treatment at A.C. Camargo Cancer Center between 2006-2020.
| Histological Subtype |
Number of patients
(n) |
Percentage
(%) |
RMS
Embryonal
Alveolar
Non-Paramenyngeal
Paramenyngeal
Total |
6
1
1
1
9 |
23.1%
|
OS
Osteoblastic
Chondroblastic
Fibroblastic
Mixed
High-Grade
Low-Grade
Total |
11
9
2
2
1
1
26 |
66.7%
|
Other HNS
Spindle
Ewing
Others
Total |
2
1
1
4 |
10.3%
|
Table 3.
CTX regime as neoadjuvant or adjuvant therapy in patients submitted to multimodal treatment for HNS at A.C. Camargo Cancer Center between 2006-2020.
Table 3.
CTX regime as neoadjuvant or adjuvant therapy in patients submitted to multimodal treatment for HNS at A.C. Camargo Cancer Center between 2006-2020.
| Chemotherapy (CTX) |
Number of patients
(n) |
Percentage
(%) |
Neoadjuvant CTX
EpSGG protocol
CDDP + Doxorubicin
IRS-IVA
IFO + ADRIA
VCR + Doxorubicin
VAC
CDDP
IFO + Doxorubicin
SIOP
Total |
3
3
1
1
1
1
1
1
1
13 |
33.3% |
Adjuvant CTX
Standard Risk
Intergroup
VC
CDDP + Doxorubicin
IFO + VP
GBTO
Total |
1
1
1
13
3
1
20 |
51.28% |
Salvage CTX
IFO + VP
IFO + Etoposide
ICE
CDDP + Doxorubicin
IFO
Irinotecan + VCR
Topotecan + Cyclophosphamide
Total |
4
2
2
1
1
1
1
12 |
30.75%
|
Non-CTX |
6 |
15.38% |
Table 4.
Chi-square test for statistical analysis from clinical, morphologic tumor criteria and therapeutic data versus histological subtype of patients diagnosed with HNS submitted to multimodal treatment at A.C. Camargo Cancer Center during 2006-2020.
Table 4.
Chi-square test for statistical analysis from clinical, morphologic tumor criteria and therapeutic data versus histological subtype of patients diagnosed with HNS submitted to multimodal treatment at A.C. Camargo Cancer Center during 2006-2020.
| Clinical, morphologic, and therapeutic data/ Histological subtype |
RMS
(n) |
OS
(n) |
Other HNS
(n) |
Gender
Male
Female
Total
p value |
7
2
9
0.52 |
15
11
26 |
3
1
4 |
Age
0-12 years
o 18 years
19 to 35 years
36 to 60 years
>61 years
Total
p value |
4
2
1
2
0
9
0.055 |
2
1
12
7
3
25 |
0
1
1
2
0
4 |
Clinical staging (cT)
Tx/T1/T2
T3/T4a/T4b
Total
p value |
8
1
9
0.08 |
12
14
26 |
3
1
4 |
Pathologic staging (pT)
TX/ T1/T2
T3/T4a/T4b
Total
p value |
8
1
9
0.13 |
13
12
25 |
3
1
4 |
Tumor size (cm)
<=4cm.
>4cm.
Total
p value |
3
2
5
0.50 |
8
17
25 |
1
3
4 |
Perineural invasion (PI)
Yes
No
Total
p value |
1
4
5
0.16 |
1
25
26 |
1
3
4 |
Extranodal extension (ENE)
Yes
No
Total
p value |
1
0
1
0.03 |
0
25
25 |
0
3
3 |
Treatment
Monotherapy
Multimodal therapy
Total
p value |
1
8
9
1.00 |
5
21
26 |
0
4
4 |
Radiotherapy (RT)
Yes
No
Total
p value |
6
3
9
0.05 |
6
20
26 |
2
2
4
|
RT technique
IMRT
Non-IMRT
Total
p value |
0
6
6
0.18 |
3
3
6 |
1
1
2 |
Total dose (Gy)
<=60Gy
>60Gy
Total
p value |
5
1
6
0.78 |
4
2
6 |
1
1
2 |
Chemotherapy (CTX)
Neoadjuvant
Adjuvant
Not performed
Total
p value |
6
3
0
9
0.058
|
5
16
5
26 |
2
1
1
4 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).