1. Background
Complex regional pain syndrome (CRPS), also known as reflex sympathetic dystrophy or causalgia, is a debilitating and persistent neuroinflammatory disorder that typically emerges in the aftermath of stressful events, such as surgery or trauma. CRPS can manifest in two forms: CRPS-I affects individuals without confirmed nerve injury, while CRPS-II affects those with associated nerve damage [
1]. Both types of CRPS are diagnoses reached by ruling out other potential causes, and they involve pain that is disproportionate to the initial injury, accompanied by the presence of signs in at least two and symptoms in at least three of the following categories: sensory, vasomotor, sudomotor, and motor or trophic [
2].
CRPS patients commonly experience decreased mobility, an inability to work, a diminished quality of life (QoL), as well as depression, anxiety, and long-term reliance on opioids. Therefore, it is crucial to provide an early diagnosis and initiate treatment promptly to curb disease progression and enhance patients’ QoL. Conservative approaches, such as medication use, physical therapy interventions, or injections are among the available treatment options for CRPS. While there is ongoing debate regarding this matter; early symptoms of CRPS are widely believed to be influenced by sympathetic activity resulting in the adoption of sympathetic blocks for symptom management purposes. Experimental treatments like intravenous immunoglobulin have yielded unsatisfactory results thus far. Tragically, 20–50% of individuals with less than one year’s worth of symptoms do not respond well enough to conservative strategies and may consequently necessitate chronic opioid use or implantation procedures involving neuromodulation devices like spinal cord stimulators or dorsal root ganglion stimulators. As CRPS progresses into its chronic stage observed efficacy from established treatment methods diminishes even further [
2].
The lack of satisfactory treatment for CRPS is due to the incomplete understanding of its root causes. Despite years of research, there is still only a partial understanding of how exactly CRPS develops. The condition involves an abnormal reaction of tissues to injury as well as enhanced sensitivity in both peripheral and central nervous systems (CNS), accompanied by inflammation and autonomic dysfunction. Furthermore, it is thought that genetic factors and psychological influences play a role in the progression of CRPS. Studies have primarily concentrated on the human leukocyte antigen system due to significant alterations in gene expression within this system [
1].
CRPS is defined by an immune reaction that promotes both inflammation and impairs neuropeptide signaling. This activation of the innate immune system leads to the proliferation of keratinocytes and the release of proinflammatory cytokines, such as interleukin (IL)-6, IL-1, and tumor necrosis factor-alpha (TNF-α). These cytokines initiate a cascade within the immune system, causing histamine-induced vasodilation which results in redness, swelling, pain, and warmth during the acute phase of CRPS [
1]. Moreover, these proinflammatory cytokines stimulate osteoblasts and osteoclasts, resulting in accelerated bone remodeling and the distinctive osteoporotic alterations observed in chronic CRPS cases. Additionally, neuropathic inflammation is thought to be a key contributor to the onset of CRPS. The activation of peripheral nociceptors situated on C-fibers leads to the transmission of pain signals to dorsal ganglia and the affected tissue. This retrograde transmission produces proinflammatory neuropeptides, such as substance P (SP) and calcitonin gene-related peptide (CGRP) [
1].
Currently, there are no known indicators that can be used to predict the likelihood of developing or recovering from CRPS. It is crucial to identify the factors that contribute to recovery to prevent and treat this debilitating condition effectively. We believe that the diversity of symptoms and response to treatment in CRPS may be linked to variations in gut microbiota. We hypothesize that the gut microbiome (GM) could play a role in both the onset and persistence of CRPS symptoms. Additionally, we suggest that studying the clinical status of individuals affected by CRPS could provide valuable insights into biomarkers related to their microbiota composition, as well as potential therapeutic approaches aimed at preventing long-term pain. We propose exploring how metabolome activity, immune system activation, and microglial activation might be influenced by or connected with changes in the gut microbiota during different stages of CRPS [
2].
The correlation between the GM and painful conditions has garnered considerable research interest. Extensive evidence suggests that GM plays a vital role in maintaining human physiological balance, impacting systemic inflammation, immunity, circadian rhythm, and hormone regulation – all of which have been linked to pain. The GM has shown associations with various types of pain including visceral pain, inflammatory pain, headache, neuropathic pain, chronic pain, and opioid tolerance. The overall genomic material from intestinal microbes has been estimated to be greater than 100 times the size of the human genome. Most gut microbiota bacteria in healthy adults are derived from two phyla,
Firmicutes and
Bacteroidetes. These populations are believed to be influenced by various factors at personal, interpersonal, environmental, and geographical levels that shape the composition of the microbiome [
3].
Recent research has sparked great interest in the possible involvement of gut microbiota in pain, as discussed in several well-regarded reviews. Animal studies have revealed that a healthy population of gut bacteria is essential for normal perception of visceral pain, while an imbalanced composition can contribute to the development of conditions including irritable bowel syndrome (IBS), neuropathic pain, and inflammatory pain. In human clinical studies, alterations in bacterial diversity or dysbiosis within the GM have been linked to various painful conditions, such as visceral pain, fibromyalgia (FM), and knee arthritis. It is worth noting that there have been multiple instances where patients with CRPS experienced improvement following the use of antibiotics known to impact the microbiome. In these cases, the individuals had been dealing with CRPS for a significant amount of time before being prescribed antibiotics for unrelated reasons. These reports suggest a potential connection between microbiota and long-term management of CRPS [
2].
Additionally, in his review titled “Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome”, Geoffrey Littlejohn suggests that neuroinflammation may have a “neurogenic” basis, possibly influenced by pain and stress. However, it is premature to solely attribute neuroinflammation and central sensitization to a primary neurogenic origin without considering the well-established presence of coexisting factors, such as small intestinal bacterial overgrowth (SIBO), mitochondrial dysfunction and vitamin D deficiency associated with gastrointestinal (GI) dysbiosis [
4].
Recently, there has been evidence of SIBO in individuals with FM, which refers to the presence of colonic bacteria in the distal small bowel. Furthermore, patients with CRPS have shown impaired intestinal permeability (IP). It is suggested that defective immune cell function plays a role in the pathophysiology of both FM and CRPS. Peripheral blood mononuclear cells from patients with FM and CRPS exhibited reduced messenger ribonucleic acid (mRNA) expression for anti-inflammatory cytokines, indicating an increased inflammatory response. It could be hypothesized that a compromised gut barrier may contribute to specific immunological reactions associated with these syndromes. There is evidence suggesting that restoring normal IP could potentially improve disease activity in certain human conditions. Another possible explanation for the elevated IP in both FM and CRPS patients might be related to distress caused by their pain. It is well-established that pain can induce stress, although the level of distress does not necessarily correlate directly with the intensity of pain. Research conducted on animal models and human mucosal investigations supports the role of stress in altering IP. One hypothesis could be that pain-related distress independently contributes to changes in IP. Therefore, this study observed a trend towards a greater increase in gastroduodenal IP among CRPS patients compared to those with FM. Altered IP may arise from various factors, such as SIBO, gut infections, gut inflammation, medication usage, stress levels, trauma, or non-steroidal anti-inflammatory drugs (NSAIDs) use [
5].
The timely initiation of therapy is crucial for improving patient prognosis in cases of CRPS, as the symptoms can vary over time. The main goals of treatment are to restore limb functionality, reduce pain, and enhance QoL. This typically involves a multidisciplinary approach that includes patient education, physical and occupational therapy, psychiatric support, and interventions from pain medicine specialists through medications or surgical procedures. To further advance our understanding of this condition’s root causes and develop more targeted therapies, larger-scale clinical studies with higher-quality data are necessary [
1]. Thus, neuroinflammation plays a role in FM and CRPS by contributing biologically to issues such as GI dysbiosis, vitamin D deficiency, and mitochondrial dysfunction. These independent factors often occur together, and each contributes to the development of both peripheral and central hyperalgesia. The consistent pain relief seen with interventions targeting intestinal dysbiosis (antibiotics), vitamin D deficiency (supplementation), and mitochondrial dysfunction (ubiquinone) demonstrates that these painful conditions have multiple underlying causes that can be addressed [
4]. Therefore, further research is necessary to explore combinations of medical and surgical treatments for future CRPS management.
2. Dysbiosis and Degradation of the Mucus Gel Layer in Complex Regional Pain Syndrome
In healthy individuals, the GM maintains a balanced and stable condition characterized by a diverse and abundant population. It is believed that in this state of equilibrium, the GM helps regulate the permeability of the intestinal wall. A healthy GM also contributes to the production of essential vitamins and nutrients, some deficiencies of which have been associated with pain (
e.g., vitamin B complex and vitamin D). When disturbances occur within the GM ecosystem, such as dysbiosis, there can be significant changes to key bacterial species. These alterations may result in modified permeability levels, disruptions in physiological functions, metabolic imbalances, and an increased susceptibility to diseases [
3].
One important role of the GM is to support the development and maintenance of a healthy intestinal barrier. This barrier consists of a protective mucosal layer and a monolayer of intestinal epithelial cells that are held together by tight junctions. In normal conditions, this barrier prevents microbes and their components from crossing into the underlying immune cells. The presence of beneficial bacteria helps protect the integrity of this barrier by preventing harmful pathogens from colonizing on the surface or invading epithelial cells and entering circulation. Additionally, microbial-produced short-chain fatty acids (SCFAs) like butyrate and acetate play a role in preserving the integrity of these tight junction proteins that keep everything in place within our intestines. Disruption of the epithelial barrier and heightened permeability are linked to alterations in gut microbiota composition. When there is an imbalance of bacteria in the intestines (bacterial dysbiosis), it can lead to a compromised intestinal barrier, allowing neuroactive compounds from microbes and immune substances to leak into the bloodstream. This leakage has an impact on peripheral inflammation [
6].
Current research suggests that GM plays a significant role in mediating systemic inflammation. This low-grade chronic inflammation has been proposed as a potential factor contributing to chronic pain and other chronic diseases. Numerous studies have also highlighted the established relationship between GM and immune-mediated inflammatory responses. The GM can modulate the immune response by influencing the function of B- and T-cells, which ultimately affects the body’s resistance to pathogens. Additionally, it should be noted that GM in humans produces various hormones or hormone-like compounds, functioning similarly to an endocrine organ. This designation is based on its ability to produce chemicals that can travel through the bloodstream and affect distant sites within the body. The first category consists of substances that regulate pain, such as SCFAs and neurotransmitters. SCFAs are produced by the GM in the large intestine through the fermentation of dietary fibers. Propionate is mainly generated by bacteria from the
Bacteroidetes phylum, while butyrate is primarily produced by bacteria from the
Firmicutes phylum. SCFAs play a crucial role in controlling intestinal inflammation and maintaining epithelial barrier function. Dysfunction at this level can contribute to “leaky gut syndrome”, which is implicated in various painful conditions. Additionally, circulating levels of SCFAs can influence systemic inflammation modulation as they enter the bloodstream. Notably, individuals with FM showed significant alterations in their serum levels of butyrate and propionate compared to healthy controls [
3].
Microbes break down resistant starches and dietary fibers through decomposition and fermentation, producing SCFAs, such as formic, pyruvic, butyric, lactic, and acetic acids that are delivered to the host. Along with metabolizing bile acids and other compounds, gut bacteria are believed to be the primary source of SCFAs. These SCFAs have been proven to regulate inflammation by recruiting leukocytes, promoting chemokine production, maintaining integrity in the intestinal wall’s tight junctions, and safeguarding the blood–brain barrier (BBB). The composition of the GM also plays a significant role in microglial homeostasis by generating fermentation products derived from microbiota activity [
2].
Microglia cells are a vital component of the structural framework of the nervous system, forming connections with astrocytes. They function as neuromodulators, influencing the excitability of central nervous cells and sensory neurons in the spinal cord. Conditions characterized by heightened sensitivity to pain, like FM and CRPS, may be influenced by the activation of microglial cells due to proinflammatory cytokines [
7]. Animal models have shown an increase in both activated microglia and their proliferation during acute, inflammatory, and neuropathic pain scenarios. Activated microglia initiate various defense mechanisms, such as clearing toxic debris through phagocytosis, processing antigens for presentation purposes, and releasing multiple cytokines. These functions are regulated not only by other glial cells and neurons but also by external factors like SCFAs produced by gut microbiota. The production of proinflammatory substances, such as TNF-α and IL-1β, contributes to nerve fiber sensitization within the CNS leading to heightened transmission of pain signals [
6].
The connection between microglia–pain, microglia–microbiome, and pain–microbiome suggests that there may be a relationship between intestinal dysbiosis and the activation of microglial cells in the development of chronic pain conditions. Moreover, patients with CRPS exhibit elevated levels of activated microglia in their spinal cord and brain as well as reduced diversity in gut microbial populations. Activation of the vagus nerve has been shown to inhibit proliferation of microglial cells and decrease expression of proinflammatory cytokines during inflammation caused by lipopolysaccharides. Consequently, signals originating from intestinal microbes may be transmitted through the vagus nerve to influence activation in these immune cells, ultimately contributing to the transmission of pain sensations [
6].
The gut–brain axis is a two-way communication system that involves the sympathetic, parasympathetic, and enteric systems. It allows for constant exchange of information between the brain and various bodily functions to maintain balance, including digestion and immune response in the gut [
8]. The autonomic nervous system’s efferent nerves play a role in regulating the GI tract and its microbiota by influencing its environment indirectly and directly through signaling molecules like SCFAs, bile acid metabolites, neuropeptides with antimicrobial properties, such as gamma-aminobutyric acid, tryptophan precursors and metabolites, serotonin, and catecholamines. In addition, the release of cytokines during the immune response to microbes can specifically communicate with host cells in the gut through receptors on local epithelial and mesenchymal cells. These factors can also transmit signals through neurological pathways (such as vagal and possibly spinal afferents) and endocrine mechanisms to target areas beyond the GI tract, including vagal afferents in the portal vein and receptors in the brain. While some of these signaling mechanisms can occur even when there is an intact epithelium, they are likely intensified and modified in situations where IP is increased due to stress or mucosal inflammation [
9].
Interestingly, the analysis of the CRPS microbiome revealed a decrease in the levels of various bacterial strains that are normally present in a healthy microbiome. These include bacteria associated with the production of SCFAs, such as
Bifidobacterium,
Eubacterium, and
Lachnospiraceae. There is also a reduction in the abundance of
Firmicutes phylum, indicating dysbiosis patterns among CRPS patients. Dysbiosis can disrupt the intestinal barrier, leading to increased interactions between bacteria and the immune system which results in localized inflammation. Maintaining an intact intestinal barrier is crucial for SCFA production, particularly butyric acid and butyrate. However, CRPS patients display lower levels of these beneficial compounds due to the decreased presence of certain bacteria like
Eubacterium species which play a role in their synthesis. The decrease in the variety of bacteria, particularly those involved in generating beneficial SCFAs, implies that this mechanism could potentially contribute to the onset of CRPS [
10].
Extensive evidence suggests the involvement of the immune system in both the onset and persistence of chronic pain. Notably, there are indications that GI bacteria significantly influences the immune system function, as individuals with CRPS exhibit lower levels of microbial diversity compared to their healthy counterparts. Moreover, decreased diversity in GI microbiota has been associated with inflammation in the GI tract, impairment of mucosal integrity, and unregulated production of proinflammatory cytokines. It has been proposed that rather than a specific pathogenic bacterium, the cause of GI inflammation is an imbalance in the distribution of healthy gut bacteria [
8]. The proximity of these bacteria to the epithelial layer of the GI tract likely plays a role in activating immune cells, which make up 70% of the body’s immune cells within the GI lymphoid tissue. This interaction leads to the production of antimicrobial proteins by epithelial and plasma cells. Previous studies on GI community structures have shown that
Firmicutes and
Bacteroidetes are major contributors at the phylum level when considering their relative abundance. The composition of
Firmicutes and
Proteobacteria varied significantly between the CRPS and control groups. Notably, individuals with CRPS had higher levels of
Proteobacteria, which consists mainly of gram-negative bacteria. Gram-negative bacteria’s cell walls contain lipopolysaccharides, triggering an innate immune response in humans characterized by cytokine production, inflammation, and sickness response. Toll-like receptor 4 (TLR4), a pattern recognition receptor crucial to the innate immune system, plays a key role in recognizing microbial elements associated with inflammation following tissue injury [
8].
Recent studies have found that individuals with FM may experience SIBO. SIBO refers to the inappropriate colonization of colonic bacteria in the distal small intestine. This can lead to an increased IP, where there is a greater chance for luminal products to pass through the epithelial layer. Abnormal access to these products can stimulate immune cells and contribute to the development of systemic diseases, such as inflammatory bowel disease (IBD), allergies, and arthritis. The observed increase in IP and dysbiosis (microbiome alterations) may have pathophysiological significance for both FM and CRPS [
5]. Previous research has shown that CRPS is associated with dysbiosis and increased IP, which are also found in SIBO. Chronic systemic inflammation is a common feature of both conditions. SP levels, which are elevated in IBS, have been implicated in the development of CRPS, particularly among women who are more susceptible to this condition. Furthermore, SIBO-induced increased IP can lead to lipopolysaccharide translocation and activation of microglia activity in CRPS. Evidence indicates that manipulating the microbiome through long-term cephalosporin therapy may be beneficial for managing CRPS symptoms based on reported cases of remission [
7].
Additionally, heightened levels of TNF-α and IL-6 proinflammatory cytokines have been observed in correlation with increased IP. The initiation of an immune response in the peripheral region can contribute to inflammation within the CNS, including the activation of microglia. Elevated levels of peripheral inflammation are linked to compromised integrity of the BBB and subsequent central inflammation. This compromised BBB allows for small bacterial components and metabolites to enter the CNS, triggering abnormal microglial activity. Disruptions in intestinal barrier integrity have been reported across various chronic pain conditions, such as FM, CRPS, and IBS. The severity of pain and plasma levels of proinflammatory cytokines, such as IL-2, IL-6, and TNF-α, are influenced by the extent of IP. These findings suggest that a compromised gut barrier contributes to both systemic and central inflammation and plays a role in the development of chronic pain. The relationship between an impaired intestinal barrier in conditions associated with chronic pain is still not fully understood; it could be either a cause or an effect of the pain response. There is likely a bidirectional mechanism where imbalances in gut bacteria lead to increased IP, which initiates chronic pain. This further disrupts gut homeostasis and alters microbial communities [
6].
The composition of intestinal microbes in adults tends to remain stable over time and is unique to each individual. However, various external factors, such as genetics, mode of birth delivery, diet, age, antibiotic treatments, and probiotics can potentially influence this microbial composition. The intestinal microbiota plays an active role in several processes including improving nutrient absorption and breaking down non-digestible compounds in the diet. Additionally, it provides essential nutrients while removing harmful toxins and non-nutritional substances. Given the rise of antibiotic-resistant pathogens and the negative impact that antibiotics have on beneficial bacteria, the development of supplementary or alternative therapies based on bacterial replacement is crucial for preserving a healthy microbiota [
11].
3. Boron: Essential Element into Host–Microbiota Symbiosis
Boron (B) was recently claimed as an essential element for the human host–microbiota healthy symbiosis and the effects of B deficiency in the microbiota could lead to: (
i)
dysbiosis, claimed to happen due to deficiency of the autoinducer-2–furanosyl borate diester (AI-2B) signaling molecule; and (
ii)
degradation of the mucus gel layer, due to the lack of B content in the mucin gel structure, which causes the interaction of the bacterial biofilm directly with the host cell membranes and therefore, their direct infection [
12,
13,
14].
Recent new insights into B’s mechanism of action (MoA) are based on claims that: (
i) B is an essential element for the symbiosis between the commensal microorganisms in the microbiome and the human and animal host; (
ii) B is not required by the human cell, the human host cells do not need B nutritionally, this element being necessary only for a healthy symbiosis between the host organism and the various microbiomes of the gut, scalp, mouth, skin and vagina; (
iii) some naturally occurring prebiotic boron complexes (PBCs) species have recently been proven to be microbiota-accessible boron complexes (MABCs) [
12,
13,
14,
15]. More than that, PBCs, such as B-containing pectic polysaccharides (BPPs) and the recently discovered borate complexes of chlorogenic acid (diester chlorogenoborate – DCB), are indigestible compounds [
14], while inorganic B compounds, such as boric acid (BA) and borate salts, are digestible and, in certain circumstances, they can be toxic [
16,
17,
18].
These observations helped to formulate a new perspective on the essentiality of B in the animal kingdom, pretending that the AI-2B signaling molecule is actually able to modulate microbiota (composition, bacteria behavior and community dynamics) as well as the mucus gel layer under conditions of dysbiosis [
19]. The production of AI-2B by one phylum could influence the gene expression of other species and facilitate interspecies communication, making bacteria to change its behavior, especially luminescence, virulence, and formation of biofilm between various species [
20]. This characteristic determines AI-2B to turn into a very good candidate for regulating interactions between cells in the human gut and the microbiota, where hundreds of bacterial phyla live together and communicate. Recently, the AI-2B molecule has been proposed as a biomarker for dysbiosis [
12,
14,
15]. In addition, B has recently been declared an essential element involved in the synthesis of the autoinducer-2 (AI-2) quorum sensing (QS) system. At the same time, the active AI-2B is being generated by the addition of borate to the AI-2 precursor, being able to amplify the activity of AI-2 and support the secretion of extracellular polymeric substances [
21,
22].
Several natural organic boron (NOB) species have been detected in bacteria (borate polyketides, borate–siderophore complexes, AI-2B), fungi (borate esters of carbohydrates) and plants (rhamnogalacturonan II–borate complex (BPP), borate esters of carbohydrates, borate esters of organic acids,
bis-
N-acetyl serine, and borate complexes of phenolic acid, such as DCB) [
14,
15,
23,
24]. From digestibility assays of NOBs, only for BPP [
25,
26] and DCB complex extracted from green coffee beans (GCB) [
14] prebiotic properties were highlighted. These organic B species are distinct from inorganic BA/borates, which aren’t prebiotic because they are digestible and could be toxic to the microbiota [
23,
27].
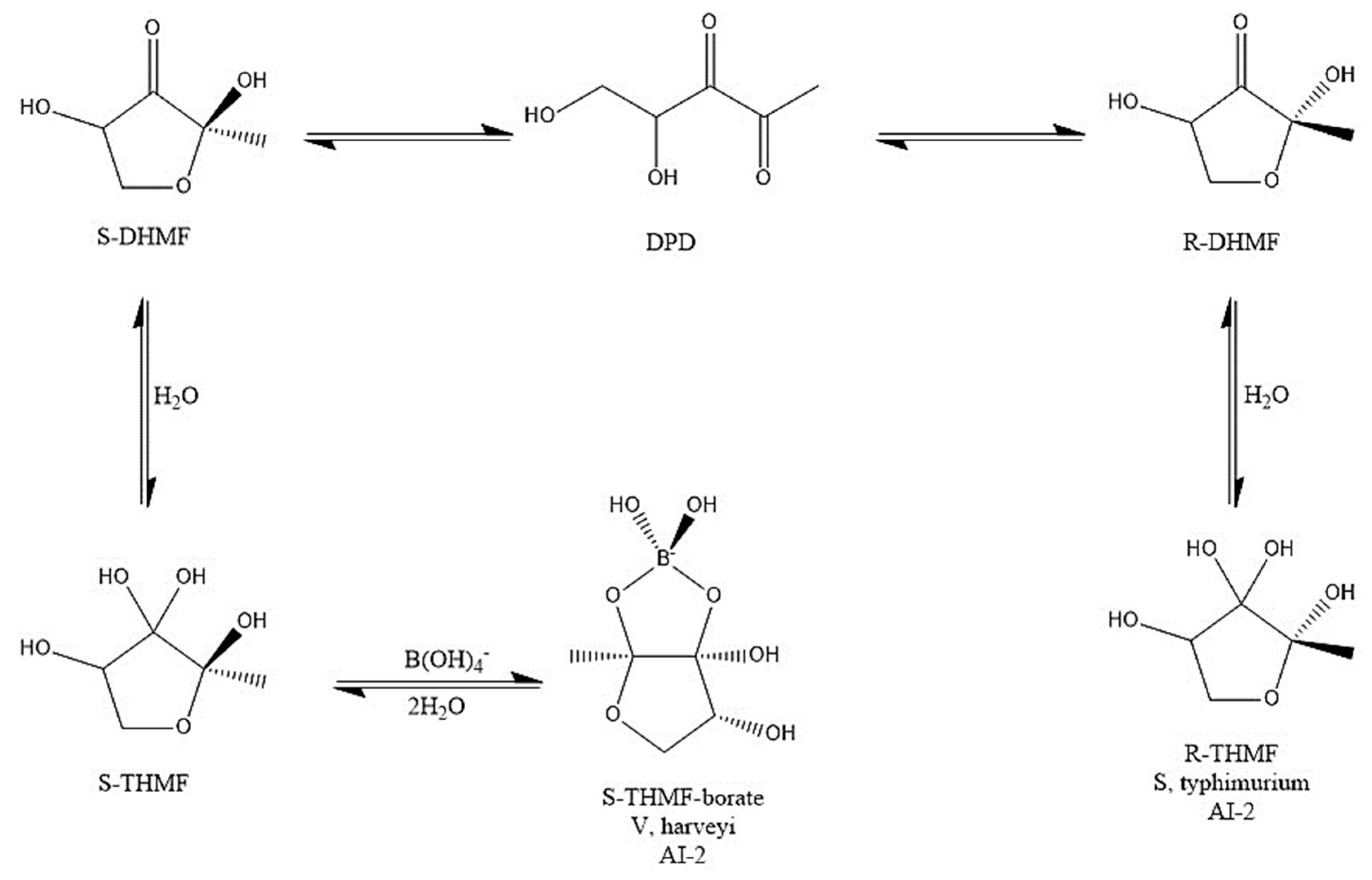
The proposed MoA of B for PBCs is connected to both the fortification of mucus glycoprotein gels with PBCs and synthesis of AI-2B molecule. Outside the cell, PBCs can react with 4,5-dihydroxy-2,3-pentanedione (DPD) or a hydrate thereof, producing the AI-2B signaling compound. In aqueous solutions, (
S)-DPD is in balance with its two stereoisomers (
R-2,4-dihydroxy-2-methyldihydro-3-furanone (
R-DHMF) and
S-DHMF) (
Figure 1).
For instance, AI-2B participates in intracellular communications with other symbiotic bacteria in the microbiome. It can also react with mucins (high molecular weight glycoproteins) to produce borate-stabilized glycoproteins. These borate-stabilized glycoproteins are being integrated by the mucus gel layer of the gut, protecting the integrity of the intestinal wall in the host organism [
12,
13,
14,
15].
The interactions of the microbiota with the human host have become an extremely active research direction in recent years, and many scientists are investigating the effect of the intestinal microbiome on several diseases in animals and humans. Researchers have noticed a strong correlation between intestine microbiota and illnesses, such as GI diseases (
e.g., IBD, colon cancer, gastroenteropancreatic neuroendocrine neoplasms), cardiovascular diseases (CVDs) (
e.g., atherosclerosis), psychiatric diseases (
e.g., multiple sclerosis (MS), Alzheimer’s disease (AD), Parkinson’s disease (PD)), metabolic diseases (
e.g., obesity and type 2 diabetes (T2D)), respiratory diseases (
e.g., chronic obstructive pulmonary disease, bronchial asthma, infectious lung diseases), immune diseases (
e.g., rheumatoid arthritis (RA) and ankylosing spondylitis) and so on [
29,
30]. A main characteristic of the microbiota is the difference between sex, which also means various nutritional needs of B between males and females. Thus, females are more exposed to illnesses caused by prebiotic B deficiency in their nutrition. Supervising the B level and/or the level of AI-2B molecule in feces will show new scientific ways for the illness’s avoidance due to prebiotic B lack in nutrition [
13,
14,
15]. The various types of GM in males and females are influenced by sex hormones, which result in different male and female immunity, as well as susceptibility to many infections and chronic diseases for them [
31]. At the same time, B amount in males and female’s hair is different, since women have almost two times less B in their hair, this means that women need about two times more B in nutrition than men [
32]. Several diseases have been explored in relation to the GM and gender differences and the effects of B in nutrition, such as osteoarthritis (OA), osteoporosis (OP), periodontal disease, polycystic ovary syndrome (POS), ovarian cancer, IBD, obesity, fatty liver disease (FLD), T2D, depression, allergic diseases, CVDs, and ischemic stroke [
31,
33].
The new findings into B essentiality for humans require a new approach of B status in science. Thus, the MoA of B needs to be identified, therefore explaining the B effect in human health. Moreover, PBCs are nutritionally essential for human host–microbiota healthy symbiosis, while inorganic B species, since they are digestible, they are not prebiotics [
12].
4. Autoinducer-2–Borate Deficiency and Dysbiosis
Recently, PBCs have been claimed to ensure communication between bacteria and the host through AI-2B as well as by B-stabilized mucins in the intestinal mucus [
12,
14]. Moreover, the connection between host–microbiota symbiosis disturbance, the so-called dysbiosis and some chronic illness is highlighted [
34]. Dysbiosis due to B deficiency in nutrition happens when the AI-2B level in microbiota decreases, the balance between commensal and pathogenic bacteria is broken, and subsequently the mucus gel is also being destroyed [
35].
B is an essential microelement for synthesis of AI-2 signaling molecule and influences bacterial general behavior. The health of the microbiota was positively correlated with a high concentration of B in the excreted feces [
15]. Thus, the level of B in the feces is not correlated with the levels of B in the body of the host organism; rather, the level of B in feces is correlated with a level of B in the gut microbiota and mucin gel layer. High levels of B in feces after administration of non-digestible PBCs indicate a human host–microbiota healthy symbiosis. When the B intake is digestible,
e.g., BA or inorganic borates, then the level of B in urine and blood is correlated with increased levels of B in the host organism, for example, an animal or a human, and can become potentially toxic to host [
12].
The MoA of PBCs is as follows: PBCs intake (
i.e., DCB and BPP) allows bacterial species that communicate using AI-2 to accumulate B in the form of AI-2B, which transfers the borate anion to glycoprotein mucins to fortify the layer of colonic mucus, helping host protection against bacterial infection. After PBCs intake, the amount of B accumulated in the gut mucus gel layer could lead to important effects on human health: boost of AI-2B level, reduction of the gut damage, and microbial flora modifications in diarrhea. Thus, many factors lead to microbiota dysbiosis, out of which the most important is the lowering of the AI-2B level, resulting in diminishing the QS system ability to keep the stability of the gut microbiota, thus leading to diarrhea [
13,
14,
15].
Some recent animals and human experiments were carried out regarding the supplementation with PBCs standardized as DCB-rich GCB natural extract and the level of AI-2B was mainly monitored [
15]. Dietary supplementation with DCB improved the diarrhea index and increased AI-2B in a rat model of castor oil-induced diarrhea.
In vivo feeding with DCB-rich natural extract was performed on three groups of male Wistar rats, six-month-old, four animals in each group, with a medium weight of 290±10 g, as follows: Group 1 (control) – rats fed with normal diet; Group 2 (castor oil-induced diarrhea) – rats with castor oil-induced diarrhea; Group 3 (DCB) – rats fed with DCB-rich GCB natural extract (as 15 ppm B) five days after the onset of diarrhea. During the study, the rats were put in individual cages and monitored under standard conditions of humidity, lighting, and temperature (12-hour light/dark cycle). AI-2B values in induced diarrhea for Group 2 are noted to be very low at an average of 1.4 μM, which corresponds to an increased diarrhea index. The DCB-rich GCB natural extract diet five days after the onset of diarrhea results in a consistent fecal AI-2B concentration of 45.3 μM and a near-normal diarrhea index [
15].
The dysbiosis index (DI) in the stool of the patients with long-term antibiotic therapy supplemented with a DCB-rich GCB natural extract was also described [
15]. Manipulation of QS AI-2 signaling affects antibiotic-treated gut microbiota. These discoveries provide the new way the AI-2 is working as a signal molecule for intraspecies and interspecies communication, as increased AI-2 counteracts antibiotic-induced dysbiosis. DCB or a DCB-rich natural extract may serve as a nutritional adjuvant for gut microbiota in patients undergoing long-term antibiotic therapy for respiratory tract infection, including tuberculosis. The use of DCB-rich natural extract in diarrhea relief increases fecal AI-2B levels and reduces dysbiosis. Group 1: 10 healthy subjects (control); Group 2: 10 subjects with standard intravenous antibiotic treatment (Sulcef); Group 3: 10 subjects with standard intravenous antibiotic treatment and supplemented with standardized DCB-rich GCB natural extract (three capsules/day – 1 mg B, approx. 65 mg DCB) during the 30 days. For the pilot study, DCB was prepared as GCB natural extract (ca. 6.5% DCB) at a standard concentration of 1000 ppm B. The DI of Group 2, treated with antibiotics, shows a strong increase and there is a corresponding decrease in the level of AI-2B, which means that the commensal bacteria are reduced in number. Group 3, supplemented with DCB-rich GCB natural extract, shows a reduction in DI, compared to Group 2, and a significant increase in the level of AI-2B. Supplementation with natural extracts rich in DCB can prevent dysbiosis induced by antibiotic treatment by increasing AI-2B in the gut microbiota. AI-2B is being proposed by the authors as a potential marker of intestinal dysbiosis [
15].
Subsequently, dysbiosis due to the deficiency of PBCs and implicitly of AI-2B determines the disruption of communication between bacteria, between bacteria and the host, and has consequently the degradation of the mucus gel. Recent results claim that cross-kingdom communication happens between bacteria and eukaryotic cells through the bacterial AI-2 QS system. By raising AI-2 levels, Thompson
et al. (2015) noticed that the evolution of
Firmicutes was increased, while the development of
Bacteroidetes was stopped, thus inverting the antibiotic-induced dysbiosis. The
Firmicutes group, which are producers of AI-2, diminish in abundance following antibiotic treatment, while of the
Bacteroidetes phylum augment in abundance [
19,
36]. More recently, in research on murine models with neonatal necrotizing enterocolitis (NEC), the concentration of AI-2 was found to have been highly reduced during the acute disease phase, but gradually raised in the remission phase [
19]. These observations prove that AI-2B and AI-2 have an impact on the balance and structure of the gut microbiota. The connection between AI-2B and intestine microbiota equilibrium shows that AI-2B can be a new biomarker to identify intestine homeostasis [
36]. Recent data notice that high levels of AI-2 are thus able to rebuild the equilibrium between
Firmicutes and
Bacteroidetes and hinder or invert dysbiosis, obesity, autism, IBD and disorders connected to aging and stress. These findings that prebiotic B boosting the activity of AI-2 through the ingestion of PBCs and the formation of AI-2B, can be used to restore the equilibria in the GM after antibiotic treatment and in addition, can be a nutritional adjuvant for a long and healthy life [
37]. It is known that in children and the elderly the
Firmicutes/
Bacteroidetes ratio is low, while for adults this ratio increases [
38], aging is finding an inducing factor of dysbiosis. Subsequently, the health condition of the microbiota symbiosis is important in increasing the healthy and long life of human hosts and increasing stress resistance. Research has proved that aging is correlated with many modifications, such as decreases in GM variation, decreases in the rates of
Firmicutes and
Bacteroidetes, abundances of pathogens and decreases of bacteria producing the SCFAs necessary to keep composition integrity and stop inflammation in the intestine [
39,
40,
41,
42,
43].
Non-digestible carbohydrates dietary fibers are used as prebiotics, but recently, polyphenols, they can also function as prebiotics to generate butyrate. Intake of polyphenols increases the abundance of butyrate-bacteria producers, such as
Faecalibacterium and members of the
Ruminococcaceae family. Other phenolic compounds, such as caffeic acid, chlorogenic acid and rutin, are also reported to boost microbial butyrate [
44,
45,
46].
Deficiency of PBCs in the diet correlates with the reduction of AI-2B levels in the microbiota with direct consequences on
Firmicutes, which are the majority producers of the butyrate, with direct effects on human host-microbiota healthy symbiosis host recent evidence shows that dysbiosis is linked to low production of butyrate [
47]. The effects of prebiotic B deficiency are translated by the decrease of
Firmicutes, the decrease of AI-2 and, implicitly, AI-2B. Over 200 species of bacteria from the
Firmicutes species make a major contribution to the commensal microbiota. B compounds accessible to the microbiota increase the level of acetate and especially butyrate with effects on the brain, bones, and the immune system of the intestine. The lack of PBCs will be felt through dysbiosis, mucus degradation and implicitly the decrease in the level of butyrate in the microbiota, and this will lead to a pleiotropic effect on the skeletal system (gut–bone and gut–cartilage axes) and the immune system (inflammation of the cardiovascular system, brain, autoimmune pathologies) [
48].
Moreover, the lack of PBCs in food favors pathogenic bacteria and causes dysbiosis and degradation of the intestinal mucus, with consequences on the health of the host through the appearance of both local and systemic diseases (GI diseases, inflammation of the cardiovascular system, brain, and skeletal system degradation), and in the end even breaking the cycle of life [
12]. A molecule is considered to be an essential nutrient if the diet deficiency results in biological dysfunction. Dysbiosis can influence the function of the gut barrier by changing the thickness of the mucus, and this leads to the progression of many illnesses. Predominantly bacteria that reside within humans are either commensal or mutual and the mucus layer is the first line of defense against the penetration of microbiota, acids and digestive enzymes, digested food components and food-associated toxins. Mucus is thought to serve as a source of nutrients for commensal microbiota [
49,
50].
Therefore, the presence of B in the microbiota through the diet with PBCs has the following MoA: (
i) increasing the level of AI-2B that prevent against the pathogenic bacteria and stimulates the growth of commensal bacteria, especially
Firmicutes [
13,
14,
36]; (
ii) improving dysbiosis and strengthening the mucus gel of the gut by increasing of B content in the mucin gel structure [
12]; (
iii) increasing the level of
Firmicutes and other AI-2-dependent bacteria (
Clostridium,
Coprococcus,
Eubacterium,
Ruminococcus) [
51]; (
iv) increasing the total amount of SCFAs, especially butyrate; (
v) increasing of B in colon, butyrate-producing colonic microbiota stimulate 1,25-dihydroxyvitamin D (vitamin D3) production by colon-resident immune cells [
52,
53].
5. Perspectives to Use the Prebiotic Boron-Containing Compounds in Complex Regional Pain Syndrome
MABCs hold promise as a potential therapeutic approach in CRPS, a debilitating chronic pain condition that affects a considerable number of individuals worldwide. Several studies have highlighted the role of gut microbiota in the modulation of pain responses and the development of chronic pain conditions, such as FM and IBS.
Recent research has shown that alterations in gut microbiota composition and IP are associated with chronic pain conditions. This suggests that targeting the microbiota–gut–brain axis could be a potential path for therapeutic intervention in CRPS. MABCs have emerged as a potential therapeutic strategy due to their ability to modulate gut microbiota composition and restore gut homeostasis. These compounds have been shown to improve IP and reduce inflammation, which are key factors in the development of chronic pain.
In our opinion, the prebiotic B deficiency in the diet causes dysbiosis and degradation of the gel mucus in the intestinal tract. Damage in the gut mucus layer aggravates gut inflammation and infection. The intestine microbiota sends bidirectional signals to organs affecting the metabolism of the whole body and is a providing factor to metabolic illness and a damaged gut mucosal barrier can be an essential interface between host and flora. PBCs also improve immunity, have antioxidant and anti-inflammatory actions on the microbiome [
54] and determined the health of the body through the main “axes” defined in the scientific literature: “microbiota–gut–brain axis”, “microbiota–gut–bone axis”, “microbiota–gut–thyroid axis”, “microbiota–gut–cartilage axis”, “microbiota–gut–heart axis” [
55]. Subsequently, the PBCs have a major role in the avoidance of certain illnesses, such as OA, OP, RA, cardiovascular inflammation, depression, obesity, T2D, thyroid diseases [
56].
The indigestible PBC species are microbiota-accessible and cause an increase in the level of volatile fatty acids, due to the increase in the activity of commensal bacteria, especially the level of butyrate producers [
57]. Most human butyrate producers belong to the
Firmicutes phylum, including species, such as
Butyrivibrio fibrisolvens,
Clostridium butyricum,
C. kluyveri,
Eubacterium limosum,
Faecalibacterium prausnitzii [
58]. In addition, other bacteria produce butyrate:
Anaerostipes spp.,
Bifidobacterium spp.,
E. hallii.
Bifidobacterium spp. generating butyrate from lactate (a product of glucose metabolism) and acetate. All primary butyrate-producing bacteria are anaerobic, meaning they can only grow in low-oxygen environments. Because oxygen levels in a healthy colon are extremely low, these organisms thrive in this ecosystem.
Firmicutes phylum has recently been shown to increase intestinal levels of AI-2 and butyrate. Acetate and propionate are the main products of the
Bacteroidetes phylum. Thus, the increase in AI-2 levels favored the expansion of
Firmicutes in the microbiota treated with antibiotics, and inhibited
Bacteroidetes, therefore counteracting the dysbiosis induced by antibiotic treatment [
19,
59].
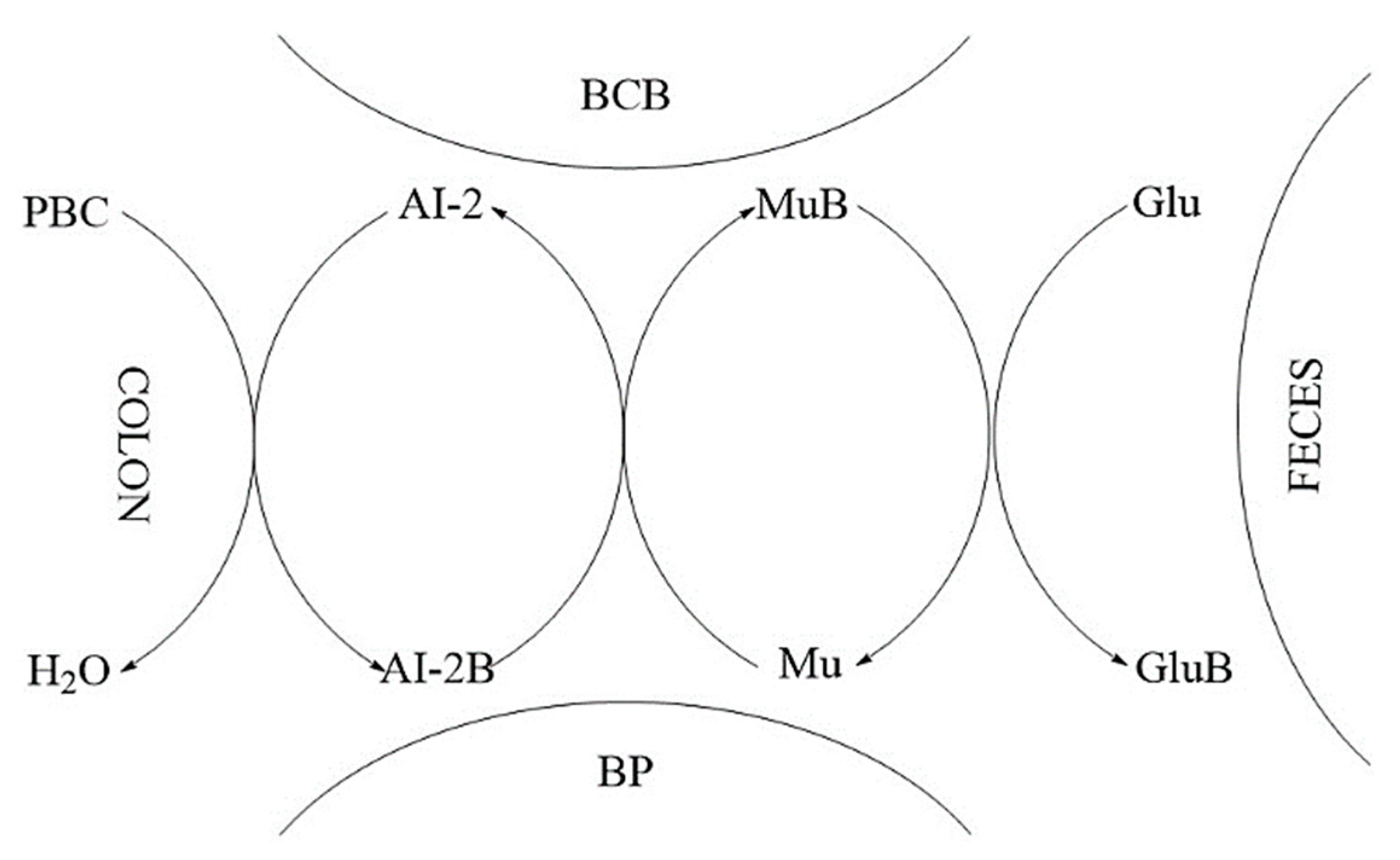
We proposed a MoA of PBCs as mediators for production of butyrate in the colon (
Figure 2). Thus, AI-2B level increases and stimulates the growth of butyrate-producing bacteria (mainly
Firmicutes) [
51], AI-2B transfers the borate anion to mucins stimulated by butyrate, and through the high level of AI-2B inhibits pathogenic bacteria (such as
Vibrio spp.) [
60,
61,
62]. The reverse process of decreasing prebiotic borate intake leads to the inhibition of butyrate-producing bacteria, and the growth of pathogenic bacteria that are stimulated by low AI-2B. In addition, the lack of PBCs in the diet accelerates the loss of B from the mucus in the feces, by complexing, mainly, with fucose and sialic acid (
Figure 2). Subsequently, PBCs are the ones that have an essential impact on host–microbial symbiosis in health, and mainly on butyrate-producing bacteria and AI-2 signaling molecules, according to the model shown in
Figure 2, in two directions: microbiota–gut–immune system and microbiota–gut–musculoskeletal system.
Butyrate is known to be a key metabolite of the GM that mediates the effects on the immune system cells (T-cells, antigen-presenting cells, monocytes, and neutrophils) and plays a key function in maintaining gut immune homeostasis, but also has potential future therapeutic for a spectrum of intestine and systemic illness [
47,
63,
64,
65,
66,
67]. Recent studies have found that butyrate-producing microbes protect against or are associated with less severe symptoms of a long list of conditions related to chronic inflammation: allergies, IBS, RA, PD, high blood pressure, insomnia, anxiety, T2D [
47]. Moreover, the butyrate is involved in boosting the mechanical and immune barrier of the intestine and promotes a healthy intestinal barrier, preventing the “leaky gut” syndrome [
68] and has been reported to be an inhibitor of histone deacetylases (HDACs). A growing number of studies have found that butyrate may exert protective effects in atherosclerosis, hypertension, and vascular health [
47].
Subsequently, butyrate-producing bacteria of
Firmicutes filum can have a key role in the healthy symbiosis of the human colon as a major energy source for the colonic mucosa, increased mucin secretion and as an important modulator of gene expression, inflammation, differentiation, and apoptosis in host cells with function in improving intestinal barrier function [
69,
70]. B stimulates butyrate-producing bacteria through AI-2B. Butyrate stimulates the
de novo synthesis of mucins and ensures the transfer of B to them and catalyzes the incorporation of B-stabilized mucins into mucus (
Figure 2). Mucins are the growth substrate for butyrate producing
Firmicutes and because mucosal butyrate producers release butyrate close to epithelium, they may increase the bioavailability of butyrate to the host [
71].
Firmicutes are the main butyrate-producing bacteria in the human colon, especially
E. rectale,
Clostridium leptum,
Roseburia spp. and
F. prausnitzii [
72,
73]. Furthermore, butyrate plays a role in controlling the synthesis of cathelicidins, which are polycationic peptides involved in the innate immune system of mammals and demonstrate wide-ranging antimicrobial capabilities against potential intestinal pathogens. Conversely, a decreased presence of butyrate-producing organisms in the intestinal environment leads to the proliferation of aerobic bacteria from the
Enterobacteriaceae genus, which is commonly associated with signs of intestinal dysbiosis [
74].
The number of articles which study the
in vitro and
in vivo effects of B on butyrate and SCFAs production are limited. There are few studies that show that B as feed additive can improve ruminal microbial fermentation, promote SCFAs formation, especially an increase in butyrate by 40%, while total SCFAs increases by over 60% [
57,
75]. A recent study proved that the B improvement the disordered gut morphology and combined treatments with probiotics reduced both oxidative damage and inflammatory processes [
76]. The latest studies have noticed that there are some close correlations between B intake, AI-2, the
Firmicutes phylum and the production of butyrate [
36,
77]. In addition, chlorogenic acid, the ligand in the DCB complex, and other phenolic compounds, such as caffeic acid and rutin, are also reported to increase microbial butyrate [
44]. Therefore, DCB, like PBCs, has in its composition both a prebiotic part of phenolic acid and B as an essential nutritional element for butyrate-producing bacteria [
14]. Thus, many of the functions of butyrate on health status are also found in the case of B ingestion in both animals and humans.
6. Microbiota–Gut–Immune System
The immune system is a complex network of biochemical molecules, cells, organs, and tissues that coordinate with each other to fight off invaders and repair damage [
78,
79,
80]. The cooperation between the gut microbiota and the immune system can take place on several main axes:
(
i)
Microbiota–gut–brain axis: There is an expanding interest in the relationship between microbiome, intestine, and brain, which is definite as the “microbiota–gut–brain axis” [
81]. The “microbiota–gut–brain axis” plays an important role not only in the health of the GI tract, but also in brain function and behavior and is modulated at different levels by enteric nervous system, CNS, hypothalamic–pituitary–adrenal axis, and the immune system through a complex communication of immune, endocrine, and neuronal factors [
82,
83]. In the context of the microbiota–gut–brain axis, a leaky gut has become an important point of interest in neurological disorders in the last decade, with the demonstration of gut microbiota dysbiosis and/or an impaired gut barrier in neurological diseases of various etiologies, such as accident stroke, Huntington’s disease, and amyotrophic lateral sclerosis (ALS). Disruption of the intestinal barrier has also been linked to cognitive impairment associated or not with neurodegeneration and gut microbiota dysbiosis is functionally linked to brain immunological dysfunctions, contributing to poor mental health, neuroinflammation by increasing bacterial metabolites and inflammatory cytokines in the intestine and BBB and the development of numerous neurodegenerative diseases, including AD, PD, MS, and ALS [
84].
The latest research showed that B is a beneficial trace element for some metabolic pathway in the body as many deficiencies of B disturb memory, anxiety level, cognitive functions, mood, sleep, modulated calcium and magnesium exchange, vitamin D and sex steroid metabolism. B can help create the intestinal organizational structure, which improves GI absorption [
85,
86,
87]. Recently, exposure to B nitride nanotubes was shown to stimulate tadpole growth and was also connected with intestine microbiota remodeling, clearly demonstrating the role of B in modulating microbiome physiology. Nutritional prebiotic B intakes have been noticed to have favorable benefits on central neurological function with the potential to treat neurological diseases, such as PD and AD. At the same time, B based diet significantly improves brain function and cognitive functioning in humans and will play a significant role in improving dysbiosis and will open new directions for researchers for perspectives of B nutrition [
88,
89]. The most impressive feature in the microbiota of the centenarian peoples is a change in the ratio of
Firmicutes and
Bacteroidetes, with the old people having a higher proportion of
Bacteroidetes, while young adults have higher proportions of
Firmicutes (butyrate-producing bacteria) which are stimulated by PBCs [
39]. The butyrate is known to contribute to modulation of brain-derived neurotrophic factor (BDNF), and it is well known that gut dysbiosis causes decreased BDNF levels, which could affect neuronal development and synaptic plasticity. The use of butyrate-producing bacteria increasing attention as and in the conditions in which the PBCs ensures the simulation of butyrate-producing bacteria (
Firmicutes and
Actinomycetetae) and this MABCs can be a promising adjuvant nutritional therapeutic for depression. Aging is associated with low levels of BDNF, suggesting that maintaining adequate levels of BDNF could help prevent or delay the onset of cognitive decline. A convenient way to increase BDNF levels is by supplementing with the PBCs, a SCFA that functions as a HDAC inhibitor [
90]. This improves brain plasticity, leading to new neural connections and growth and increased cell survival [
91,
92]. It has recently been shown that adequate B supplementation, can support the brain development of ostrich chicks by boosting the BDNF expression and lowering cell apoptosis process, but, a high dose of B intake, damages the neuron structure of chick ostrich brain by inhibiting BDNF expression and increasing cell apoptosis [
93]. Moreover, an
in vitro approach has shown that B-containing compounds could be neuroprotective agents, for the management of AD [
94].
The MoA of B neuroprotection could relate to: (
i) reversed dysbiosis and stopping of degradation of mucus by B-stabilized mucins by AI-2B; (
ii) the regulation of BDNF and acetylcholinesterase expression levels by gut microbiota-derived metabolite butyrate induced of the PBCs [
15,
95].
(
ii)
Microbiota–gut–heart axis: CVDs are illnesses that affect the blood vessels and heart,
i.e., hypertension, heart failure (HF), atherosclerosis and stroke. Numerous
in vitro and
in vivo recent studies proved that cardiovascular protective capabilities of butyrate and especially protective effects in atherosclerosis,
i.e., higher stroke risk relates to reduced level of butyrate-producing bacteria in the intestine and reduced fecal butyrate concentrations [
47,
96,
97]. Recent results show that B had a positive impact on myocardial infarction-induced HF, attenuated apoptosis and cardiac fibrosis and has potential to induce cardiac tissue regeneration after injury [
27,
98,
99].
(
iii)
Microbiota–gut–thyroid axis: The butyrate increases the expression of thyroid hormone receptors in mice being a well-recognized inhibitor of HDAC, thus butyrate increases iodine uptake and the expression of thyroid hormone receptors in mice [
100,
101]. Regarding the nutritional intake of B, the most important researches regarding the supplementation of the diet with B in animals and humans have shown the following: (
a) healthy women fed with a diet rich in B (10 mg B per day) have a significant decrease of 25% in the thyroid-stimulating hormone (TSH, thyrotropin) level [
102]; (
b) B shows an iodine-like effect while investigating the influence on tail maturation of
Xenopus laevis frogs,
i.e., tail maturation in
X. laevis is modulated by the thyroid axis [
103,
104]; (
c) dietary supplementation with BA (400 mg/kg) during a 4-month period increased the level of triiodothyronine (T3) of the rams in the serum samples compared with control [
105]. There were also some contradictory results, but in general, favorable effects were observed with the B diet in animals and humans [
106]. However, the B has a great beneficial role in increasing the secretory activity of the thyroid gland and recently, on finding that commensal microbes function as a T3 deposits and may prevent thyroid hormone fluctuation and thus may be able to reduce the need for thyroxine (T4) supplementation. Moreover, B intake inhibits TSH by dopamine (DA)-producer bacteria [
107]. How the gut causes thyroid disease may be explained by damage to the gut barrier due to dysbiosis and the subsequent increase in gut permeability, allowing antigens to pass through more easily and activate the immune system or cross-react with extraintestinal tissues, respectively [
108]. The lack of PBCs in food determines B deficiency in the large intestine, that is, where 90% of the commensal bacteria are, dysbiosis and implicitly cellular permeabilization at colon level, with devastating effects for the human body. B ensures a healthy symbiosis and is involved in the storage of T3 hormone in the microbiota and with direct action on the DA hormone that controls thyroid function. DA is a physiological regulator of TSH secretion. There are recent data that demonstrate the link between the level of DA and the gut microbiota-derived metabolite butyrate, DA having a regulatory effect on the synthesis of colonic mucus [
109]. Therefore, the presence of B in the microbiota through the diet with MABCs, has the following MoA: (
i) B increases the level of AI-2B; (
ii) it improves dysbiosis and strengthens the mucus gel of the cell wall; (
iii) it increases the level of butyrate, which causes an increase in DA, which will reduce the level of TSH with major implications on the metabolism of iodine and therefore of T3 hormone [
110]; (
iv) B increases T3 deposits from the microbiota, with the increase in the level of AI-2B and butyrate [
15,
87,
111].
7. Microbiota–Gut–Musculoskeletal System
The musculoskeletal system comprises muscles, bones, tendons, ligaments, cartilage, and other connective tissues, and its primary roles include providing structural support to the body, safeguarding vital organs, and enabling bodily movement [
112]. The relation between the GM and the musculoskeletal system can take place on two main axes: microbiota–gut–cartilage and microbiota–gut–bone.
(
i)
Microbiota–gut–cartilage axis: In the research paper entitled “Essentiality of boron for healthy bones and joints”, Newnham wrote that “in areas of the world where boron intake is 1.0 mg or less/day, the incidence of arthritis varies from 20 to 70%, while that in areas where boron intake is 3 to 10 mg, the incidence of arthritis ranges from 0 to 10%” and B levels in sera was lower in patients with OA [
113,
114,
115]. A recent approach showing that oral and intraperitoneal injection of BA is beneficial for rats with induced OA. The effect of BA on cartilage lesions was investigated and showed that the healing process of cartilage lesions could be improved by injecting BA into the knee joint of rabbits [
116,
117]. Moreover, it has been proved that B showed significant improvement in knee OA symptoms and reduces blood levels of C-reactive protein in patients suffering from OA [
23,
118].
In recent years, emerging evidence has indicated that dysbiosis and changes in butyrate metabolite production are closely related to OA pathogenesis. These findings provide insight into potential strategies targeting butyrate-producing bacteria for the prevention and treatment of OA [
109]. Butyrate reduces pain caused by OA, has chondroprotective effects and reduced articular cartilage degeneration [
119,
120]. Butyrate modulates levels of inflammatory mediators, anabolic factors, and catabolic factors in OA. These findings provide insight into potential strategies targeting the stimulation of butyrate-producing bacteria by PBCs and reverse dysbiosis and permeabilization stopping by B-stabilized mucins for the prevention and treatment of OA [
120].
(
ii)
Microbiota–gut–bone axis: Within the microbiota–gut–bone axis, butyrate promotes osteoblast differentiation and stimulates the formation of mineralized nodules to support bone growth and has been reported to directly stimulate osteoblast activation and bone formation, can promote calcium absorption, and significantly improve bone strength and bone mineral density [
121,
122,
123,
124]. Furthermore, elevated levels of free fucose and sialic acid have been documented during antibiotic therapy. This could be attributed, at least in part, to the heightened presence of mucin-degrading
Bacteroides, which has been observed to proliferate following antibiotic treatment [
125,
126]. In short, butyrate has a positive effect on the maintenance of bone health by regulating the immune function of the animal body and mitigating bone destruction and loss and as a result the beneficial effects of B on bone health can be correlated with a healthy symbiosis of the
Firmicutes phylum the largest producer of butyrate [
47,
68,
127,
128]. B helps the body use vitamin D more efficiently, acting as a stabilizer for this otherwise ephemeral nutrient. It’s not just the advantages that B provides to bone health; it also plays a crucial role in maintaining hormone balance, supporting brain health, and exhibiting anti-inflammatory properties. Nevertheless, numerous human health studies indicate that the beneficial effects of B are noticeable when the daily dose equals or exceeds 3 mg. When it comes to incorporating B into your diet, like many other essential nutrients, the key is to consume a variety of fresh, whole fruits, vegetables, and nuts. Foods, such as avocados, apricots, cashews, almonds, dates, peanuts, prunes, raisins, lentils, and hazelnuts, are prime examples of vitamin B-rich foods and rank among the finest sources of dietary prebiotic B [
129]. Excessive alcohol intake, heightened stress, heavy smoking, and a family background of OP are factors associated with elevated urinary B levels. This increase in B excretion could potentially lead to greater bone deterioration, especially in postmenopausal individuals. Among females and males over 40, urinary levels of cortisol, adrenaline, and DA were found to be elevated and correlated with increased calcium excretion. This implies that the heigh levels of catecholamines in these individuals may contribute to calcium loss and potentially compromise bone health as they age [
130]. Subsequently, for the microbiota–gut–bone axis, a nutritional adjuvant PBCs stimulate bone formation by microbial metabolite butyrate and may represent a therapeutic strategy to enhance bone anabolism [
131].
8. Conclusions
The intricate relationship between the GM and neuropathic pain in CRPS reveals a fascinating and potentially pivotal aspect of this debilitating condition. The growing body of research exploring how gut dysbiosis and alterations in the microbiota–gut–brain axis may contribute to neuropathic pain underscores the need for further investigation. While much remains to be understood about the precise mechanisms and causal links involved, these findings hold significant promise for the development of novel therapeutic strategies targeting the GM to alleviate neuropathic pain in CRPS patients. As we delve deeper into this complex interplay, we open new paths for potential relief and improved QoL for those affected by CRPS, offering hope for more effective treatments in the future.
B is a crucial element for improving the symbiotic relationships between commensal microorganisms in the microbiome and their human or animal hosts. It is important to note that human or animal host cells don’t have a nutritional requirement for B themselves. Instead, B is essential solely for maintaining a healthy symbiotic relationship between the human host and the diverse microbiomes found in the gut, scalp, mouth, skin, and vagina.
In the future, butyrate and AI-2B have the potential to serve as markers for detecting gut dysbiosis. The consumption of PBCs (such as BPPs or DCB) found in fruits and vegetables can help restore the balance between Firmicutes and Bacteroidetes and prevent or reverse dysbiosis. There is a significant link between nutrition rich in PBCs, AI-2B, the Firmicutes phylum, and other butyrate-producing bacteria. A deficiency of these prebiotic compounds in the diet can lead to dysbiosis and the breakdown of the mucus barrier in the intestinal tract.
The lack of prebiotic B will be felt through dysbiosis, the degradation of mucus and implicitly the decrease in the level of butyrate and AI-2B in the feces, and this will lead to a pleiotropic effect on the skeletal system (gut–bone and gut–cartilage axes) and the immune system (inflammation of the cardiovascular system, brain, autoimmune pathologies – MS, thyroiditis, etc.).
An essential feature of the microbiome is the difference between the sexes, which also translates into different nutritional requirements for B between the sexes. Several diseases have been explored in relation to the GM and gender differences and the effects of B in nutrition, such as OA, OP, periodontal disease, ovarian cancer, POS, obesity, IBD, T2D, FLD, allergic diseases, depression, ischemic stroke, and CVDs.
Consequently, evidence suggests that B may hold promise as a potential therapeutic pathway for individuals suffering from CRPS. While further research is needed to establish the precise mechanisms of B’s action and its optimal dosage for CRPS management, the potential benefits it offers in terms of pain relief, anti-inflammatory activity, and overall health support make it an intriguing area for future investigation. As science continues to unravel the complexities of CRPS, B remains a noteworthy candidate for inclusion in the arsenal of treatments aimed at improving the QoL for those afflicted by this challenging condition.
Author Contributions
Conceptualization, C.E.B., I.R.S. and P.L.C.; methodology, C.E.B., A.F.V., A.E.M., A.B. and Ş.C.D.; writing—original draft preparation, C.E.B., A.F.V., A.E.M., V.C.D., Ş.C.D., C.C., A.L.B. and A.F.; writing—review and editing, C.E.B., A.F.V., A.E.M., G.D.M., Ş.C.D., C.C., A.L.B., A.F. and P.L.C.; supervision, C.E.B., I.R.S. and P.L.C.; funding acquisition, C.E.B. and P.L.C. All authors have read and agreed to the published version of the manuscript.