Submitted:
20 September 2023
Posted:
22 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
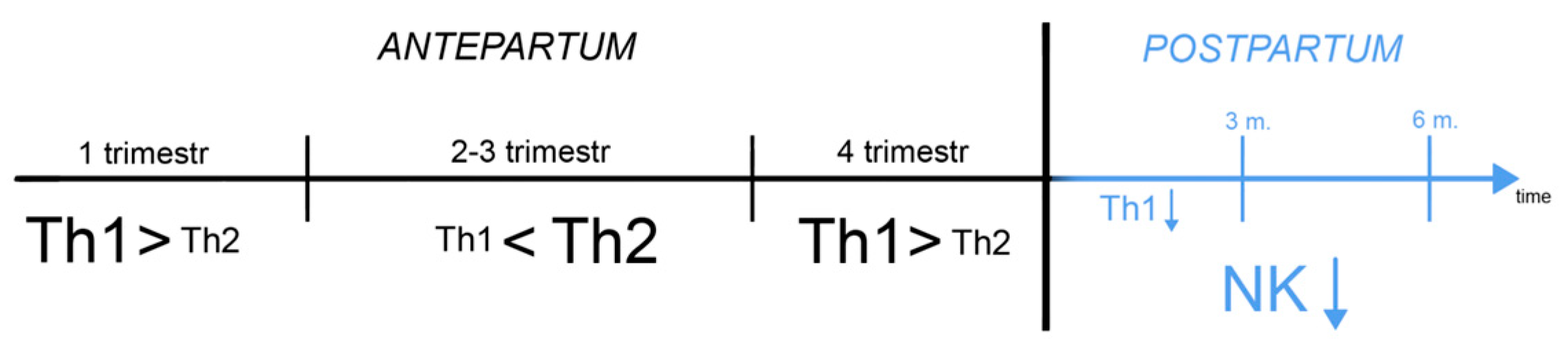
3. Immunological Markers: Relevance to PPD
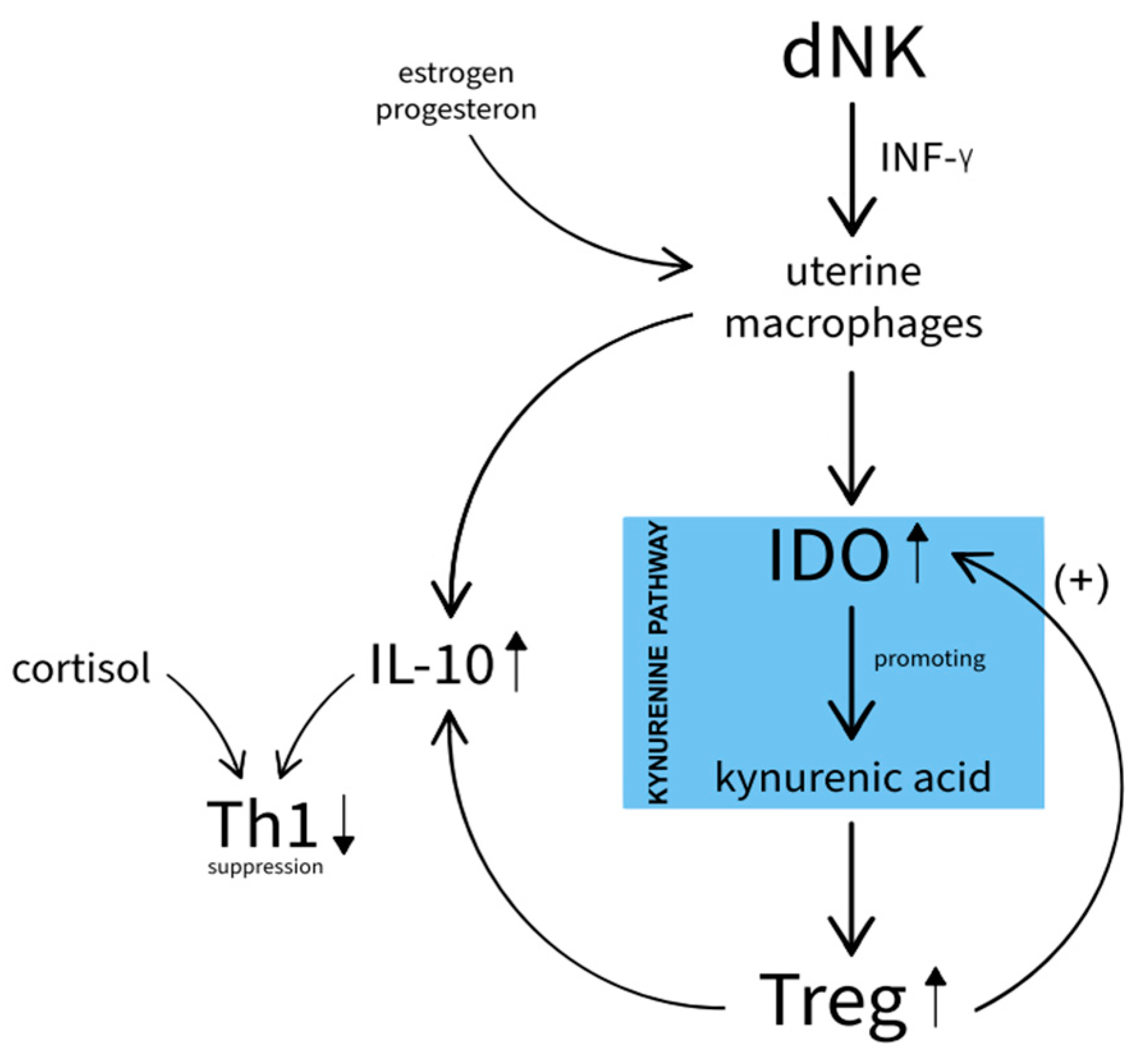
3.1. Kynurenine pathway
3.2. Hormone markers: relevance to PPD
4. Review of Hormonal Markers
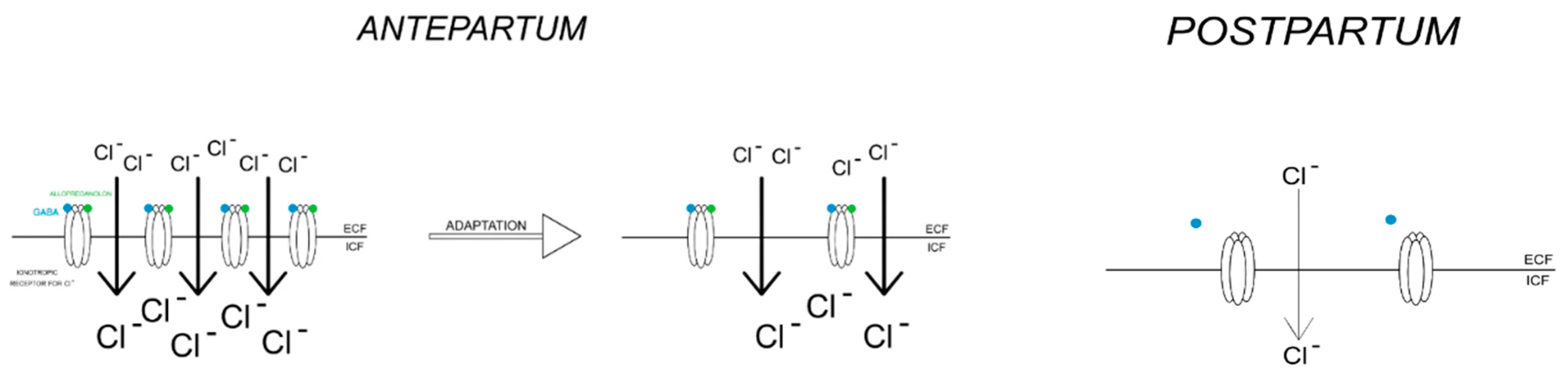
4.1. Estrogen and Progesterone, Allopreganolone
4.2. HPA Axis
4.3. Prolactin and Oxytocin
4.4. Thyroid Hormones
4.5. Conclusions
| Hormone | Concentration in PPD | |
|---|---|---|
| estrogen | ↓ | |
| progesteron | ||
| allopreganolon | ||
| HPA | CRH | ↓ |
| ACTH | ||
| Cortisol | ↓/↑ | |
5. Review of Inflammatory Markers
5.1. Tumor Necrosis Factor α (TNF-α)
5.2. CRP Protein
5.3. Transforming Growth Factor Beta (TGF-Beta β)
5.4. IL-10
5.5. IL-18
5.6. Chemokines
5.7. IL-1β
5.8. IFN-γ
5.9. IL-6
5.10. IL-2 and Soluble Interleukin-2 Receptor (sIL-2R)
5.11. IL-4
5.12. IL-8
5.13. IL-17
5.14. BDNF
5.15. IL-3
5.16. Conclusions
| Inflammation biomarker | Concentration in PPD | |
|---|---|---|
| Chemokines | CX3CL1 | ↓ |
| CXCL8 | ↑ | |
| CCL2 | ↑ | |
| CCR2 | ↓ | |
| CCL4 | ↑ | |
| CCL5 | ↑ | |
| CCL11 | ↑ | |
| TNF-α | ↑ | |
| TGF-β1 | ↓ | |
| TGF-β2 | ↑ | |
| TGF-β3 | ↓ | |
| INF-γ | ↓ | |
| Interleukins | IL-1β | ↑ |
| IL-2 i IL-2R | ↑ | |
| IL-3 | ↑ | |
| IL-4 | ↓ | |
| IL-6 | ↑ | |
| IL-8 | ↑ | |
| IL-10 | ↓ | |
| IL-17 | ↑ | |
| IL-18 | ↑ | |
| BDNF | ↓ | |
| CRP | ↑ | |
6. Nutrients
6.1. Vitamin A
6.2. B vitamins
6.3. Vitamin C
6.4. Vitamin D
6.5. Vitamin E
6.6. Zinc
6.7. Iron
6.8. Selenium
6.9. Magnesium
6.10. Conclusions
| Nutrient | Concentration in PPD | |
|---|---|---|
| vitamin A | ↓ | |
| vitamin B6 | ||
| vitamin B9 | ||
| vitamin B12 | ||
| vitamin C | ||
| vitamin D | ||
| vitamin E | ||
| elements | Zinc | ↓ |
| Iron | ||
| Selenium | ||
| Magnesium | ||
7. Review of Metabolic Markers and other Metabolic Substances
7.1. Insulin
7.2. Uric Acid
7.3. Homocysteine
7.4. Tyrosine
7.5. Vanillymandelic Acid
7.6. Alanine
7.7. Conclusions
| Metabolic markers and other metabolic substances | Concentration in PPD |
|---|---|
| Insulin | ↓ |
| Uric acid | ↓ |
| Homocysteine | ↓ |
| Tyrosine | ↓ |
| Vanillin-malic acid | ↓ |
| Alanine | ↓ |
8. Metabolic Lipid Markers
8.1. PUFAs
8.2. Cholesterol
8.3. Conclusions
| Lipid marker | Concentration in PPD |
|---|---|
| PUFA | ↓ |
| cholesterol | ↓ |
9. Oxidative Stress in Pregnancy
9.1. MPO
9.2. NO i NOS
9.3. MnSOD
9.4. Lipid Peroxidase
9.5. MDA
9.6. Conclusions
| Enzyme | Concentration in PPD |
|---|---|
| MPO | ↑ |
| NO i NOS | ↑ |
| MnSOD | ↑ |
| lipid peroxidase | ↑ |
| MDA | ↑ |
10. Genetic and Epigenetic Factors
11. Limitations
12. Funding
13. Conclusions
References
- Zhu, J.; Jin, J.; Tang, J. Inflammatory pathophysiological mechanisms implicated in postpartum depression. Front Pharmacol 2022, 13, 955672. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Mentally Sick or Not-(Bio)Markers of Psychiatric Disorders Needed. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Maes, M.; Stevens, W.; Peeters, D.; DeClerck, L.; Scharpe, S.; Bridts, C.; Schotte, C.; Cosyns, P. A study on the blunted natural killer cell activity in severely depressed patients. Life Sci 1992, 50, 505–513. [Google Scholar] [CrossRef]
- Grosse, L.; Carvalho, L.A.; Birkenhager, T.K.; Hoogendijk, W.J.; Kushner, S.A.; Drexhage, H.A.; Bergink, V. Circulating cytotoxic T cells and natural killer cells as potential predictors for antidepressant response in melancholic depression. Restoration of T regulatory cell populations after antidepressant therapy. Psychopharmacology (Berl) 2016, 233, 1679–1688. [Google Scholar] [CrossRef]
- Groer, M.W.; Morgan, K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology 2007, 32, 133–139. [Google Scholar] [CrossRef]
- Groer, M.W.; El-Badri, N.; Djeu, J.; Williams, S.N.; Kane, B.; Szekeres, K. Suppression of natural killer cell cytotoxicity in postpartum women: time course and potential mechanisms. Biol Res Nurs 2014, 16, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Postpartum depression: psychoneuroimmunological underpinnings and treatment. Neuropsychiatr Dis Treat 2013, 9, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Colucci, F.; Kieckbusch, J. Maternal uterine natural killer cells nurture fetal growth: in medio stat virtus. Trends Mol Med 2015, 21, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Colucci, F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest 2014, 124, 1872–1879. [Google Scholar] [CrossRef]
- Ren, S.; Correia, M.A. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch Biochem Biophys 2000, 377, 195–203. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol 1999, 461, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Walter, M.; Gos, T.; Guillemin, G.J.; Bernstein, H.G.; Sarnyai, Z.; Mawrin, C.; Brisch, R.; Bielau, H.; Meyer zu Schwabedissen, L.; et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 2011, 8, 94. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Larocca, L.; Ramhorst, R.; Roca, V.; Calafat, M.; Aisemberg, J.; Franchi, A.; Pérez Leirós, C. Neuroimmune-endocrine interactions during early pregnancy in an autoimmune context: focus on macrophage activation. Neuroimmunomodulation 2008, 15, 84–90. [Google Scholar] [CrossRef]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. Journal of Clinical Medicine 2020, 9, 3793. [Google Scholar] [CrossRef]
- Amin, S.; Peterson, E.J.; Reed, A.M.; Mueller, D.L. Pregnancy and rheumatoid arthritis: insights into the immunology of fetal tolerance and control of autoimmunity. Curr Rheumatol Rep 2011, 13, 449–455. [Google Scholar] [CrossRef]
- Oracz, A.; Modzelewski, S.; Iłendo, K.; Sokół, A. Brexanolone and current methods of treating postpartum and perinatal depression. Pharmacotherapy in Psychiatry and Neurology/Farmakoterapia w Psychiatrii i Neurologii 2023, 39, 53–64. [Google Scholar] [CrossRef]
- Balan, I.; Patterson, R.; Boero, G.; Krohn, H.; O'Buckley, T.K.; Meltzer-Brody, S.; Morrow, A.L. Brexanolone therapeutics in post-partum depression involves inhibition of systemic inflammatory pathways. EBioMedicine 2023, 89, 104473. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Daly, R.C.; Rubinow, D.R. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 2003, 44, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Freire-Garabal, M.; Núñez, M.J.; Balboa, J.; López-Delgado, P.; Gallego, R.; García-Caballero, T.; Fernández-Roel, M.D.; Brenlla, J.; Rey-Méndez, M. Serotonin upregulates the activity of phagocytosis through 5-HT1A receptors. Br J Pharmacol 2003, 139, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Worthen, R.J.; Beurel, E. Inflammatory and neurodegenerative pathophysiology implicated in postpartum depression. Neurobiol Dis 2022, 165, 105646. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Liu, Y.; Li, Z.; Li, X. TLR4-NF- κ B Signal Involved in Depressive-Like Behaviors and Cytokine Expression of Frontal Cortex and Hippocampus in Stressed C57BL/6 and ob/ob Mice. Neural Plast 2018, 2018, 7254016. [Google Scholar] [CrossRef]
- Sha, Q.; Madaj, Z.; Keaton, S.; Escobar Galvis, M.L.; Smart, L.; Krzyzanowski, S.; Fazleabas, A.T.; Leach, R.; Postolache, T.T.; Achtyes, E.D.; et al. Cytokines and tryptophan metabolites can predict depressive symptoms in pregnancy. Transl Psychiatry 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.J.; Escobar Galvis, M.L.; Lim, C.K.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav Immun 2020, 83, 239–247. [Google Scholar] [CrossRef]
- Wang, S.; Quan, C.; Tan, Y.; Wen, S.; Zhang, J.; Duan, K. [Correlation between kynurenine metabolites and postpartum depression]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2018, 43, 725–731. [Google Scholar] [CrossRef]
- Tulchinsky, D.; Hobel, C.J.; Yeager, E.; Marshall, J.R. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol 1972, 112, 1095–1100. [Google Scholar] [CrossRef]
- Smith, R.S. The macrophage theory of depression. Med Hypotheses 1991, 35, 298–306. [Google Scholar] [CrossRef]
- Malyala, A.; Kelly, M.J.; Rønnekleiv, O.K. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids 2005, 70, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Maguire, J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits 2014, 8, 3. [Google Scholar] [CrossRef]
- Walton, N.; Maguire, J. Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiol Stress 2019, 11, 100198. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.; Ferando, I.; Simonsen, C.; Mody, I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci 2009, 29, 9592–9601. [Google Scholar] [CrossRef]
- Patchev, V.K.; Shoaib, M.; Holsboer, F.; Almeida, O.F. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994, 62, 265–271. [Google Scholar] [CrossRef]
- Stirone, C.; Duckles, S.P.; Krause, D.N.; Procaccio, V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 2005, 68, 959–965. [Google Scholar] [CrossRef]
- Scharfman, H.E.; MacLusky, N.J. Estrogen-growth factor interactions and their contributions to neurological disorders. Headache 2008, 48 Suppl 2, S77–89. [Google Scholar] [CrossRef]
- Khodadad, M.; Bahadoran, P.; Kheirabadi, G.R.; Sabzghabaee, A.M. Can Vitamin B6 Help to Prevent Postpartum Depression? A Randomized Controlled Trial. Int J Prev Med 2021, 12, 136. [Google Scholar] [CrossRef]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci 2015, 9, 37. [Google Scholar] [CrossRef]
- Mastorakos, G.; Ilias, I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci 2003, 997, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Torpy, D.J.; Gold, P.W. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 1998, 129, 229–240. [Google Scholar] [CrossRef]
- Ouakinin, S.R.S.; Barreira, D.P.; Gois, C.J. Depression and Obesity: Integrating the Role of Stress, Neuroendocrine Dysfunction and Inflammatory Pathways. Front Endocrinol (Lausanne) 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bosmans, E.; Meltzer, H.Y.; Scharpé, S.; Suy, E. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry 1993, 150, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; McEwen, B.S.; Ledoux, J.E.; Nader, K. Fear learning transiently impairs hippocampal cell proliferation. Neuroscience 2005, 130, 17–24. [Google Scholar] [CrossRef]
- Lee, A.R.Y.B.; Tariq, A.; Lau, G.; Tok, N.W.K.; Tam, W.W.S.; Ho, C.S.H. Vitamin E, Alpha-Tocopherol, and Its Effects on Depression and Anxiety: A Systematic Review and Meta-Analysis. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 1995, 15, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Hanson, N.D.; Owens, M.J.; Nemeroff, C.B. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology 2011, 36, 2589–2602. [Google Scholar] [CrossRef]
- Workman, J.L.; Barha, C.K.; Galea, L.A. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 2012, 126, 54–72. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Colquhoun, H.; Riesenberg, R.; Epperson, C.N.; Deligiannidis, K.M.; Rubinow, D.R.; Li, H.; Sankoh, A.J.; Clemson, C.; Schacterle, A.; et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 2018, 392, 1058–1070. [Google Scholar] [CrossRef]
- Stefaniak, M.; Dmoch-Gajzlerska, E.; Jankowska, K.; Rogowski, A.; Kajdy, A.; Maksym, R.B. Progesterone and Its Metabolites Play a Beneficial Role in Affect Regulation in the Female Brain. Pharmaceuticals (Basel) 2023, 16. [Google Scholar] [CrossRef]
- Management of Premenstrual Syndrome: Green-top Guideline No. 48. BJOG 2017, 124, e73–e105. [Google Scholar] [CrossRef]
- O'Hara, M.W.; Schlechte, J.A.; Lewis, D.A.; Varner, M.W. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol 1991, 100, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Harris, B. Biological and hormonal aspects of postpartum depressed mood. Br J Psychiatry 1994, 164, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, M.T.; Ghubash, R.; Karim, L.; Krymski, M.; Bhai, I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology 1998, 23, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Nott, P.N.; Franklin, M.; Armitage, C.; Gelder, M.G. Hormonal changes and mood in the puerperium. Br J Psychiatry 1976, 128, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Johns, S.; Fung, H.; Thomas, R.; Walker, R.; Read, G.; Riad-Fahmy, D. The hormonal environment of post-natal depression. Br J Psychiatry 1989, 154, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Zonana, J.; Gorman, J.M. The neurobiology of postpartum depression. CNS Spectr 2005, 10, 792–799. [Google Scholar] [CrossRef]
- Pearson Murphy, B.E.; Steinberg, S.I.; Hu, F.Y.; Allison, C.M. Neuroactive ring A-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5 alpha-dihydroprogesterone in depressed patients during the latter half of pregnancy. J Clin Endocrinol Metab 2001, 86, 5981–5987. [Google Scholar] [CrossRef]
- Goland, R.S.; Wardlaw, S.L.; Stark, R.I.; Brown, L.S.; Frantz, A.G. High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. J Clin Endocrinol Metab 1986, 63, 1199–1203. [Google Scholar] [CrossRef]
- Thomson, M. The physiological roles of placental corticotropin releasing hormone in pregnancy and childbirth. J Physiol Biochem 2013, 69, 559–573. [Google Scholar] [CrossRef]
- Greenwood, J.; Parker, G. The dexamethasone suppression test in the puerperium. Aust N Z J Psychiatry 1984, 18, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Schmidt, P.J.; Danaceau, M.; Murphy, J.; Nieman, L.; Rubinow, D.R. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000, 157, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Yim, I.S.; Glynn, L.M.; Dunkel-Schetter, C.; Hobel, C.J.; Chicz-DeMet, A.; Sandman, C.A. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry 2009, 66, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Brody, S.; Stuebe, A.; Dole, N.; Savitz, D.; Rubinow, D.; Thorp, J. Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD). J Clin Endocrinol Metab 2011, 96, E40–47. [Google Scholar] [CrossRef] [PubMed]
- Melón, L.C.; Hooper, A.; Yang, X.; Moss, S.J.; Maguire, J. Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology 2018, 90, 182–193. [Google Scholar] [CrossRef]
- Kammerer, M.; Taylor, A.; Glover, V. The HPA axis and perinatal depression: a hypothesis. Arch Womens Ment Health 2006, 9, 187–196. [Google Scholar] [CrossRef]
- Lamers, F.; de Jonge, P.; Nolen, W.A.; Smit, J.H.; Zitman, F.G.; Beekman, A.T.; Penninx, B.W. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 2010, 71, 1582–1589. [Google Scholar] [CrossRef]
- Szpunar, M.J.; Parry, B.L. A systematic review of cortisol, thyroid-stimulating hormone, and prolactin in peripartum women with major depression. Arch Womens Ment Health 2018, 21, 149–161. [Google Scholar] [CrossRef]
- Ystrom, E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth 2012, 12, 36. [Google Scholar] [CrossRef]
- Deems, N.P.; Leuner, B. Pregnancy, postpartum and parity: Resilience and vulnerability in brain health and disease. Front Neuroendocrinol 2020, 57, 100820. [Google Scholar] [CrossRef]
- Parry, B.L.; Sorenson, D.L.; Meliska, C.J.; Basavaraj, N.; Zirpoli, G.G.; Gamst, A.; Hauger, R. Hormonal basis of mood and postpartum disorders. Curr Womens Health Rep 2003, 3, 230–235. [Google Scholar] [PubMed]
- Okun, M.L.; Luther, J.; Prather, A.A.; Perel, J.M.; Wisniewski, S.; Wisner, K.L. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord 2011, 130, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Hibbeln, J.R.; Wisner, K.L.; Brumbach, B.H.; Watchman, M.; Gelenberg, A.J. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr Scand 2006, 113, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.H.; Backes, K.A.; Schuette, S.A. PLASMA OXYTOCIN CONCENTRATION AND DEPRESSIVE SYMPTOMS: A REVIEW OF CURRENT EVIDENCE AND DIRECTIONS FOR FUTURE RESEARCH. Depress Anxiety 2016, 33, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Wysokiński, A.; Kłoszewska, I. Level of thyroid-stimulating hormone (TSH) in patients with acute schizophrenia, unipolar depression or bipolar disorder. Neurochem Res 2014, 39, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- O'Leary, P.C.; Boyne, P.; Atkinson, G.; Mileham, K.J.; James, I. Longitudinal study of serum thyroid hormone levels during normal pregnancy. Int J Gynaecol Obstet 1992, 38, 171–179. [Google Scholar] [CrossRef]
- Miller, E.S.; Sakowicz, A.; Roy, A.; Yang, A.; Sullivan, J.T.; Grobman, W.A.; Wisner, K.L. Plasma and cerebrospinal fluid inflammatory cytokines in perinatal depression. Am J Obstet Gynecol 2019, 220, 271–e271. [Google Scholar] [CrossRef] [PubMed]
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The Role of TNF-α and Anti-TNF-α Agents during Preconception, Pregnancy, and Breastfeeding. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Corwin, E.J.; Pajer, K.; Paul, S.; Lowe, N.; Weber, M.; McCarthy, D.O. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun 2015, 49, 86–93. [Google Scholar] [CrossRef]
- Buglione-Corbett, R.; Deligiannidis, K.M.; Leung, K.; Zhang, N.; Lee, M.; Rosal, M.C.; Moore Simas, T.A. Expression of inflammatory markers in women with perinatal depressive symptoms. Arch Womens Ment Health 2018, 21, 671–679. [Google Scholar] [CrossRef]
- Simpson, W.; Steiner, M.; Coote, M.; Frey, B.N. Relationship between inflammatory biomarkers and depressive symptoms during late pregnancy and the early postpartum period: a longitudinal study. Braz J Psychiatry 2016, 38, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Nousiainen, N.; Scheinin, N.M.; Maksimow, M.; Salmi, M.; Lehto, S.M.; Tolvanen, M.; Lukkarinen, H.; Karlsson, H. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy-the FinnBrain Birth Cohort Study. Arch Womens Ment Health 2017, 20, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Szpakowski, M.; Nowak, M.; Oszukowski, P.; Wieczorek, A.; Skotnicka, A. [C-reactive protein in normal pregnancy]. Ginekol Pol 1996, 67, 17–20. [Google Scholar]
- Liu, H.; Zhang, Y.; Gao, Y.; Zhang, Z. Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Res 2016, 243, 43–48. [Google Scholar] [CrossRef]
- Miller, E.S.; Hoxha, D.; Pinheiro, E.; Grobman, W.A.; Wisner, K.L. The association of serum C-reactive protein with the occurrence and course of postpartum depression. Arch Womens Ment Health 2019, 22, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Corwin, E.J.; Murray-Kolb, L.E.; Beard, J.L. Low hemoglobin level is a risk factor for postpartum depression. J Nutr 2003, 133, 4139–4142. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 2009, 9, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.G.; Brown, D.A.; Fairlie, W.D.; Bauskin, A.R.; Brown, P.K.; Munier, M.L.C.; Russell, P.K.; Salamonsen, L.A.; Wallace, E.M.; Breit, S.N. The Transforming Growth Factor-β Superfamily Cytokine Macrophage Inhibitory Cytokine-1 Is Present in High Concentrations in the Serum of Pregnant Women1. The Journal of Clinical Endocrinology & Metabolism 2000, 85, 4781–4788. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhou, L.; Chen, J.; Li, M.; Xie, R. [Association between postpartum depression and concentrations of transforming growth factor-β in human colostrum: a nested cohort study]. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 1426–1430. [Google Scholar] [CrossRef]
- Gałecki, P.; Talarowska, M. Inflammatory theory of depression. Psychiatr Pol 2018, 52, 437–447. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, H.-F.; Chen, J.; Li, Z.-B.; Han, Y.-S.; Chen, J.-X.; Li, J.-C. Postpartum Depression: Current Status and Possible Identification Using Biomarkers. Frontiers in Psychiatry 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Frontiers in Immunology 2013, 4. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001, 19, 423–474. [Google Scholar] [CrossRef] [PubMed]
- Löb, S.; Ochmann, B.; Ma, Z.; Vilsmaier, T.; Kuhn, C.; Schmoeckel, E.; Herbert, S.L.; Kolben, T.; Wöckel, A.; Mahner, S.; et al. The role of Interleukin-18 in recurrent early pregnancy loss. J Reprod Immunol 2021, 148, 103432. [Google Scholar] [CrossRef]
- Trifu, S.; Vladuti, A.; Popescu, A. THE NEUROENDOCRINOLOGICAL ASPECTS OF PREGNANCY AND POSTPARTUM DEPRESSION. Acta Endocrinol (Buchar) 2019, 15, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Fransson, E.; Dubicke, A.; Byström, B.; Ekman-Ordeberg, G.; Hjelmstedt, A.; Lekander, M. Negative Emotions and Cytokines in Maternal and Cord Serum at Preterm Birth. American Journal of Reproductive Immunology 2012, 67, 506–514. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Camacho-Arroyo, I.; Flores-Ramos, M.; Mancilla-Herrera, I.; Cruz, F.M.C.; Hernández-Ruiz, J.; Diaz, G.P.; Labonne, B.F.; Del Pilar Meza-Rodríguez, M.; Gelman, P.L. Chemokine profile in women with moderate to severe anxiety and depression during pregnancy. BMC Pregnancy Childbirth 2021, 21, 807. [Google Scholar] [CrossRef]
- Edvinsson, Å.; Bränn, E.; Hellgren, C.; Freyhult, E.; White, R.; Kamali-Moghaddam, M.; Olivier, J.; Bergquist, J.; Boström, A.E.; Schiöth, H.B.; et al. Lower inflammatory markers in women with antenatal depression brings the M1/M2 balance into focus from a new direction. Psychoneuroendocrinology 2017, 80, 15–25. [Google Scholar] [CrossRef]
- Maes, M.; Abe, Y.; Sirichokchatchawan, W.; Suwimonteerabutr, J.; Sangkomkamhangd, U.; Almulla, A.F.; Satthapisit, S. The Cytokine, Chemokine, and Growth Factor Network of Prenatal Depression. Brain Sciences 2023, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Satyam, A.; Sharma, J.B. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol 2007, 58, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Falzone, L.; Bramanti, P.; Nicoletti, F.; Basile, M.S. Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Exp Ther Med 2019, 18, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.A.; Air, T.; Pradhan, A.; Johnston, J.; Lavretsky, H.; Stuart, M.J.; Baune, B.T. A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2016, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kudo, K.; Mabuchi, T.; Takemoto, K.; Fujimaki, K.; Wati, H.; Iguchi, H.; Tezuka, H.; Kanba, S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology 2006, 31, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Corwin, E.J.; Johnston, N.; Pugh, L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs 2008, 10, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Fišar, Z.; Medina, M.; Scapagnini, G.; Nowak, G.; Berk, M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 2012, 20, 127–150. [Google Scholar] [CrossRef]
- Sunico, C.R.; Portillo, F.; González-Forero, D.; Moreno-López, B. Nitric-oxide-directed synaptic remodeling in the adult mammal CNS. J Neurosci 2005, 25, 1448–1458. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Glaser, R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res 2002, 53, 873–876. [Google Scholar] [CrossRef]
- Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 1995, 332, 1351–1362. [Google Scholar] [CrossRef]
- Lenz, K.M.; Post, C.; Castaneda, A.J.; Banta, P.; Nelson, L.H.; Saulsbery, A.I.; Leuner, B. Abstract # 3185 Central immune alterations in a gestational stress animal model of postpartum depression. Brain, Behavior, and Immunity 2019, 76, e38. [Google Scholar]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.E.; Jevitt, C.; Ji, M. Immune changes and dysphoric moods across the postpartum. Am J Reprod Immunol 2015, 73, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Han, Q.Q.; Gong, W.Q.; Pan, D.H.; Wang, L.Z.; Hu, W.; Yang, M.; Li, B.; Yu, J.; Liu, Q. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation 2018, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Solmi, M.; Sanches, M.; Machado, M.O.; Stubbs, B.; Ajnakina, O.; Sherman, C.; Sun, Y.R.; Liu, C.S.; Brunoni, A.R.; et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl Psychiatry 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.A.; Prêle, C.M.; Nicholson, S.E.; Kolesnik, T.B.; Hart, P.H. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology 2010, 131, 118–127. [Google Scholar] [CrossRef]
- Lu, L.; Hu, X.; Jin, X. IL-4 as a potential biomarker for differentiating major depressive disorder from bipolar depression. Medicine (Baltimore) 2023, 102, e33439. [Google Scholar] [CrossRef]
- Min, Z.; Li, Y.; Ying, H. Blood T-helper 17 cells and interleukin-17A correlate with the elevated risk of postpartum depression and anxiety. J Clin Lab Anal 2022, 36, e24559. [Google Scholar] [CrossRef]
- Szpunar, M.J.; Malaktaris, A.; Baca, S.A.; Hauger, R.L.; Lang, A.J. Are alterations in estradiol, cortisol, and inflammatory cytokines associated with depression during pregnancy and postpartum? An exploratory study. Brain Behav Immun Health 2021, 16, 100309. [Google Scholar] [CrossRef]
- Hong, J.; Hutton, G.J. Regulatory effects of interferon-β on osteopontin and interleukin-17 expression in multiple sclerosis. J Interferon Cytokine Res 2010, 30, 751–757. [Google Scholar] [CrossRef]
- Saraykar, S.; Cao, B.; Barroso, L.S.; Pereira, K.S.; Bertola, L.; Nicolau, M.; Ferreira, J.D.; Dias, N.S.; Vieira, E.L.; Teixeira, A.L.; et al. Plasma IL-17A levels in patients with late-life depression. Braz J Psychiatry 2018, 40, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.I.; Park, J.I.; Kim, Y.K.; Yang, J.C. Decreased Plasma BDNF Levels of Patients with Somatization Disorder. Psychiatry Investig 2016, 13, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, J.; Yao, H.; Cai, Y.; Cheng, R. Serum BDNF concentration after delivery is associated with development of postpartum depression: A 3-month follow up study. J Affect Disord 2016, 200, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, K.H.; Lee, B.H.; Kim, Y.K. Plasma level of brain-derived neurotrophic factor (BDNF) in patients with postpartum depression. Prog Neuropsychopharmacol Biol Psychiatry 2021, 109, 110245. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Berk, M.; Turck, C.W.; Steiner, J.; Gonçalves, C.A. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol Psychiatry 2014, 19, 750–751. [Google Scholar] [CrossRef]
- Xiu, M.H.; Lin, C.G.; Tian, L.; Tan, Y.L.; Chen, J.; Chen, S.; Tan, S.P.; Wang, Z.R.; Yang, F.D.; Chen, D.C.; et al. Increased IL-3 serum levels in chronic patients with schizophrenia: Associated with psychopathology. Psychiatry Res 2015, 229, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Rupanagunta, G.P.; Nandave, M.; Rawat, D.; Upadhyay, J.; Rashid, S.; Ansari, M.N. Postpartum depression: aetiology, pathogenesis and the role of nutrients and dietary supplements in prevention and management. Saudi Pharm J 2023, 31, 1274–1293. [Google Scholar] [CrossRef]
- Sparling, T.M.; Waid, J.L.; Wendt, A.S.; Gabrysch, S. Depression among women of reproductive age in rural Bangladesh is linked to food security, diets and nutrition. Public Health Nutr 2020, 23, 660–673. [Google Scholar] [CrossRef]
- Mousa, A.; Naqash, A.; Lim, S. Macronutrient and Micronutrient Intake during Pregnancy: An Overview of Recent Evidence. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Wisner, K.L. Nutrition and depression: implications for improving mental health among childbearing-aged women. Biol Psychiatry 2005, 58, 679–685. [Google Scholar] [CrossRef]
- MacDonald, K.; Krishnan, A.; Cervenka, E.; Hu, G.; Guadagno, E.; Trakadis, Y. Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. Am J Med Genet B Neuropsychiatr Genet 2019, 180, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Rouillon, F.; Thalassinos, M.; Miller, H.D.; Lemperiere, T. Folates and post partum depression. J Affect Disord 1992, 25, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, M.T.; Ghubash, R.; Karim, L.; Krymski, M.; Anderson, D.N. The role of pterins and related factors in the biology of early postpartum depression. Eur Neuropsychopharmacol 1999, 9, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.C.; Downey, L.A.; Simpson, T.; McPhee, G.; Oliver, C.; Stough, C. The Effect of a High-Dose Vitamin B Multivitamin Supplement on the Relationship between Brain Metabolism and Blood Biomarkers of Oxidative Stress: A Randomized Control Trial. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Rogne, T.; Tielemans, M.J.; Chong, M.F.; Yajnik, C.S.; Krishnaveni, G.V.; Poston, L.; Jaddoe, V.W.; Steegers, E.A.; Joshi, S.; Chong, Y.S.; et al. Associations of Maternal Vitamin B12 Concentration in Pregnancy With the Risks of Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analysis of Individual Participant Data. Am J Epidemiol 2017, 185, 212–223. [Google Scholar] [CrossRef]
- Pierce, M.A.; Johnson, M.D.; Maciunas, R.J.; Murray, M.J.; Allen, G.S.; Harbison, M.A.; Creasy, J.L.; Kessler, R.M. Evaluating contrast-enhancing brain lesions in patients with AIDS by using positron emission tomography. Ann Intern Med 1995, 123, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, M.; Tveden-Nyborg, P.; Lykkesfeldt, J. Regulation of vitamin C homeostasis during deficiency. Nutrients 2013, 5, 2860–2879. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Lykkesfeldt, J. Does vitamin C deficiency increase lifestyle-associated vascular disease progression? Evidence based on experimental and clinical studies. Antioxid Redox Signal 2013, 19, 2084–2104. [Google Scholar] [CrossRef]
- Nauser, T.; Koppenol, W.H.; Schöneich, C. Protein thiyl radical reactions and product formation: a kinetic simulation. Free Radic Biol Med 2015, 80, 158–163. [Google Scholar] [CrossRef]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J Nutr Biochem 2020, 85, 108459. [Google Scholar] [CrossRef]
- Ward, M.S.; Lamb, J.; May, J.M.; Harrison, F.E. Behavioral and monoamine changes following severe vitamin C deficiency. J Neurochem 2013, 124, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008, 88, 491S–499S. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Weaver, C.M. Calcium and vitamin D. Endocrinol Metab Clin North Am 2003, 32, 181–194. [Google Scholar] [CrossRef]
- Deluca, H.F.; Cantorna, M.T. Vitamin D: its role and uses in immunology. FASEB J 2001, 15, 2579–2585. [Google Scholar] [CrossRef]
- Amini, S.; Jafarirad, S.; Amani, R. Postpartum depression and vitamin D: A systematic review. Crit Rev Food Sci Nutr 2019, 59, 1514–1520. [Google Scholar] [CrossRef]
- Lansdowne, A.T.; Provost, S.C. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl) 1998, 135, 319–323. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev 2009, 67, 481–492. [Google Scholar] [CrossRef]
- Prohan, M.; Amani, R.; Nematpour, S.; Jomehzadeh, N.; Haghighizadeh, M.H. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-CRP levels in relation to depression scales in university male students. Redox Rep 2014, 19, 133–139. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Pioli, R.; Demedts, P.; Wauters, A.; Neels, H.; Christophe, A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord 2000, 58, 241–246. [Google Scholar] [CrossRef]
- Gautam, M.; Agrawal, M.; Sharma, P.; Gautam, A.S.; Gautam, S. Role of antioxidants in generalised anxiety disorder and depression. Indian J Psychiatry 2012, 54, 244–247. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Klabnik, J.J.; O'Donnell, J.M. Novel therapeutic targets in depression and anxiety: antioxidants as a candidate treatment. Curr Neuropharmacol 2014, 12, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Evers, S.E.; Avison, W.R.; Campbell, M.K. Higher zinc intake buffers the impact of stress on depressive symptoms in pregnancy. Nutr Res 2010, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.J.; Gong, B.; Xu, F.Y.; Luo, Y. Four trace elements in pregnant women and their relationships with adverse pregnancy outcomes. Eur Rev Med Pharmacol Sci 2015, 19, 4690–4697. [Google Scholar] [PubMed]
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Mahieu, B.; Nowak, G.; Maes, M. Lower Serum Zinc and Higher CRP Strongly Predict Prenatal Depression and Physio-somatic Symptoms, Which All Together Predict Postnatal Depressive Symptoms. Mol Neurobiol 2017, 54, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Imai, K.; Owaki, T.; Kobayashi-Nakano, T.; Ushida, T.; Iitani, Y.; Nakamura, N.; Kajiyama, H.; Kotani, T. The Possible Effects of Zinc Supplementation on Postpartum Depression and Anemia. Medicina (Kaunas) 2022, 58. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddin, N.; Zavoshy, R.; Noroozi, M.; Sarichloo, M.E.; Jahanihashemi, H. The Association Between Postpartum Depression and Pica During Pregnancy. Glob J Health Sci 2015, 8, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Mokhber, N.; Namjoo, M.; Tara, F.; Boskabadi, H.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Majdi, M.R.; Tavallaie, S.; Azimi-Nezhad, M.; et al. Effect of supplementation with selenium on postpartum depression: a randomized double-blind placebo-controlled trial. J Matern Fetal Neonatal Med 2011, 24, 104–108. [Google Scholar] [CrossRef]
- Abumaria, N.; Yin, B.; Zhang, L.; Li, X.Y.; Chen, T.; Descalzi, G.; Zhao, L.; Ahn, M.; Luo, L.; Ran, C.; et al. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J Neurosci 2011, 31, 14871–14881. [Google Scholar] [CrossRef] [PubMed]
- Iosifescu, D.V.; Bolo, N.R.; Nierenberg, A.A.; Jensen, J.E.; Fava, M.; Renshaw, P.F. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry 2008, 63, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.S.; Sakowicz, A.; Roy, A.; Wang, A.; Yang, A.; Ciolino, J.; Grobman, W.A.; Wisner, K.L.; Yee, L.M. Is peripartum magnesium sulfate associated with a reduction in postpartum depressive symptoms? Am J Obstet Gynecol MFM 2021, 3, 100407. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Littenberg, B.; MacLean, C.D.; Kennedy, A.G.; Daley, C. Role of magnesium supplementation in the treatment of depression: A randomized clinical trial. PLoS One 2017, 12, e0180067. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Overland, S.; Stewart, R.; Tell, G.S.; Bjelland, I.; Mykletun, A. Association between magnesium intake and depression and anxiety in community-dwelling adults: the Hordaland Health Study. Aust N Z J Psychiatry 2009, 43, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yary, T.; Lehto, S.M.; Tolmunen, T.; Tuomainen, T.P.; Kauhanen, J.; Voutilainen, S.; Ruusunen, A. Dietary magnesium intake and the incidence of depression: A 20-year follow-up study. J Affect Disord 2016, 193, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Guintivano, J.; Aberg, K.A.; Clark, S.L.; Rubinow, D.R.; Sullivan, P.F.; Meltzer-Brody, S.; van den Oord, E.J.C.G. Transcriptome-wide association study for postpartum depression implicates altered B-cell activation and insulin resistance. Mol Psychiatry 2022, 27, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, N.L.; Kenna, H.A.; Williams, K.E.; Powers, B.; Wroolie, T.; Schatzberg, A.F. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. ScientificWorldJournal 2010, 10, 321–328. [Google Scholar] [CrossRef]
- Rasgon, N.L.; Kenna, H.A.; Reynolds-May, M.F.; Stemmle, P.G.; Vemuri, M.; Marsh, W.; Wang, P.; Ketter, T.A. Metabolic dysfunction in women with bipolar disorder: the potential influence of family history of type 2 diabetes mellitus. Bipolar Disord 2010, 12, 504–513. [Google Scholar] [CrossRef]
- Sepanjnia, K.; Modabbernia, A.; Ashrafi, M.; Modabbernia, M.J.; Akhondzadeh, S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology 2012, 37, 2093–2100. [Google Scholar] [CrossRef]
- Kim, C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabetic Medicine 2014, 31, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ruohomäki, A.; Toffol, E.; Upadhyaya, S.; Keski-Nisula, L.; Pekkanen, J.; Lampi, J.; Voutilainen, S.; Tuomainen, T.P.; Heinonen, S.; Kumpulainen, K.; et al. The association between gestational diabetes mellitus and postpartum depressive symptomatology: A prospective cohort study. J Affect Disord 2018, 241, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Tokuda, N.; Kobayashi, Y.; Tanaka, H.; Sawai, H.; Shibahara, H.; Takeshima, Y.; Shima, M.; Group, J.E.a.C.s.S. Association between the serum insulin-like growth factor-1 concentration in the first trimester of pregnancy and postpartum depression. Psychiatry Clin Neurosci 2021, 75, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X. A Novel Review of Homocysteine and Pregnancy Complications. Biomed Res Int 2021, 2021, 6652231. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Fernandez-Ballart, J.D. Homocysteine in pregnancy. Adv Clin Chem 2011, 53, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, W.; Huang, Y.; Wen, X.; Huang, J.; Wang, Y.; Sheng, X. A Preliminary Study of Uric Metabolomic Alteration for Postpartum Depression Based on Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry. Dis Markers 2019, 2019, 4264803. [Google Scholar] [CrossRef]
- Liu, Y. Demonstrations of AIDS-associated malignancies and infections at FDG PET-CT. Ann Nucl Med 2011, 25, 536–546. [Google Scholar] [CrossRef]
- McLean, A.; Rubinsztein, J.S.; Robbins, T.W.; Sahakian, B.J. The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology (Berl) 2004, 171, 286–297. [Google Scholar] [CrossRef]
- Doornbos, B.; Fekkes, D.; Tanke, M.A.; de Jonge, P.; Korf, J. Sequential serotonin and noradrenalin associated processes involved in postpartum blues. Prog Neuropsychopharmacol Biol Psychiatry 2008, 32, 1320–1325. [Google Scholar] [CrossRef]
- Mukta, F.Y.; Akhter, Q.S.; Islam, S.; Layla, K.N.; Azad, A.B.; Rahman, K.L.; Sarker, S.; Shahid, S.T.B. The evaluation of urinary vanillylmandelic acid level in patients with generalized anxiety disorder. 2021, 001–005.
- Wang, W.; Guo, H.; Zhang, S.X.; Li, J.; Cheng, K.; Bai, S.J.; Yang, D.Y.; Wang, H.Y.; Liang, Z.H.; Liao, L.; et al. Targeted Metabolomic Pathway Analysis and Validation Revealed Glutamatergic Disorder in the Prefrontal Cortex among the Chronic Social Defeat Stress Mice Model of Depression. J Proteome Res 2016, 15, 3784–3792. [Google Scholar] [CrossRef]
- Jiang, R.; Wu, S.; Fang, C.; Wang, C.; Yang, Y.; Liu, C.; Hu, J.; Huang, Y. Amino acids levels in early pregnancy predict subsequent gestational diabetes. J Diabetes 2020, 12, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F. Phospholipid metabolism and depression: the possible roles of phospholipase A2 and coenzyme A-independent transacylase. Hum Psychopharmacol 2001, 16, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Essential fatty acid transfer and fetal development. Placenta 2005, 26 Suppl A, S70–75. [Google Scholar] [CrossRef]
- Tahaei, H.; Gignac, F.; Pinar, A.; Fernandez-Barrés, S.; Romaguera, D.; Vioque, J.; Santa-Marina, L.; Subiza-Pérez, M.; Llop, S.; Soler-Blasco, R.; et al. Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Multi-Centre Population-Based Birth Cohort Study in Spain. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Christophe, A.; Delanghe, J.; Altamura, C.; Neels, H.; Meltzer, H.Y. Lowered omega3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res 1999, 85, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef]
- Wahab, R.J.; Jaddoe, V.W.V.; Mezzoiuso, A.G.; Gaillard, R. Maternal polyunsaturated fatty acid concentrations during pregnancy and childhood liver fat accumulation. Clin Nutr 2022, 41, 847–854. [Google Scholar] [CrossRef]
- Hamazaki, K.; Matsumura, K.; Tsuchida, A.; Kasamatsu, H.; Tanaka, T.; Ito, M.; Inadera, H.; Group, J.E.a.C.s.S. Dietary intake of fish and n-3 polyunsaturated fatty acids and risk of postpartum depression: a nationwide longitudinal study - the Japan Environment and Children's Study (JECS). Psychol Med 2020, 50, 2416–2424. [Google Scholar] [CrossRef]
- Llorente, A.M.; Jensen, C.L.; Voigt, R.G.; Fraley, J.K.; Berretta, M.C.; Heird, W.C. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. Am J Obstet Gynecol 2003, 188, 1348–1353. [Google Scholar] [CrossRef]
- Freeman, M.P.; Davis, M.; Sinha, P.; Wisner, K.L.; Hibbeln, J.R.; Gelenberg, A.J. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J Affect Disord 2008, 110, 142–148. [Google Scholar] [CrossRef]
- Marangell, L.B.; Martinez, J.M.; Zboyan, H.A.; Chong, H.; Puryear, L.J. Omega-3 fatty acids for the prevention of postpartum depression: negative data from a preliminary, open-label pilot study. Depress Anxiety 2004, 19, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.; Smeeth, D.; Milner, Y.; Thure, S. The Role of Lipid Biomarkers in Major Depression. Healthcare (Basel) 2017, 5. [Google Scholar] [CrossRef]
- Ramachandran Pillai, R.; Wilson, A.B.; Premkumar, N.R.; Kattimani, S.; Sagili, H.; Rajendiran, S. Low serum levels of High-Density Lipoprotein cholesterol (HDL-c) as an indicator for the development of severe postpartum depressive symptoms. PLoS One 2018, 13, e0192811. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Oxygen, the Janus gas; its effects on human placental development and function. J Anat 2009, 215, 27–35. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, H.; Qiu, Y.; Liu, Y.; Wen, L.; Fu, Y.; Qi, H.; Baker, P.N.; Tong, C. Reactive Oxygen Species are Essential for Placental Angiogenesis During Early Gestation. Oxid Med Cell Longev 2022, 2022, 4290922. [Google Scholar] [CrossRef]
- Hubel, C.A.; Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rogers, G.M.; McLaughlin, M.K. Lipid peroxidation in pregnancy: new perspectives on preeclampsia. Am J Obstet Gynecol 1989, 161, 1025–1034. [Google Scholar] [CrossRef]
- Cranfield, L.M.; Gollan, J.L.; White, A.G.; Dormandy, T.L. Serum antioxidant activity in normal and abnormal subjects. Ann Clin Biochem 1979, 16, 299–306. [Google Scholar] [CrossRef]
- Hansson, M.; Olsson, I.; Nauseef, W.M. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch Biochem Biophys 2006, 445, 214–224. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 2002, 181-182, 223–227. [Google Scholar] [CrossRef]
- Hammer, A.; Desoye, G.; Dohr, G.; Sattler, W.; Malle, E. Myeloperoxidase-dependent generation of hypochlorite-modified proteins in human placental tissues during normal pregnancy. Lab Invest 2001, 81, 543–554. [Google Scholar] [CrossRef]
- Kindzelskii, A.L.; Clark, A.J.; Espinoza, J.; Maeda, N.; Aratani, Y.; Romero, R.; Petty, H.R. Myeloperoxidase accumulates at the neutrophil surface and enhances cell metabolism and oxidant release during pregnancy. Eur J Immunol 2006, 36, 1619–1628. [Google Scholar] [CrossRef]
- Vaccarino, V.; Brennan, M.L.; Miller, A.H.; Bremner, J.D.; Ritchie, J.C.; Lindau, F.; Veledar, E.; Su, S.; Murrah, N.V.; Jones, L.; et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry 2008, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Boeldt, D.S.; Yi, F.X.; Bird, I.M. eNOS activation and NO function: pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium--new insights into eNOS regulation through adaptive cell signaling. J Endocrinol 2011, 210, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Lee, K.H.; Im, M.W.; Kim, Y.J.; Ha, E.H. Placental superoxide dismutase, genetic polymorphism, and neonatal birth weight. J Prev Med Public Health 2004, 37, 306–311. [Google Scholar]
- Scapagnini, G.; Davinelli, S.; Drago, F.; De Lorenzo, A.; Oriani, G. Antioxidants as antidepressants: fact or fiction? CNS Drugs 2012, 26, 477–490. [Google Scholar] [CrossRef]
- Marcus, C.; Feizi, P.; Hogg, J.; Summerfield, H.; Castellani, R.; Sriwastava, S.; Marano, G.D. Imaging in Differentiating Cerebral Toxoplasmosis and Primary CNS Lymphoma With Special Focus on FDG PET/CT. AJR Am J Roentgenol 2021, 216, 157–164. [Google Scholar] [CrossRef]
- Gupta, S.; Aziz, N.; Sekhon, L.; Agarwal, R.; Mansour, G.; Li, J.; Agarwal, A. Lipid peroxidation and antioxidant status in preeclampsia: a systematic review. Obstet Gynecol Surv 2009, 64, 750–759. [Google Scholar] [CrossRef]
- Bilici, M.; Efe, H.; Köroğlu, M.A.; Uydu, H.A.; Bekaroğlu, M.; Değer, O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 2001, 64, 43–51. [Google Scholar] [CrossRef]
- Gałecki, P.; Szemraj, J.; Bieńkiewicz, M.; Florkowski, A.; Gałecka, E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol Rep 2009, 61, 436–447. [Google Scholar] [CrossRef]
- Kodydková, J.; Vávrová, L.; Zeman, M.; Jirák, R.; Macásek, J.; Stanková, B.; Tvrzická, E.; Zák, A. Antioxidative enzymes and increased oxidative stress in depressive women. Clin Biochem 2009, 42, 1368–1374. [Google Scholar] [CrossRef]
- Herken, H.; Gurel, A.; Selek, S.; Armutcu, F.; Ozen, M.E.; Bulut, M.; Kap, O.; Yumru, M.; Savas, H.A.; Akyol, O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res 2007, 38, 247–252. [Google Scholar] [CrossRef]
- Stefanescu, C.; Ciobica, A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord 2012, 143, 34–38. [Google Scholar] [CrossRef]
- Jiménez-Fernández, S.; Gurpegui, M.; Díaz-Atienza, F.; Pérez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry 2015, 76, 1658–1667. [Google Scholar] [CrossRef]
- Mazereeuw, G.; Herrmann, N.; Andreazza, A.C.; Khan, M.M.; Lanctôt, K.L. A meta-analysis of lipid peroxidation markers in major depression. Neuropsychiatr Dis Treat 2015, 11, 2479–2491. [Google Scholar] [CrossRef]
- da Cruz, K.L.O.; Salla, D.H.; de Oliveira, M.P.; da Silva, L.E.; Dela Vedova, L.M.; Mendes, T.F.; Bressan, C.B.C.; Costa, A.B.; da Silva, M.R.; Réus, G.Z.; et al. The impact of obesity-related neuroinflammation on postpartum depression: A narrative review. Int J Dev Neurosci 2022, 82, 375–384. [Google Scholar] [CrossRef]
- Suzuki, K.; Masuya, M.; Matsumoto, T.; Ito, N.; Ohishi, K.; Maeda, M.; Katayama, N. High-intensity signals in the basal ganglia from gadolinium-enhanced T1-weighted MRI as an early change in toxoplasma encephalitis in an AIDS patient. J Infect Chemother 2010, 16, 135–138. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Anderson, G.; Carvalho, A.F.; Duleu, S.; Geffard, M.; Maes, M. IgM-mediated autoimmune responses to oxidative specific epitopes, but not nitrosylated adducts, are significantly decreased in pregnancy: association with bacterial translocation, perinatal and lifetime major depression and the tryptophan catabolite (TRYCAT) pathway. Metab Brain Dis 2017, 32, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Roomruangwong, C.; Anderson, G.; Berk, M.; Stoyanov, D.; Carvalho, A.F.; Maes, M. A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 81, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Ait Tayeb, A.E.K.; Poinsignon, V.; Chappell, K.; Bouligand, J.; Becquemont, L.; Verstuyft, C. Major Depressive Disorder and Oxidative Stress: A Review of Peripheral and Genetic Biomarkers According to Clinical Characteristics and Disease Stages. Antioxidants 2023, 12, 942. [Google Scholar] [CrossRef]
- Goldberg, D. The heterogeneity of "major depression". World Psychiatry 2011, 10, 226–228. [Google Scholar] [CrossRef]
- Pasco, J.A.; Nicholson, G.C.; Williams, L.J.; Jacka, F.N.; Henry, M.J.; Kotowicz, M.A.; Schneider, H.G.; Leonard, B.E.; Berk, M. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry 2010, 197, 372–377. [Google Scholar] [CrossRef]
- Mondin, T.C.; de Azevedo Cardoso, T.; Moreira, F.P.; Wiener, C.; Oses, J.P.; de Mattos Souza, L.D.; Jansen, K.; da Silva Magalhães, P.V.; Kapczinski, F.; da Silva, R.A. Circadian preferences, oxidative stress and inflammatory cytokines in bipolar disorder: A community study. J Neuroimmunol 2016, 301, 23–29. [Google Scholar] [CrossRef]
- Gadad, B.S.; Jha, M.K.; Czysz, A.; Furman, J.L.; Mayes, T.L.; Emslie, M.P.; Trivedi, M.H. Peripheral biomarkers of major depression and antidepressant treatment response: Current knowledge and future outlooks. J Affect Disord 2018, 233, 3–14. [Google Scholar] [CrossRef]
- Ripke, S.; Wray, N.R.; Lewis, C.M.; Hamilton, S.P.; Weissman, M.M.; Breen, G.; Byrne, E.M.; Blackwood, D.H.; Boomsma, D.I.; Cichon, S.; et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry 2013, 18, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Couto, T.C.; Brancaglion, M.Y.; Alvim-Soares, A.; Moreira, L.; Garcia, F.D.; Nicolato, R.; Aguiar, R.A.; Leite, H.V.; Corrêa, H. Postpartum depression: A systematic review of the genetics involved. World J Psychiatry 2015, 5, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fan, W.; Zhang, X.; Dong, E. Gestational stress induces depressive-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics 2016, 11, 150–162. [Google Scholar] [CrossRef] [PubMed]
- D'Addario, C.; Dell'Osso, B.; Galimberti, D.; Palazzo, M.C.; Benatti, B.; Di Francesco, A.; Scarpini, E.; Altamura, A.C.; Maccarrone, M. Epigenetic modulation of BDNF gene in patients with major depressive disorder. Biol Psychiatry 2013, 73, e6–7. [Google Scholar] [CrossRef]
- Comasco, E.; Sylvén, S.M.; Papadopoulos, F.C.; Sundström-Poromaa, I.; Oreland, L.; Skalkidou, A. Postpartum depression symptoms: a case-control study on monoaminergic functional polymorphisms and environmental stressors. Psychiatr Genet 2011, 21, 19–28. [Google Scholar] [CrossRef]
- Alvim-Soares, A.; Miranda, D.; Campos, S.B.; Figueira, P.; Romano-Silva, M.A.; Correa, H. Postpartum depression symptoms associated with Val158Met COMT polymorphism. Arch Womens Ment Health 2013, 16, 339–340. [Google Scholar] [CrossRef]
- Doornbos, B.; Dijck-Brouwer, D.A.; Kema, I.P.; Tanke, M.A.; van Goor, S.A.; Muskiet, F.A.; Korf, J. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog Neuropsychopharmacol Biol Psychiatry 2009, 33, 1250–1254. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





