1. Introduction
Malaria, caused by Plasmodium parasites and transmitted through mosquito bites, remains a significant global health concern, particularly in regions with high endemicity. In 2021, there were an estimated 247 million malaria cases worldwide, marking an increase from the 2020 count of 245 million, primarily observed in countries within the WHO African Region. In 2015, the Global Technical Strategy for Malaria 2016–2030 (GTS) set a baseline of 230 million cases, aiming for a reduction of at least 75% in case incidence and mortality rate by 2025, and 90% by 2030 from the 2015 baseline. However, the GTS 2020 milestones for morbidity and mortality were not met globally, and current trends suggest that the 2025 targets are also at risk. The observed malaria case incidence of 59 cases per 1000 population at risk in 2021 deviates significantly from the expected 31 cases per 1000, indicating a 48% deviation from the set goal [
1].
The signs and symptoms of malaria are non-specific, primarily characterized by fever or a history of fever. However, relying solely on clinical features for diagnosis lacks specificity and often leads to overtreatment. Travelers acquiring malaria, particularly those from non-endemic areas, face distinct challenges. They are typically non-immune individuals residing in urban settings of endemic countries or visitors from non-endemic regions. When these travelers return to non-endemic countries and present with malaria, case fatality rates tend to be higher due to delayed diagnosis, unfamiliarity with malaria among healthcare professionals, and limited availability of effective antimalarial drugs. This underscores the critical need for awareness and preparedness in non-endemic areas [
2].
In regions with high chloroquine or multidrug resistance, doxycycline at 100 mg, administered one day prior to travel, daily during travel, and continued for four weeks post-return, is a recommended chemoprophylactic regimen. However, prophylactic failures are attributed to inadequate dosing, patient non-compliance, and emerging resistance [3, 4].
Acute kidney injury (AKI) is a frequent complication of severe malaria, occurring in approximately 40% of severe P. falciparum cases in endemic areas, with a mortality rate of around 75%. The pathogenesis involves mechanisms such as acute tubular necrosis, infection-triggered proinflammatory reactions, and metabolic disturbances. Renal insufficiency typically manifests 3–7 days after fever onset, with serum creatinine improvement taking an average of 17 ± 6 days. Electrolyte imbalances, including acidosis, hyponatremia, and hyperkalemia, are common, and primarily attributed to tissue hypoxia, initial internal dilution, and hemolysis. Additionally, anemia and thrombocytopenia arise from the malarial infection. Some patients develop proteinuria due to glomerulonephritis. P. falciparum is the primary causative agent, contributing significantly to AKI cases [5, 6, 7].
Advanced age, referral from another hospital, hyperbilirubinemia, inotropic drug requirement, hospital-acquired secondary infection, hyperkalemia, jaundice, altered consciousness level, leukocytosis, oligo-anuria, and
P. falciparum infection are identified risk factors for AKI in malaria. Oligo-anuria is a common clinical presentation (46-76% of cases), accompanied by severe metabolic acidosis and hypercatabolic state. Notably, hyponatremia and hyperkalemia are frequent electrolyte abnormalities in malaria-associated AKI, occurring in 30-50% of cases [
6].
The primary goal in treating severe malaria is preventing mortality, necessitating prompt administration of effective parenteral antimalarial treatment followed by a full course of oral artemisin-based combination therapy (ACT). For severe cases, parenteral (IV or IM) artesunate is recommended for at least 24 hours, switching to oral treatment once tolerated. If artesunate is unavailable, artemether is the preferred alternative over quinine [
2]. Additionally, supportive care, fluid replacement, renal replacement therapy, and avoidance of nephrotoxic drugs are crucial components in managing malarial acute renal failure (ARF) [
9].
This case report highlights the challenges associated with diagnosing and treating severe P. falciparum malaria with renal involvement, especially in non-endemic and developing countries. The delayed-onset symptoms despite doxycycline prophylaxis underscore the need for continued vigilance and effective management strategies, particularly in travelers from non-endemic regions. Implementing timely and appropriate interventions remains critical in improving outcomes for patients with severe malaria and renal complications.
2. Case Presentation Section
A 55-year-old patient, without a previous medical history, was admitted to the hospital due to cough, fever (39oC), extreme fatigue, and sudden loss of consciousness. Although the patient resides in Pristina, he is a businessman who visited Guinea for a month and a half and returned some ten days ago. The patient was prescribed prophylactic doxycycline 100 mg/day (brand name Dovicin) by the family doctor, which, according to the patient, was taken regularly, starting two days before the trip and continuing throughout his 1.5-month stay in Guinea, as well as an additional 4 days after returning from home. During this time, he used bed nets and mosquito repellants. He did not take any medications that could interact with doxycycline. However, he discontinued the medication on the 5th day upon not feeling well and experiencing cough and fever. Initially considered as having atypical pneumonia, the patient was treated empirically as an outpatient by the family physician with azithromycin 100 mg qd for a few days. Because his condition did not improve, on the contrary, it has been worsening with extreme fatigue and sudden loss of consciousness, he was hospitalized.
On admission, the patient was alert, Glasgow coma scale of 15, blood pressure was 100/60mmHg, pulse rate was 98/min, respiratory rate was 20/min, febrile with body temperature was 39oC, and oxygen saturation was 92% on room air. Physical examination revealed a normal lung and heart condition, slightly enlarged liver and spleen, no joint swelling, no skin rash, no enlarged lymph nodes, with signs of moderate dehydration and moderate jaundice of sclera. He noticed less urination in the last two days, but he related this to the high temperature and sweating he had.
The patient's laboratory results on admission to the hospital showed a lower limit of the red blood cell (RBC) count with a value of 3.93 x 10
12/L (3.80-5.80 x 10
12/L), which during the first week of treatment decreased to the level of 2.4 x 10
12/L; hemoglobin (Hgb) level of 9.1 g/dL (11.00-16.5 g/dL), which further decreased to the levels of 6.5 g/dL; platelet levels (PL) of 86 x 10
9/L (150-390 x 10
9/L), which further decreased to 77 x 10
9/L; and white blood cells (WBC) of 9.2 x 10
9/L (3.5-10.0 x 10
9/L, which later increased to 17.5 x 10
9/L. The patient's C-reactive protein (CRP) was 113.1 mg/dL, later increased to 212 mg/dL; erythrocyte sedimentation rate (ESR) was 20 mm/h, liver enzymes were elevated with aspartate aminotransferase (AST) 90 U/L (8-48 U/L), later increased to 183 UI/L, and alanine transaminase (ALT) 82 U/L, (7-55 U/L) increased to 165 U/L, total bilirubin was 99.9 µmol/L (1.71-20.5 µmol/L and direct bilirubin was 77.3 µmol/L (<5.1 µmol/L), both elevated, later gradually returned to normal; glycemia 3.88 mmol/L (3.9 – 5.6 mmol/L), which later returned to normal levels; urea (19.45 mmol/L, and later 39.15 mmol/L) and creatinine (223.7 µmol/L, and later 557.8 µmol/L); total proteins/albumins 5.6/3.4 g/dL (6-8/3-5 g/dL), later 4.6/2.5 g/dl; LDH 1075 U/L (normal range 140-280 U/L), later increased to 1897 U/L, and then decreased to 630 U/L; D-dimer 6650 ng/mL (normal <500 ng/mL), decreased to 933 ng/dL; PT 88 sec (normal 11-13.5 sec); pH 7.37, later 7.41; PaCO2 28 - 44 mmHg (normal range 35-45 mmHg); PaO2 35 -135 mmHg (normal 75-100 mmHg); sodium 133 – 157 mEq/L (135-145 mEg/L); potassium 3.5 -4.1 mmol/L (normal 3.6-5.3 mmol/L); calcium 0.95 – 1.19 mmol/L (normal 2.13-2.55 mmol/L) (
Table 1).
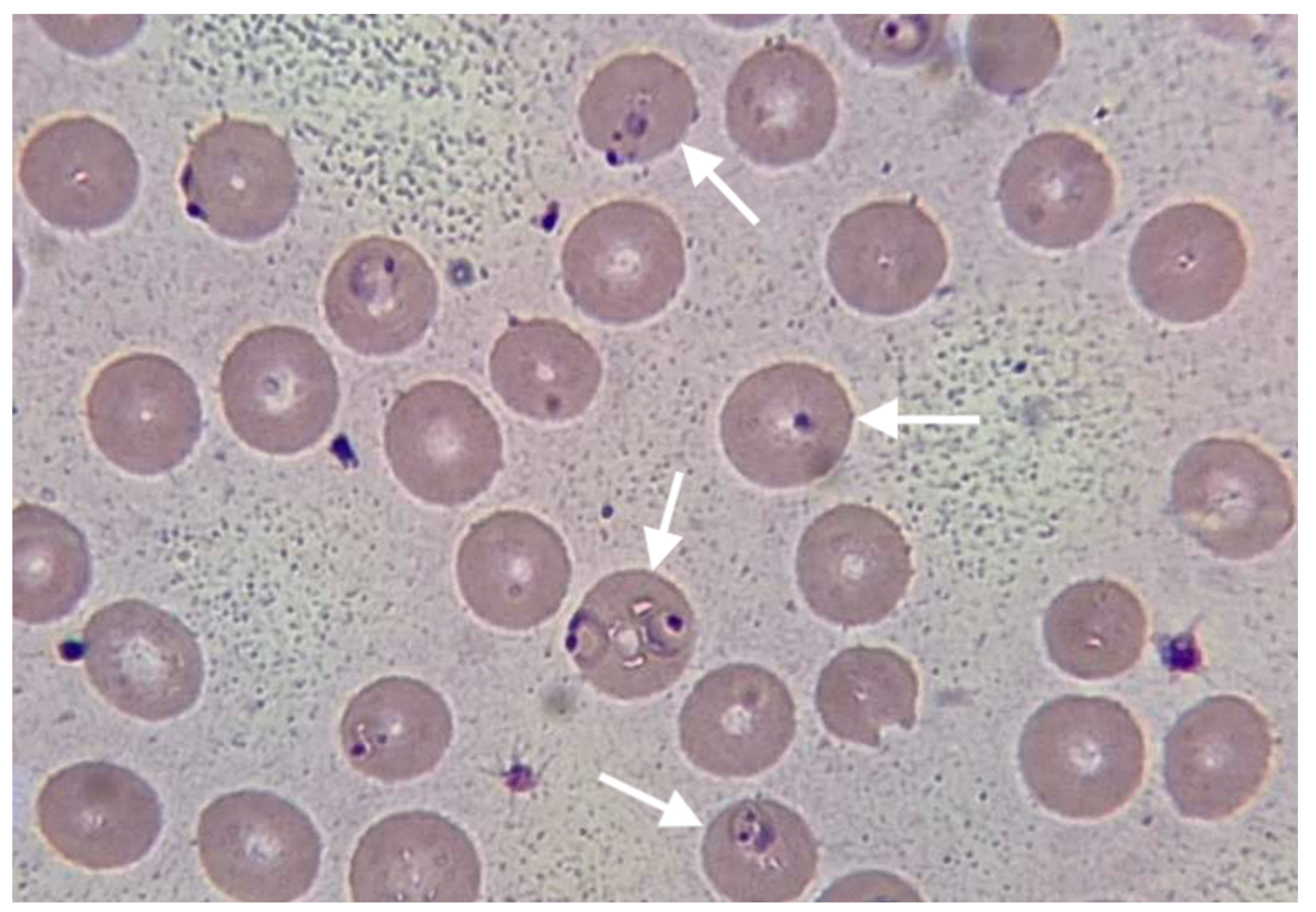
Chest X-ray revealed non-homogeneous opacities in the right lung; abdominal USG revealed hepato-splenomegaly, increased size of the kidney and loss of kidney corticomedullary differentiation. Blood cultures were negative, as well as a rapid immunochromatographic test for IgM/IgG antibodies for Leishmania and Leptospira. The rapid immunochromatographic test for malaria (The Right Sign Malaria Pf test, Biotest, Hangzhou Biotest Biotech Co, China) was positive. Thick and thin blood smear examination revealed crescent-shaped gametocytes of
Plasmodium falciparum (
Figure 1).
The treatment with artemether 80mg/lumefantrine 480mg was initiated on the first day of hospitalization, for 6 consecutive days. This regimen, which is not the recommended regimen for the treatment of severe malaria, was used due to the lack of parenteral antimalarial medications and the impossibility of providing them within a short period of time. Empirical therapy with IV ceftriaxone was also initiated, along with symptomatic, rehydration (2000ml/day), and supportive therapy.
The patient was febrile for the first three days of hospitalization while being afebrile until the end of the treatment. Lab results deteriorated during the first week of hospitalization and gradually improved during the second week. Because of the serious deterioration of renal functions, the patient underwent five cycles of dialysis starting from day 3 of hospitalization. Urine production got better from the 8th day of hospitalization and was normalized on discharge.
After 14 days of intensive care, the patient was discharged in an improved health condition and normalized lab results, with red blood cell (RBC) count with a value of 3.95 x 1012/L (3.80-5.80 x 1012/L), hemoglobin (Hgb) level of 11.1 g/dL (11.00-16.5 g/dL), white blood cells (WBC) within the reference range with a count of 7.6 x 109/L (3.5-10.0 x 109/L), C-reactive protein (CRP) was 16.8 mg/dL, aspartate aminotransferase (AST) = 35 UI/L, alanine transaminase (ALT) = 40 UI/L, bilirubin total 10.2 µmol/L, direct bilirubin 4.3 µmol/L, urea 12.9 mmol/L, urea 12.9 mml/L, and creatinine 112 µmol/L.
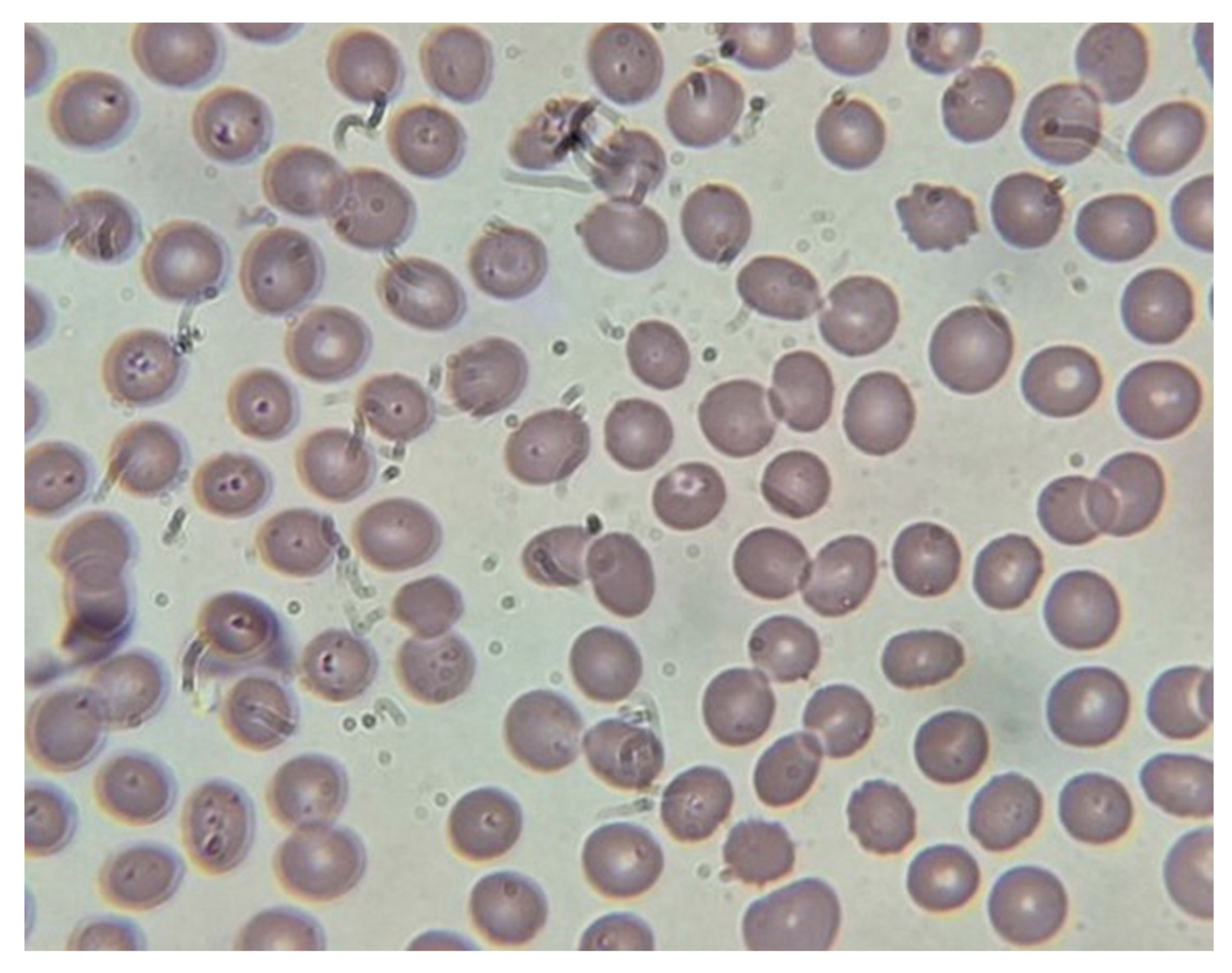
Peripheral smear done on the last day of hospitalization showed no signs of parasitemia (
Figure 2).
The control examination two weeks after hospitalization shows complete clinical and laboratory recovery of the patient.
3. Discussion
The presented case highlights several important aspects of the prophylaxis failure as well as the diagnosis and management of malaria in non-endemic resource-limited countries. Despite receiving prophylactic doxycycline treatment, the patient experienced symptoms consistent with
Plasmodium falciparum infection. Doxycycline is a commonly prescribed prophylactic medication for travelers visiting malaria-endemic regions. However, failures of doxycycline prophylaxis have been reported in the literature, suggesting a need for further evaluation of its efficacy [3, 4]. In the presented case, the reasons for prophylactic and clinical failure of doxycycline against
P. falciparum as inadequate dose and poor patient compliance due to simply forgetting and possible side effects, were excluded after a detailed history was taken. The delay in symptom onset after completing doxycycline prophylaxis has been described and should raise concerns about the potential for the delayed presentation of malaria [
3]. The clinical presentation of our case is similar to cases of severe malaria with AKI presented in various studies and case presentations [7-14]. The laboratory findings in this case revealed significant abnormalities, including a low red blood cell count, elevated liver enzymes, and impaired renal function. These findings are consistent with the WHO criteria of severe malaria and the involvement of multiple organs in severe malaria cases [6 - 14]. The presence of
Plasmodium falciparum ring forms on thin and thick blood smears confirmed the diagnosis of falciparum malaria, known for its potentially severe and complicated course [
2]. Prompt initiation of appropriate antimalarial therapy is crucial in severe malaria cases. In the case presented, the six-day course of oral artemether-lumefantrine resulted in clinical improvement and the resolution of symptoms, although this regimen is not recommended for the treatment of severe malaria [
2]. The reason for using the regimen not recommended by the WHO for severe malaria was the lack of parenteral artesunate and artemether in our country and the impossibility of obtaining medications from abroad within a reasonable time. This obstacle is well recognized by the World Health Organization (WHO) [
2]. Supportive care played a vital role in managing this case. The patient also received antibiotics, IV fluids, several blood transfusions and transfusions of fresh frozen plasma. Five dialysis sessions were applied to the patient, which indicates the serious involvement of the kidneys, a recognized complication of severe malaria [6, 7, 8]. The importance of supportive treatment and dialysis in the treatment of cases of severe malaria with AKI has been proven once again in our case, described widely in different papers dealing with severe malaria with AKI [7 – 16]. The improvement of the patient's condition is closely related to the immediate administration of antimalarial therapy (even oral) and the timely initiation of dialysis.
Supportive therapy in our case is in line with WHO recommendations as well as experiences presented in various studies [2, 5, 6, 9-16]. The study provides valuable insights into the clinical features and management of severe malaria cases, emphasizing the importance of intensive care unit management [
8]. This case report also raises concerns about the efficacy of doxycycline prophylaxis. Similar reports of prophylaxis failure have been documented in the literature [3, 4]. These findings call for further research and evaluation of alternative preventive strategies, especially in areas with drug-resistant strains.
This case report is subject to some limitations. Firstly, it is impeded by the challenge of quantifying parasite density due to insufficient training of laboratory personnel in a non-endemic malaria region like Kosovo. Secondly, there is a notable constraint in providing appropriate parenteral therapy for cases of severe malaria, primarily stemming from financial constraints and suboptimal operation of healthcare institutions in developing nations undergoing prolonged transitions, exemplified by the situation in Kosovo.
4. Conclusions
This case report highlights the challenges in diagnosing and treating severe malaria in a patient who had recently travelled to a malaria-endemic region. Despite receiving doxycycline prophylaxis, the patient developed symptoms of falciparum malaria. Healthcare providers should be aware of the potential for prophylaxis failure and consider the possibility of malaria even in patients who have regularly received prophylactic treatment. The presentation of this case also highlights the difficulties in treating malaria, especially severe malaria, in non-endemic countries with a lack of parenteral antimalarial medications recommended by the WHO. In the presented case, the imposed treatment with oral artemether-lumefantrine resulted in the clinical improvement of the patient; this was not confirmed by the daily determination of parasitemia density, due to the insufficiently trained laboratory staff, as a result of the rarity of malaria cases in our country, which are all imported.
Author Contributions
Conceptualization, Writing - original draft preparation and approving final version, Gramoz Bunjaku: Data curation, review, Murati Mehmet: Data curation, Reviewing, Editing, Yllka Begolli: Investigation, Visualization.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective nature of the study.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data presented in this case report are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World malaria report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- World Health Organization. WHO guidelines for malaria. 16 February 2021. World Health Organization. 2021 (WHO/UCN/GMP/2021.01). Licence: CC BY-NC-SA 3.0 IGO.
- Javelle E, Madamet M, Gaillard T, Velut G, Surcouf C, Michel R, Garnotel E, Simon F, Pradines B. Delayed Onset of Plasmodium falciparum Malaria after Doxycycline Prophylaxis in a Soldier Returning from the Central African Republic. Antimicrob Agents Chemother. 2016; 60(4), 2592-2593. [CrossRef]
- Madamet M, Gaillard T, Velut G, Ficko C, Houzé P, Bylicki C, Mérat S, Houzé S, Taudon N, Michel R, Pasquier P, Rapp C, Pradines B. Malaria Prophylaxis Failure with Doxycycline, Central African Republic, 2014. Emerg Infect Dis. 2015; 21(8), 1485-1486. [CrossRef]
- Brown DB, Solomon S, Lerner D, Del Rio M. Malaria and acute injury. Pediatric Nephrology. 2020; 35;603-608. [CrossRef]
- Silva GB Jr, Pinto JR, Barros EJG, Farias GMN, Daher EF. Kidney involvement in malaria: an update. Rev Inst Med Trop Sao Paulo. 2017; 59, e53. [CrossRef]
- Koopmans LC, van Wolfswinkel ME, Hesselink DA, Hoorn EJ, Kolelewijn R, van Hellemond JJ, van Genderen PJJ. Acute kidney injury in imported Plasmodium falciparum malaria. Malar J. 2015; 14:523. [CrossRef]
- Al Farsi F, Chandwani J, Mahdi AS, Petersen E.. Severe imported malaria in an intensive care unit: A case series. IDCases. 2019; 17, e00544. [CrossRef]
- Das, BS. Renal failure in malaria. J Vector Borne Dis. 2008, 45:83-97.
- Baswin A, Siregar ML, Jamil KF. IOP Conf Series: Earth and Environmental Science. 2018; 125: 012070. [CrossRef]
- Anghan H, Sethi P, Soneja M, Mahajan S, Wig N. Clinical and laboratory features associated with acute kidney injury in severe malaria. Indian J Crit Care Med. 2018; 22:718-22. [CrossRef]
- Kurnianingrum NMA, Ayu NP. Severe falciparum malaria with acite kidney injury: a case report. IOP Conf Series: Earth and Environmental Science. 2018; 434: 01316. [CrossRef]
- Meremo AJ, Kilonzo SM, Munisi D, Kapinga J, Juma M, Mwanakulya S, Mpondo B. Acute renal failure in a Caucasian traveller with severe malaria: a case report. Clinica Case Reports. 2014; 2(3): 82-85.
- Trošelj-Vukić B, Vuksanović-Mikuličić S, Sladoje-Martinović B, Milotić I, Slavuljica I. Unrecognised Malaria in Its Consequences – A Case Report of Severe Malaria with Acute Renal Failure. Coll. Antropol. 2013; 37(2): 611-613.
- Gumasana H, Otieno W. Case report: Acute Kidney Injury, Liver impairment, Severe Anemia in child with Malaria and Hyperparasitemia. Glob J Medical Clin Case Rep. 2021; 8(1):001-004. IOP Conf Series: Earth and Environmental Science. 2018, 125: 012070. [CrossRef]
- Bereda, G. A Case Report of Severe Malaria Associated Acute Renal Failure in an Adult. Preprint from Research Square, 19 Dec 2022. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).