1. Introduction
In our daily life, we are exposed to different health threats, as pathogens (viruses, bacteria, fungi, etc.) [
1], [
2] and hazardous chemicals (mercury, dioxins, lead, etc.) [
3], [
4]. Since these threats are ubiquitous and can pose risk to public health, taking fast and effective actions to identify and mitigate them is requested in order to reduce their potential impact on human health and food safety.
Currently, health care systems are facing new challenges in diagnosing diseases with similar clinical symptoms caused by different pathogens, like respiratory viruses as Influenza virus A/B (IVA/IVB), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or respiratory syncytial virus (RSV), which all can cause acute respiratory infections [
5]. Despite the resemblance between these infections, they differ in the management and treatment strategies. Therefore, simultaneous on-site detection of different analytes from single specimen, known as multiplexed point-of-care testing, is of paramount importance for efficient clinical diagnostics [
6].
Nowadays, the polymerase chain reaction (PCR) is the gold standard method for virus detection [
7]. However, PCR has relevant drawbacks as it requires long time between the collection of samples and their processing, needs clinical laboratory with trained staff and it has a high economic cost per test. Also, traditional PCR is at best semi-quantitative. Although there is a PCR technique named quantitative PCR (qPCR) [
8] which also offers multiplexed test, the quantitative determination by qPCR is indirect, providing relative quantification of viral load and being normally limited to 2-3 analytes per tube/assay.
Same needs emerge in the food sector [
9], where the food industry is facing the crucial challenge of ensuring that the food is safe for consumers (Food Safety), while maintaining a production process within environmental constraints. For this purpose, analytical control on the levels of chemicals and microbiological contaminants is mandatory to document compliance with maximum levels in the legislation [
10]. The problems leading to food safety issues should ideally be identified early in the value chain (ideally in real time), from raw materials to the final products, allowing to the food industry to minimize their impact through corrective actions.
Currently, depending on the contaminant, different methods are used, including immunological techniques (ELISA), culture-based methodologies, and molecular recognition (PCR) for microbial contaminations [
11] and different physio-chemical methods depending on the chemical contaminant, being high-performance liquid chromatography (HPLC) and mass spectrometric (MS) techniques the most frequently reported [
12]. Those methods are typically time-consuming and labor-intensive, and the results are often not available until after several hours or even days. Moreover, the working principles of the methods for different contaminants are often totally incompatible with each other, increasing the effort needed as several separated analyses are required. Consequently, an easy-to-use solution that allows rapid detection and quantitative analysis of multiple analytes with high sensitivity and in a single test, providing reliable results in a faster, easier, and cheaper way than the current state-of-the-art methods is being highly demanded by the food industry.
Biosensors devices have emerged as one of the most relevant diagnostic techniques for different fields[
13], [
14] due to their specificity, ease of mass fabrication, economics, and fast quantitative analysis [
15], [
16].
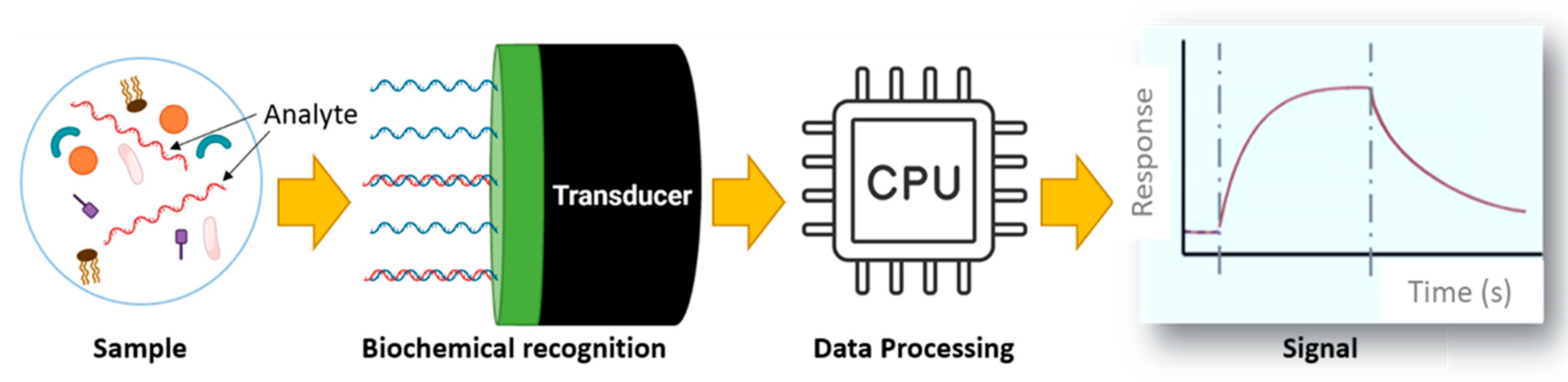
Figure 1 summarizes the working mechanisms of the biosensors which includes sample collection, biochemical-recognition, data processing and signal obtention [
17,
18,
19,
20].
Current transducers involved in biosensors include different mechanisms such as Electromechanical (potentiometric, amperometric or calorimetric), Piezoelectric, Thermal sensors and Optical/optoelectronic biosensors (surface plasmon resonance and Localized surface plasmon Resonance (SPR/LSPR), luminescence and fluorescence or evanescence waves) [
21,
22]. Among the abovementioned techniques, SPR/LSPR presents several advantages. First, SPR and LSPR-based sensors can be fabricated by classical micro/nanofabrication CMOS compatible processes, but they do not require complex building blocks as in optical waveguide or resonator-based biosensors. In addition, SPR/LSPR biosensor-based tests provide high sensitivity (low detection limit (DL)), selectivity and cost-effective analyses. Different SPR sensors with promising DL ranging from 10
−5 to 10
−8 RIU have been reported [
22]. Even, although still limited, there are SPR based market products (as Biacore instrument [
23] with a DL between 1 × 10
−6 to 1 × 10
−7 RIU). All these SPR developments have good DL that can satisfy most research requirements, but there still exist three potential problems that will limit their applications in many fields.
First, the evanescent field in those basic SPR structures only penetrates the surrounding medium for about 100 nm, and thus it is very difficult to detect the large target molecules like cells and bacteria.
Second, SPR systems, as the Biacore system, requires of complex interrogation instruments based on prism coupling what requires of expensive adaptive optics and thermal controls.
Finally, there are some sensitive SPR biosensors, but most of them only can detect one analyte. In that sense, the SPR imaging (SPRI) technique is so far, the most promising tool for high throughput multianalyte detection with a DL of approximately 10
−5 RIU [
20]. The typical SPRI sensor is however also based on complex prism coupling instrumentation, in which monochromatic incident light is expanded, passes through a prism, and strikes the interface of the thin film and prism at the coupling angle, exciting a broad area of the sensing surface.
Recent progress in nanostructure fabrication techniques has paved the route toward the development of highly sensitive and label-free optical transducers using the localized surface plasmon resonance (LSPR) of metal nanostructures. The use of such Localized Surface Plasmon Resonance (LSPR) structures has been proposed to overcome the described problems of current SPR systems. LSPR structures will increase the detection of smaller targets, as bulk effect is suppressed, and additionally, will drastically reduce the instrumentation complexity required in SPR systems avoiding the use of prisms and other complex optics systems [
24].
Regarding biochemical recognition, new approaches as gated materials have recently been drawing attention due to their applications in fields as biomedicine and molecular recognition [
14,
25]. These materials combine porous nanomaterials with a supramolecular entity called molecular gate, able to finely tune the movement of chemical or biochemical species from the voids of the porous supports to a solution in response to a predefined stimulus. Concretely, the use of molecular gates and mesoporous supports have been proved to have meaningful applications in biotechnology and biomedicine such as drug delivery [
26], diagnosis [
27,
28] or chemical communication [
29]. When gated materials are used as probes, the gating mechanism is designed to be controlled by the target species. In this way, once the molecular or supramolecular gates are attached to the outer surface of the porous substrate, upon the external stimulus (target species), the gate is opened allowing the release of previously entrapped molecules or dyes, which usually acts as reporter.
With the aim of developing an adaptable diagnostics solution based on a very innovative approach, the PHOTONGATE project has emerged [
30]. The PHOTONGATE is based on the combination of molecular gates as biorecognition elements [
31], and Local Surface Plasmonic Resonance (LSPR) structures as transducers, [
32] to detect multiple analytes with high sensitivity. This paper offers an overview of the PHOTONGATE project, focused on the explanation of the different parts of the sensor and the innovation of the PHOTONGATE approach.
2. PHOTONGATE Overall Concept
PHOTONGATE is focused on the detection of diagnostic targets that may cause respiratory infections, such as IVA, IVB, [
33], [
34], SARS-CoV-2 [
35] RSV [
36], and the detection of two of the main food chemical contaminants in fresh fish (histamine [
37] and methylmercury/MeHg [
38]) and one of the most virulent foodborne pathogens (
Listeria monocytogenes [
39]).
Conventional detection methods as culture-based techniques, ELISA, qPCR and HPLC(-MS) are very sensitive and allow identification of the compounds and microorganisms of interest and their quantification [
11], [
12]. However, these methods are time consuming, methodologically challenging or require specific skills or/and equipment to be used [
40,
41,
42]. In addition, these methods are highly dependent on the targets and do not allow multiple targets in the same analysis. For instance, there is a quantitative PCR technique (qPCR) which also offers multiplex tests, being 6 the maximum targets per test allowed. However, PCR multiplex is normally limited to a 2-3 analytes per tube/assay.
The PHOTONGATE project will develop a novel biosensor device and a readout platform based in molecular gates and LSPR structures. The PHOTONGATE concept is based on the combination of the following technologies:
Molecular gates containing the sensitive probes able to react to the analytes of interest; viruses, bacteria, and chemical hazards [
31], [
43], [
44].
LSPR structures for sensing, the label-free optical detectors based on refractive index changes [
32], [
45].
Porous nanomaterial [
46], [
47] filled with cargo and closed with the molecular gates. Chemical interactions between the targeted analyte and probe will trigger the opening of the gates allowing the release of the cargo, which is sensed by the LSPR structures, amplifying in this way the weak chemical interactions.
Polymeric microfluidic system that allows the flow from the sample to the sensing system [
48,
49,
50].
Optical readout platform, own produced for this project, this system includes optical emitters and a spectrometer device, being able to display the sensing signal for up to 12 analytes.
As shown in
Table 1, with this approach, PHOTONGATE will provide faster results (around 30 minutes) with sensitivities comparable to conventional methods for multiple analytes (up to 12 targets). Furthermore, as shown in
Table 1, PHOTONGATE sensing system will provide additional advantages as improve cost-effectiveness relation and portability, allowing to on-site analysis.
PHOTONGATE innovation
PHOTONGATE is innovating on many levels, with the primary developments being the following:
The PHOTONGATE device will be capable to detect different chemical and microbial contaminants and viral hazards, being able to work for different fields as health care and food control. In addition, it requires little training on the part of the personnel, since there is a minimum preprocess of the samples and it will offer an easy reading of the results.
The LSPR sensors used in the device do not require the use of any fluorescent label (label-free detection).
The use of molecular gates mechanism will improve the specificity and selectivity of biosensors.
The sensing mechanism involving the opening of pores by the probe-receptor interaction produce a strong change in refractive index. This mechanism of signal amplification will increase the sensitivity, allowing lower detection limits.
The analysis will require 30 min or less. Additionally, it evaluates multiple targets with no risk of cross-reactions.
The fabrication at wafer scale will ease a high integration and cost-effective devices.
The portable and easy-to use read-out platform of PHOTONGATE avoids complex components of current SPR commercial systems, enabling to be used by small clinics, labs and farms or food producers.
3. PHOTONGATE Concept Overall Design and Architecture
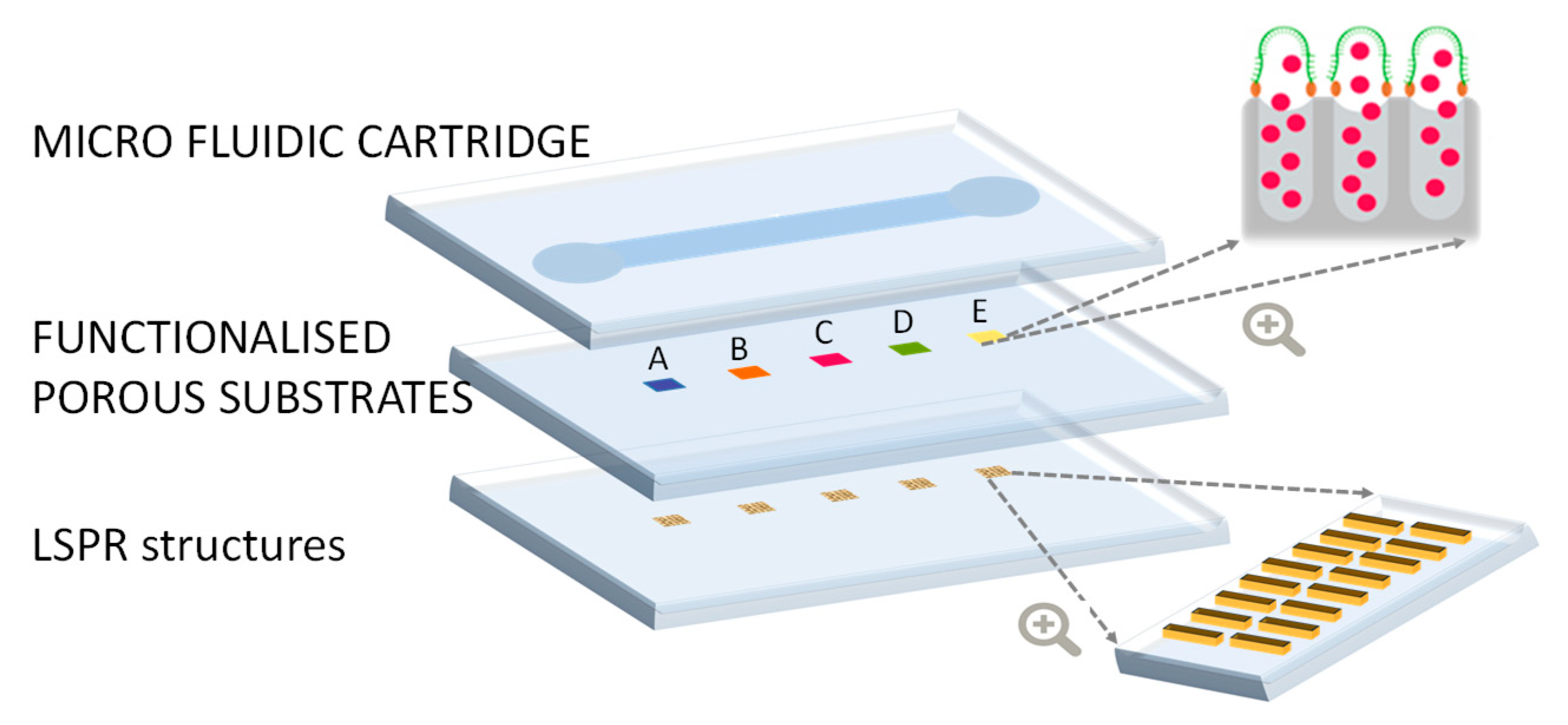
In this section, we present the overall design and specifications of the PHOTONGATE concept. The PHOTONGATE concept consists of two different parts: the sensing module and the readout platform, illustrated in
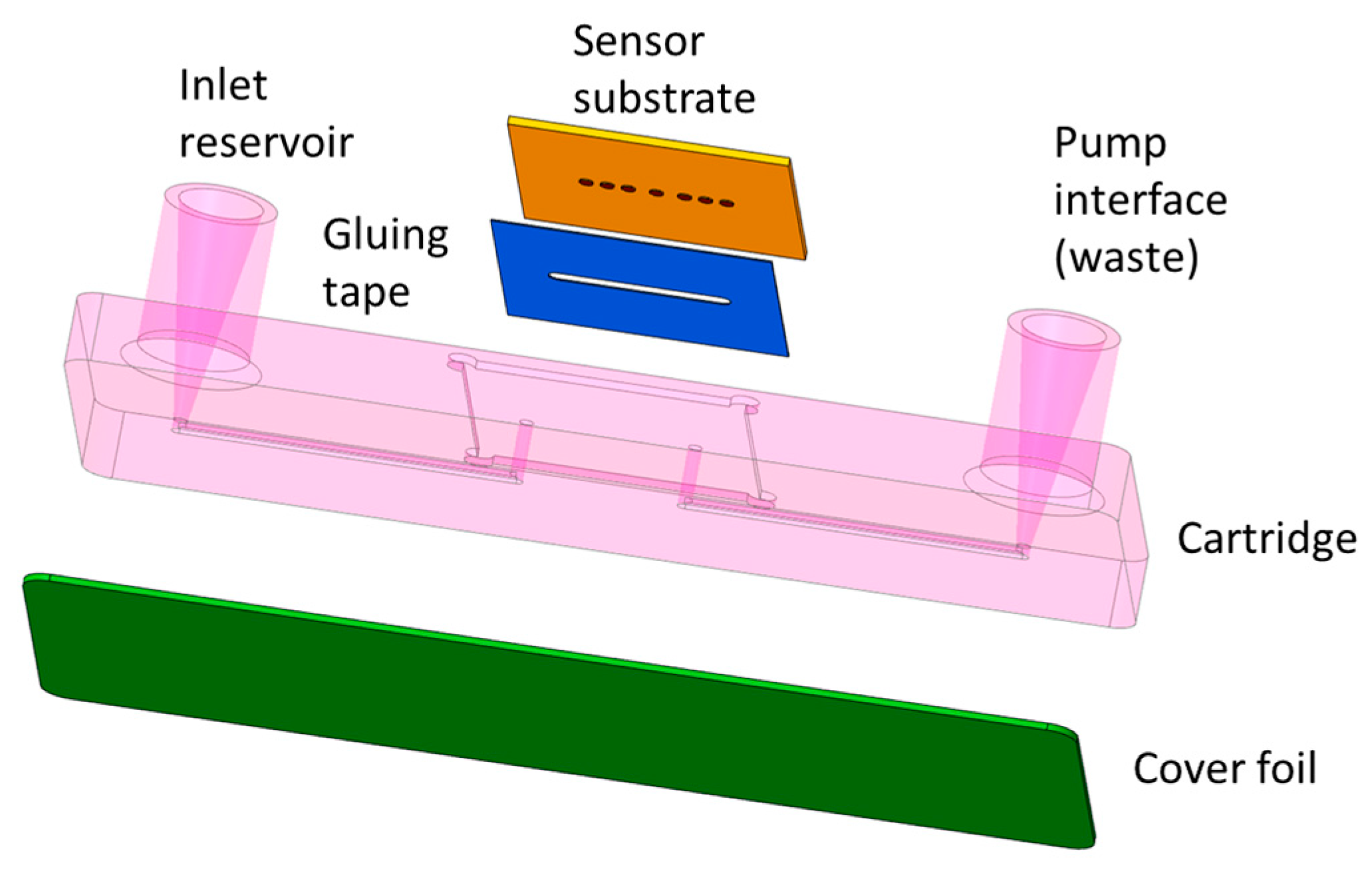
Figure 2 and Figure 5, respectively. The sensing module consists of three parts integrated in a single piece: the LSPR structures, the functionalized porous substrate, and the microfluidic cartridge.
3.1. Functionalised porous substrate
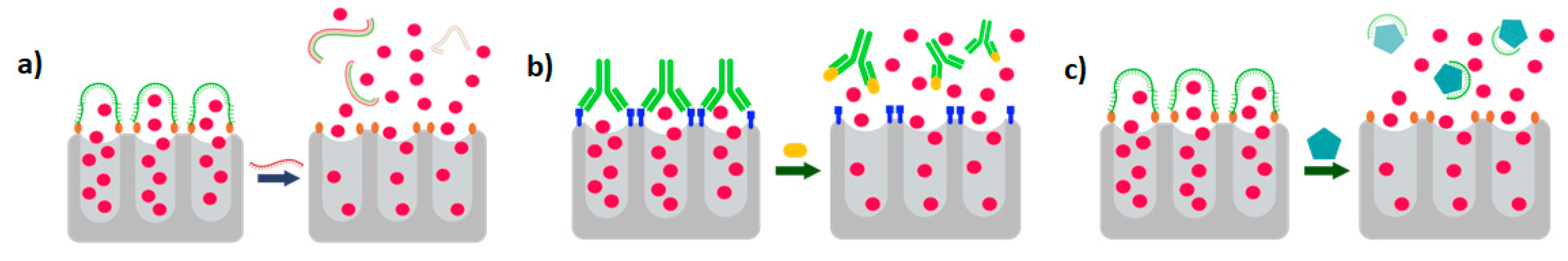
Biosensors obtain their specificity from the biological binding interaction between the analyte and its complementary receptor, which is immobilized onto the transducer surface. In the case of PHOTONGATE, a combination of molecular gates with porous nanomaterials are used for this purpose, being the molecular gates, which confer the specificity to the biosensor. They can be adapted to the desired analyte, working as receptors, and will be used to close nanostructured porous materials, once filled with the selected molecules, as well, as shown in
Figure 3. The opening mechanism of the gate is triggered when the attached receptor interacts with the specific analyte, this external stimulus opens the pores allowing the release of the previously entrapped molecules, giving rise to a strong change in the refractive index inside the porous substrate and producing an amplification of the analyte receptor recognition event, and consequently reducing the detection limit. Thus, this technology will offer an enormous potential to develop a multi-analyte system when they will be integrated with a photonic technology based on refractive index sensing such as LSPR structures.
3.2. Localized Surface Plasmon Resonance (LSPR) substrates
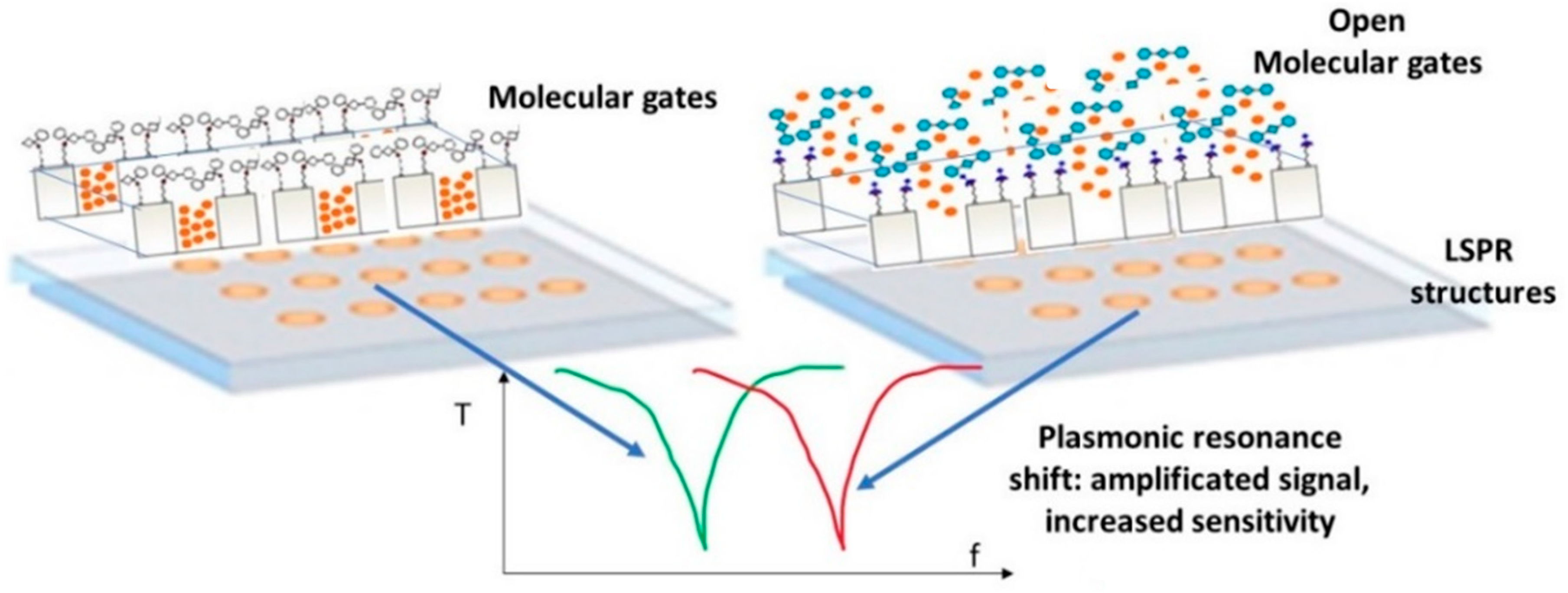
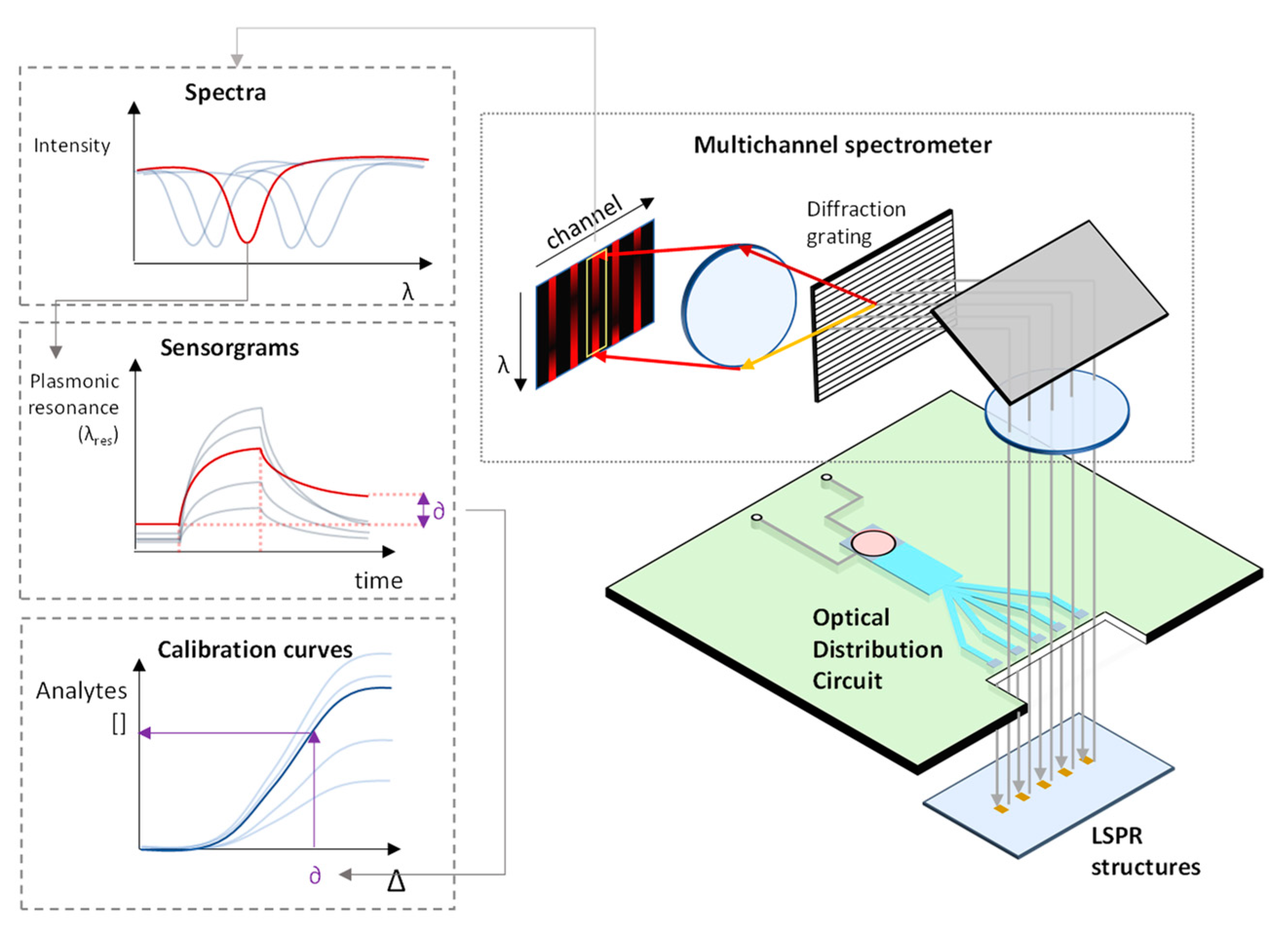
PHOTONGATE will use LSPR substrates as transducers, a periodic array of metallic tailor-made nanostructures fabricated by electron beam lithography (EBL) on a bulk substrate. The light interaction with the LSPR structures gives rise to a plasmonic resonance which appears as a peak in the optical response signal of the photonic system, shown in
Figure 4. These plasmon peaks are very sensitive to changes in the refractive index of the LSPR environment. When the probes react to the analyte the molecular gates open and the entrapped molecules are released, therefore, consequently the refractive index of the medium surrounding the metallic nanostructures undergoes a change and a shift in the plasmon peak is recorded. The amplified effect obtained by the molecular gates mechanism added to the LSPR resonance, will allow to detect and quantify the presence of the target analyte, improving the detection and quantification limits obtained with current biosensors.
Figure 4 shows the proposed PHOTONGATE biosensor mechanisms.
Besides, the reduced size of the sensors will allow the integration of multiple sensors (each one with the aim to detect a different target) in a single sensing chip.
3.3. Polymeric microfluidic system
The microfluidic system will be designed to flow the sample that contains the analyte over the bio-sensing surface (the porous substrates filled with molecules and closed with the molecular gates). It will be properly bonded to the LSPR substrate combined with the bio-sensing surface avoiding any possible cross-contamination. The fluidic system will essentially consist of a microfluidic channel having an inlet and an outlet at both ends of the channel, which connects to the sample reservoir and the waste, respectively. The sensor substrate is glued in a cartridge notch allowing accessibility for the optical interrogation of the sensor spots from the visible up to the near-infrared spectrum.
With this system, performing a test will only require the insertion of some droplets of the liquid sample into the corresponding inlet reservoir, the insertion of the cartridge in the PHOTONGATE platform, and the start the of assay from the photonic read-out platform. If the sample preparation chemicals allow, the cartridge will preferably be made from inexpensive thermoplastics like Polypropylene (PP), Polystyrene (PS) or Polymethacrylate (PMMA) or alternatively from more chemically resistant plastics like for example Polyether ether ketone (PEEK). For first evaluation tests, cartridges can also be made by using rapid-prototyping 3D printing materials. The inlet reservoir will have typical dimensions below 1mm which is compatible with the expected flow rates of several microliters per minute, generated by the connected vacuum pump. The channels will be closed to the outside world using a cover foil and a suitable bonding process to attach it to the cartridge surface surrounding the channels.
3.4. Optical readout platform and sensing data analysis/algorithms
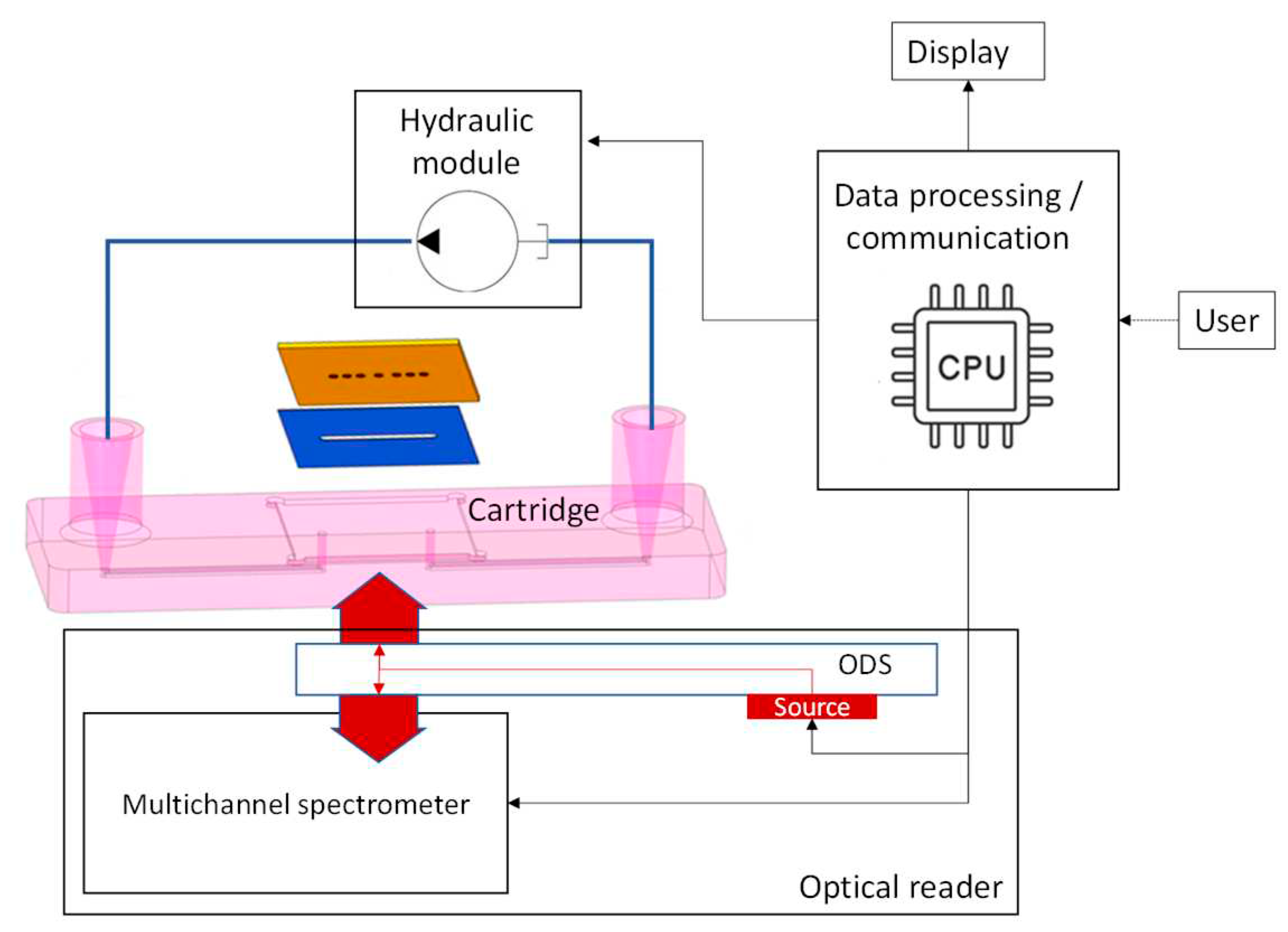
The PHOTONGATE readout platform (
Figure 6) will automatically carry out the assay once the sensing module is inserted. It is planned to be composed of three subsystems: the optical reader, the hydraulic module and the embedded data processing and communication module.
The hydraulic module will be responsible for flowing the sample over the photonic sensors with fine control on the flow rate. The optical reader will be based on a multi-channel near infrared (NIR) broadband spectrometer and an optical distribution circuit (ODS). It will be able to measure up to 12 optical spectra at a time with sub 10 picometer resolution for the resonant feature extractions. Raw spectra will be processed using specifically developed algorithms directly executed on the embedded data processing module. Spectral features as a function of time (sensorgrams) will be shown in parallel on the platform’s touch screen. The automated and embedded analysis of the sensorgrams will result in the quantitative concentrations of the target analytes in less than 30 minutes, after insertion of the cartridge in the platform. A schematic of the optical reader is shown in
Figure 7.
Sensing data analysis/algorithms
The raw data obtained with the read-out module will be processed by the Mass Data pro-cessing and communication module. The concentration of each target will be determined through the acquisition of a pair of measurements: i) its own mathematical model, where the signals acquired by the readout platform will be the input and the prediction of the parameter will be the output; and ii) reference analytical method.
For the construction of the models, two types of models will be used: partial least squares methods (PLS) and neural networks (NN). These two methods are the most used algorithms for the adjustment of linear (PLS) and nonlinear (NN) functions. To improve the performance of the mathematical model pre-treatments of raw data will be applied (Smoothing, Normalization, Savitzky-Golay, MSC, SNV, Detrend, etc.) as well as variable selection methodologies (Genetic algorithms). Combinations of previous methods with the two considered regression methods will be analysed to achieve the final mathematical model.
As result, using specifically developed algorithms and software, the spectral features will be converted into quantitative response of the target concentrations, that will be plot as an individual graphical representation over time (sensorgrams). Through this system, quantitative results will be provided 30 minutes after introducing the sample in the platform.
4. Validation
Since this technology will be cost-effective and portable, it can be implemented in different scenarios such as small farms, food manufacturing industries, clinics, or health primary care centers. A preliminary validation of PHOTONGATE concept will be performed in food (fish control) and health safety (respiratory infection) applications at laboratory with pattern samples for the proper calibration and adjustments. Finally, the platform will be placed in the research facilities of two different EU countries (Spain and Denmark) for their respective validation against golden standard techniques used in their respective areas. Additionally, PHOTONGATE system will be validated in real processing food scenarios can be emulated for full food analysis.
Before the final validation, an initial test and troubleshooting phase will be carried out. In the case of targets pathogen (L. monocytogenes) and chemical contaminants (MeHg, and histamine) the complete PHOTONGATE system will be tested with real samples (including incurred material as well as spiked samples). So, in the cases of L. monocytogenes, the selected food samples will be inoculated with known counts of the pathogen and L. innocua strain. In the case of MeHg and histamine, different amounts will be added to the samples in low (close to limit of quantification), medium (close to legal limits), and high (above legal limits) concentrations ranges, and the recovery will be used to evaluate method performance.
A preliminary evaluation of the analytical measurement range and other critical performance characteristics will be performed in relation to regulatory guidelines and international guidelines. The obtained results will be compared with results obtained with reference analytical methods for each target contaminant: i)
L. monocytogenes: the count will be carried out through the validation protocol AFNOR, i.e., in a chromogenic agar culture medium (ALOA) detecting all bacteria related to the genus Listeria through the ß-glucosidase activity determination (
L. monocytogenes is differentiated as result of the formation of a phospholipid precipitation halo derived from phospholipase activity); ii) MeHg: selective extraction (principles in EN17266 [51 and Cressy et al., 2020 [
52]]) followed by ICPMS detection and HPLC-ICPMS method; and iii) histamine: fluorometric method with HPLC/HPLC-UV (EU regulation Reg. (EC) 2073/2005).
The final validation of read out platform at relevant environments will be carried out by using the system for the analysis of real samples. In the case of the detection of respiratory viruses, virus stocks produced in cell culture will be used. This represents a higher titer of the pathogens in a relatively simple matrix (tissue culture media) for the initial optimization/testing of the molecular gates. Later on, PHOTONGATE system will be tested by using real human nasopharyngeal/nasal and oropharyngeal samples collected in virus transport medium (VTM), which will be tested in parallel to a gold-standard reference test (multiplex-RT-PCR virus screening platform). A panel of 50-positives and 50-negatives for each of the four viruses (IVA, IVB, RSV, SARS-CoV-2) to assess the sensitivity, specificity and cross-reactivity for each viral analyte will be used. In addition, an additional panel of 50 samples with mixed viral infection, to assess the sensitivity for multiplex detection, will be analyzed. Finally, 100 samples collected prospectively using the real-time RT-PCR gold-standard and the PHOTONGATE system in parallel will be assessed.
In parallel, PHOTONGATE technology will be validated for food samples (different fish samples). For this purpose, quantitative microbiologic methods (L. monocytogenes) accuracy (or recovery) and precision under reproducibility conditions will be determined, allowing to estimate the method uncertainty. In the case of histamine and MeHg contaminants, the parameters to be evaluated will be accuracy and precision under reproducibility conditions, linear range, limit of detection and quantification, and measurement uncertainty. Concerning quantitative methods, the validation will be carried out along the whole defined range, i.e., high, medium, and low concentrations. Furthermore, whenever possible, reference materials with certified target values for the contaminants will be used during the validation process.
5. Conclusions
In this paper, we describe the preliminary architecture and design of the PHOTONGATE device. This biosensor is based on two validated technologies: molecular gates technology (the biological receptor) and LSPR structures (the transducer element based on refractive index sensing). With this novel approach, PHOTONGATE will develop a biosensor device and readout platform that allow the label-free optical detection for multiple analytes with high sensitivity, also improving the time-analysis and cost-effectiveness of the current sensing methods and offering an easy reading of the results. Moreover, it will be an easy-to-use technology, which will require little training on the part of the personnel, since there is no need to preprocess the samples.
Finally, PHOTONGATE system will be validated using internationally recognized methods to assess relevant method performance characteristics. Trueness, repeatability, reproducibility, detection limits, linearity, and analytical measurement range as well as reporting range will be assessed using realistic sample material. Specificity will be assessed by testing a range of theoretically relevant chemical moieties, individually and in combination. Robustness will be assessed both in relation to experimental conditions and to matrix variability (species type, sample conditions etc.).
Author Contributions
Conceptualization, A.G. and M.G.-G..; methodology, A.G., M.G.-G, E.A., R.M.-M., F.D., F.X.L.-L., B.M.-C., J.J.S., K.L., A.M., R.G., T.K., M.C.H.B., J.M.B.B., S.P.; software, A.M., N.P., M.H., F.D., D.B.; validation, E.A., I.C., E.G., R.M.-M., B.M.-C., F.X.L.-L., J.J.S., K.L., L.D.-O., A.F.L., I.F.S., J.M.B.B.; formal analysis, O.N., D.O.d.Z., A.G., M.G.-G., E.A., R.M.-M., F.D., F.X.L.-L., B.M.-C., J.J.S., K.L., A.M., R.G., T.K., M.C.H.B., J.M.B.B., S.S.; investigation, all authors; resources, A.G., M.G.-G., E.A., R.M.-M., F.D., F.X.L.-L., B.M.-C., J.J.S., K.L., A.M., R.G., T.K., M.C.H.B., J.M.B.B., S.S.; data curation, A.M., N.P., M.H.; writing—original draft preparation, O.N.; writing—review and editing, M.G.-G., A.G; visualization, O.N., D.O.d.Z., A.G., M.G.-G.; supervision, A.G., M.G.-G.; project administration, A.G., M.G.-G.; funding acquisition, A.G., M.G.-G., E.A., R.M.-M., F.D., F.X.L.-L., B.M.-C., J.J.S., K.L., A.M., R.G., T.K., M.C.H.B., J.M.B.B., S.S.; All authors have read and agreed to the published version of the manuscript.
Funding
“This research project has received funding from the European Union’s HORIZON-CL4-2022 research and innovation programme under grant agreement ID 101093042, PHOTONGATE project”.
Acknowledgments
“The micro nanofabrication capabilities required in PHOTONGATE project are funded by the Pluri-Regional FEDER funding Plan 2014-2020 European Commission”.
References
- T. Vos et al., “Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019,” The Lancet, vol. 396, no. 10258, pp. 1204–1222, Oct. 2020. [CrossRef]
- W. Liu, R. Wang, V. Vedarethinam, L. Huang, and K. Qian, “Advanced materials for precise detection and antibiotic-free inhibition of bacteria,” Mater Today Adv, vol. 13, p. 100204, Mar. 2022. [CrossRef]
- M. Kampa and E. Castanas, “Human health effects of air pollution,” Environmental Pollution, vol. 151, no. 2, pp. 362–367, Jan. 2008. [CrossRef]
- N. B. Scott and N. S. Pocock, “The Health Impacts of Hazardous Chemical Exposures among Child Labourers in Low- and Middle-Income Countries,” Int J Environ Res Public Health, vol. 18, no. 10, p. 5496, May 2021. [CrossRef]
- G. Gradisteanu Pircalabioru et al., “Advances in the Rapid Diagnostic of Viral Respiratory Tract Infections,” Front Cell Infect Microbiol, vol. 12, Feb. 2022. [CrossRef]
- “https://www.nature.com/articles/d42473-021-00318-w.” https://www.nature.com/articles/d42473-021-00318-w (accessed May 02, 2023).
- M. Mackay, “Real-time PCR in virology,” Nucleic Acids Res, vol. 30, no. 6, pp. 1292–1305, Mar. 2002. [CrossRef]
- J. S. Dymond, “Explanatory Chapter,” in Methods in Enzymology, Academic Press Inc., 2013, pp. 279–289. [CrossRef]
- “https://food.ec.europa.eu/safety/rasff_en.” https://food.ec.europa.eu/safety/rasff_en (accessed Apr. 19, 2023).
- A. Rather, W. Y. Koh, W. K. Paek, and J. Lim, “The Sources of Chemical Contaminants in Food and Their Health Implications,” Front Pharmacol, vol. 8, no. NOV, Nov. 2017. [CrossRef]
- M. Nemati, A. Hamidi, S. Maleki Dizaj, V. Javaherzadeh, and F. Lotfipour, “An Overview on Novel Microbial Determination Methods in Pharmaceutical and Food Quality Control,” Adv Pharm Bull, vol. 6, no. 3, pp. 301–308, Sep. 2016. [CrossRef]
- F. Barzegar, M. Kamankesh, and A. Mohammadi, “Recent Development in Formation, Toxic Effects, Human Health and Analytical Techniques of Food Contaminants,” Food Reviews International, pp. 1–27, Jul. 2021. [CrossRef]
- H. H. Nguyen, S. H. Lee, U. J. Lee, C. D. Fermin, and M. Kim, “Immobilized Enzymes in Biosensor Applications,” Materials, vol. 12, no. 1, p. 121, Jan. 2019. [CrossRef]
- P. J. Conroy, S. Hearty, P. Leonard, and R. J. O’Kennedy, “Antibody production, design and use for biosensor-based applications,” Semin Cell Dev Biol, vol. 20, no. 1, pp. 10–26, Feb. 2009. [CrossRef]
- K. Saha, S. S. Agasti, C. Kim, X. Li, and V. M. Rotello, “Gold Nanoparticles in Chemical and Biological Sensing,” Chem Rev, vol. 112, no. 5, pp. 2739–2779, May 2012. [CrossRef]
- K. Singh, M. Anwar, R. Pradhan, M. S. Ashar, N. Rai, and S. Dey, “Surface plasmon resonance based-optical biosensor: Emerging diagnostic tool for early detection of diseases,” J Biophotonics, Mar. 2023. [CrossRef]
- M. C. Estevez, M. Alvarez, and L. M. Lechuga, “Integrated optical devices for lab-on-a-chip biosensing applications,” Laser and Photonics Reviews, vol. 6, no. 4. pp. 463–487, Jul. 2012. [CrossRef]
- K. Singh, M. Anwar, R. Pradhan, M. S. Ashar, N. Rai, and S. Dey, “Surface plasmon resonance based-optical biosensor: Emerging diagnostic tool for early detection of diseases,” J Biophotonics, Mar. 2023. [CrossRef]
- K. Saha, S. S. Agasti, C. Kim, X. Li, and V. M. Rotello, “Gold Nanoparticles in Chemical and Biological Sensing,” Chem Rev, vol. 112, no. 5, pp. 2739–2779, May 2012. [CrossRef]
- J. M. Yang, K. R. Kim, and C. S. Kim, “Biosensor for Rapid and Sensitive Detection of Influenza Virus,” Biotechnology and Bioprocess Engineering, vol. 23, no. 4, pp. 371–382, Aug. 2018. [CrossRef]
- Thakur, M.S., Ragavan, K.V., “Biosensors in food processing,” Journal of Food Science and Technology,vol. 50, no. 4, pp.625-641, 2013. [CrossRef]
- C. Chen, J. Wang,” Optical biosensors: an exhaustive and comprehensive review,” Analyst, vol.145, no. 5, pp.1605-1628, 2020. [CrossRef]
- . [CrossRef]
- Takemura, K., “Surface Plasmon Resonance (SPR)- and Localized SPR (LSPR)-Based Virus Sensing Systems: Optical Vibration of Nano- and Micro-Metallic Materials for the Development of Next-Generation Virus Detection Technology”, Biosensors, vol. 11, no. 250, 2021, 11. [CrossRef]
- L. Polo, N. Gómez-Cerezo, E. Aznar, J-L. Vivancos, F. Sancenón, D. Arcos, M. Vallet-Regí, R. Martínez-Máñez, “Molecular gates in mesoporous bioactive glasses for the treatment of bone tumors and infection,” Acta Biomaterialia, vol. 50, pp. 114-126, 2017. [CrossRef]
- García-Fernández, A., Aznar, E., Martínez-Máñez, R., Sancenón, F., “New Advances in In Vivo Applications of Gated Mesoporous Silica as Drug Delivery Nanocarriers,” Small, vol. 16(3), no. 1902242, Dec. 2019,. [CrossRef]
- Caballos, I., Aranda, M.N., López-Palacios, A., Pla, L., Santiago-Felipe, S., Hernández-Montoto, A., Tormo-Mas, M.Á. Pemán, J., Gómez-Ruiz, M.D., Calabuig, E., Sánchez-Sendra, B., Francés-Gómez, C., Geller, R., Aznar, E., Martínez-Máñez, R., “Aptamer-Capped Nanoporous Anodic Alumina for SARS-CoV-2 Spike Protein Detection,” Advanced Materials Technologies, vol.8(11), no.2201913. [CrossRef]
- Hernández-Montoto, A., Aranda, M.N., Caballos, I., López-Palacios, A., Tormo-Mas, M.Á., Pemán, J., Rodríguez, M.P., Picornell, C., Aznar, E., Martínez-Máñez, R.,” Human Papilloma Virus DNA Detection in Clinical Samples Using Fluorogenic Probes Based on Oligonucleotide Gated Nanoporous Anodic Alumina Films,” Advanced Healthcare Materials, no. 2192-2640, pp. 2192-2640, 2023. [CrossRef]
- De Lui, B., Llopis-Lorente, A., Rincón, P., Gadea, J., Sancenón, F., Aznar, E., Villalonga, R., Murguía, J.R., Martínez-Máñez, R., “An Interactive Model of Communication between Abiotic Nanodevices and Microorganisms,” Angewandte Chemie, vol. 58 (42), pp. 14986-14990, 2019. [CrossRef]
- “https://www.photongate.eu/.” https://www.photongate.eu/ (accessed Apr. 19, 2023).
- E. Aznar, M. Oroval, L. Pascual, J. R. Murguía, R. Martínez-Máñez, and F. Sancenón, “Gated Materials for On-Command Release of Guest Molecules,” Chem Rev, vol. 116, no. 2, pp. 561–718, Jan. 2016. [CrossRef]
- P. J. Rodríguez-Cantó et al., “Demonstration of near infrared gas sensing using gold nanodisks on functionalized silicon,” Opt Express, vol. 19, no. 8, p. 7664, Apr. 2011. [CrossRef]
- W. Kong et al., “Novel reassortant influenza viruses between pandemic (H1N1) 2009 and other influenza viruses pose a risk to public health,” Microb Pathog, vol. 89, pp. 62–72, Dec. 2015. [CrossRef]
- J. Tan, G. Asthagiri Arunkumar, and F. Krammer, “Universal influenza virus vaccines and therapeutics: where do we stand with influenza B virus?,” Curr Opin Immunol, vol. 53, pp. 45–50, Aug. 2018. [CrossRef]
- N. Zhu et al., “A Novel Coronavirus from Patients with Pneumonia in China, 2019,” New England Journal of Medicine, vol. 382, no. 8, pp. 727–733, Feb. 2020. [CrossRef]
- Y. Perk and M. Ozdil, “Respiratory syncytial virüs infections in neonates and infants,” Turk Pediatri Ars, vol. 53, no. 2, pp. 63–70, Jul. 2018. [CrossRef]
- L. Maintz and N. Novak, “Histamine and histamine intolerance,” Am J Clin Nutr, vol. 85, no. 5, pp. 1185–1196, May 2007. [CrossRef]
- S. Ceccatelli, E. Daré, and M. Moors, “Methylmercury-induced neurotoxicity and apoptosis,” Chem Biol Interact, vol. 188, no. 2, pp. 301–308, Nov. 2010. [CrossRef]
- R. Berche, “Physiopathologie des infections Listeria monocytogenes*,” 1995.
- W. Jiang and W. Wang, “A selective and sensitive ‘turn-on’ fluorescent chemodosimeter for Hg2+ in aqueous media via Hg2+ promoted facile desulfurization-lactonization reaction,” Chemical Communications, no. 26, pp. 3913–3915, 2009. [CrossRef]
- J. M. Hungerford, “Scombroid poisoning: A review,” Toxicon, vol. 56, no. 2, pp. 231–243, Aug. 2010. [CrossRef]
- V. Velusamy, K. Arshak, O. Korostynska, K. Oliwa, and C. Adley, “An overview of foodborne pathogen detection: In the perspective of biosensors,” Biotechnology Advances, vol. 28, no. 2. pp. 232–254, Mar. 2010. [CrossRef]
- F. Sancenón, L. Pascual, M. Oroval, E. Aznar, and R. Martínez-Máñez, “Gated Silica Mesoporous Materials in Sensing Applications,” ChemistryOpen, vol. 4, no. 4, pp. 418–437, Aug. 2015. [CrossRef]
- E. Climent et al., “The Determination of Methylmercury in Real Samples Using Organically Capped Mesoporous Inorganic Materials Capable of Signal Amplification,” Angewandte Chemie International Edition, vol. 48, no. 45, pp. 8519–8522, Oct. 2009. [CrossRef]
- F. J. Rodríguez-Fortuño et al., “Highly-sensitive chemical detection in the infrared regime using plasmonic gold nanocrosses,” Appl Phys Lett, vol. 98, no. 13, p. 133118, Mar. 2011. [CrossRef]
- Carvalho, P. J. Sebastião, I. Fonseca, J. Matos, and M. C. Gonçalves, “Silica and silica organically modified nanoparticles: Water dynamics in complex systems,” Microporous and Mesoporous Materials, vol. 217, pp. 102–108, Jun. 2015. [CrossRef]
- M. C. Gonçalves, “Sol-gel silica nanoparticles in medicine: A natural choice. design, synthesis and products,” Molecules, vol. 23, no. 8. MDPI AG, Aug. 13, 2018. [CrossRef]
- R. D. Allert et al., “Microfluidic quantum sensing platform for lab-on-a-chip applications,” Lab Chip, vol. 22, no. 24, pp. 4831–4840, Nov. 2022. [CrossRef]
- K. Jaruwongrungsee et al., “Microfluidic-based Split-Ring-Resonator Sensor for Real-time and Label-free Biosensing,” Procedia Eng, vol. 120, pp. 163–166, 2015. [CrossRef]
- S. Mross, S. Pierrat, T. Zimmermann, and M. Kraft, “Microfluidic enzymatic biosensing systems: A review,” Biosens Bioelectron, vol. 70, pp. 376–391, Aug. 2015. [CrossRef]
-
EN 17266. Foodstuffs. Determination elements and their chemical species. Determination of organomercury in seafood by elemental mercury analysis. 2019.
- P. Cressey, G. Miles, D. Saunders, and A.J. Pearson. “Mercury, methylmercury and long-chain polyunsaturated fatty acids in selected fish species and comparison of approaches to risk-benefit analysis”, Food and Chemical Toxicology, vol. 146, 111788, 2020. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).