1. Introduction
Diabetes mellitus (DM) significantly increases the risk of developing cardiovascular disease (CVD) [
1]. While the American Diabetes Association (ADA) defines the diagnosis of DM as having a fasting blood glucose (FBG) ≥ 126 mg/dl or an HbA1c value ≥ 6.5%, it has classified people with FBG between 100 and 125 as having prediabetes [
2]. Similar to DM, it has also been shown that prediabetes, which is also called impaired FBG and is considered to be part of the onset of DM, increases the risk of CVD [
1].
One of the diseases caused by impaired FBG is heart failure (HF) [
3]. The relationship between impaired left ventricular (LV) diastolic function (DD) parameters and impaired FBG, which is one of the earliest changes that lead to this condition, has been proven in several previous studies [
4,
5]. This precedes systolic dysfunction and associated HF with reduced ejection fraction (EF) [
4].
Left atrial strain (LAS) is a new parameter that is used in the evaluation of LV filling pressure and LV diastolic function and is divided into three parts: reservoir, conduit and contraction [
6,
7]. LAS, which provides important prognostic information in various cardiac pathologies, instantly reflects left atrial (LA) pressure and shows prognosis better than the LA volume index (LAVI) [
7,
8,
9].
Although diastolic function studies in patients with impaired FBG were generally conducted with LVDD parameters, we planned to investigate whether there is a difference in LAS findings between individuals with normal FBG and impaired FBG without known diabetes in our study.
2. Methods
Study population
This was a single-centre, retrospective study of echocardiographic measures of LV diastolic function and LAS. A total of 148 individuals without any known chronic disease who were admitted to our cardiology outpatient clinic between January 2021 and January 2022 were included in the study. The patients were divided into two groups: those with FBG <100 mg/dl and those with FBG between 100-125 mg/dl after at least 8 hours of overnight fasting. The criterion for impaired FBG was determined according to the criteria of the ADA [
2]. The study was approved by the local ethics committee of our own institution. The study was conducted according to the guidelines of the Declaration of Helsinki, and by the local ethics committee of our own institution.
The physical examination findings, electrocardiography, transthoracic echocardiography (TTE) data and laboratory values of the patients included in the study were obtained from the hospital database. Those with FBG <70 mg/dl and >125 mg/dl and those whose echocardiographic images were insufficient for speckle tracking analysis were excluded from the study. Patients with valvular disease, pulmonary hypertension, or left or right ventricular dysfunction on echocardiography, or with symptoms or signs of cardiovascular disease, arterial hypertension, heart failure, previous myocardial infarction, moderate or severe valvular disease, arrhythmias, congenital heart diseases, asthma, chronic obstructive lung disease, neoplastic disease, morbid obesity, cirrhosis, or renal failure were excluded from the study.
Echocardiographic assessment
The TTE examinations were performed by an experienced cardiologist with a Philips Epiq 7C ultrasound machine according to the recommendations of the American Society of Echocardiography [
10]. All echocardiography examinations included standard M-mode images, two-dimensional imaging, tissue Doppler evaluation at the septal and lateral mitral annulus and strain imaging in all patients at rest in the left decubitus position.
LVEF was calculated by using the standard Simpson method. Transmitral Doppler inflow and tissue pulsed Doppler velocities were obtained in the apical 4-chamber view. Pulsed Doppler measurements included the transmitral early and late diastolic peak flow velocities (Em and Am) and their ratio (E/Am). The average of the peak early diastolic relaxation velocities (e’) of the septal and lateral mitral annulus was calculated.
Strain analysis
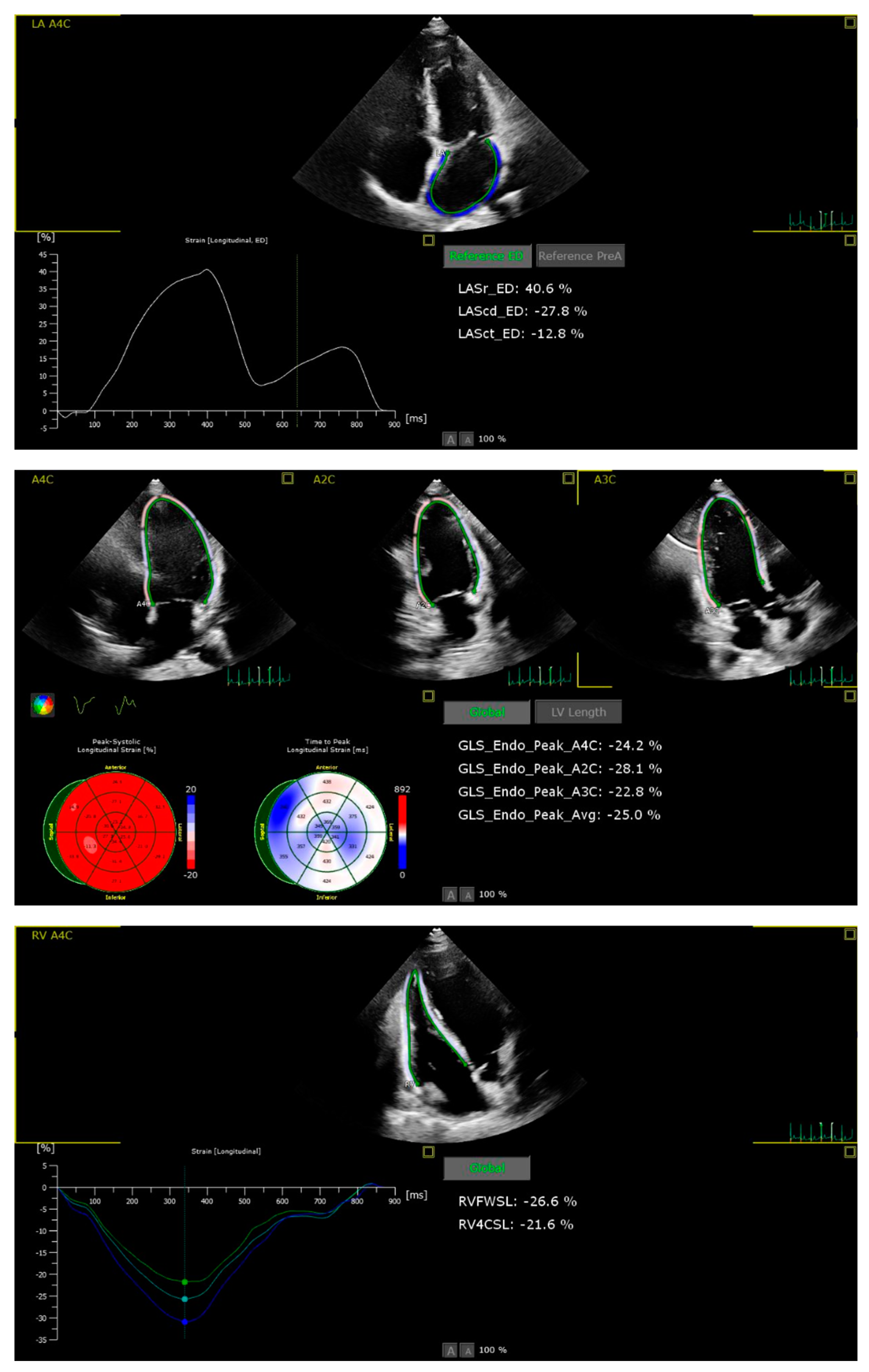
Patients with suboptimal image quality for strain analysis were excluded from the study. The echocardiographic images were analysed by a cardiologist who is particularly interested and trained in cardiovascular imaging and strain echocardiography. The strain values, including the right ventricle GLS (RVGLS) and right ventricle free wall strain (RV-FWSL), the LVGLS, and components of the LAS (atrial reservoir (LAS-r), conduit (LAS-cd), and contractile (LAS-ct) function), were examined from the stored echocardiographic images using a commercially available workstation (QLAB version 13) and by following the recommendations of the EACVI [
11] (
Figure 1).
Speckle tracking echocardiography (STE) was performed using four consecutive cardiac cycles of two-dimensional LV images from the three standard apical views of LV, LA, and RV focused apical 4 chamber, according to the latest guidelines [
12]. For strain analysis, a novel 2D strain analytical software (AutoStrain, Philips) was used that automatically defines the region for tracking by forming a line around the region while still allowing manual changes afterwards. Tracking quality was checked by comparing the motion of the endocardium by the investigator. Manual adjustments were performed after visual assessment of the tracking results throughout the entire cardiac cycle. In segments with poor tracking results, the border was readjusted manually until the best tracking was provided.
Statistical analysis
All statistical analyses were performed using SPSS v25.0. Continuous variables are presented as the mean and standard deviation, and categorical data are presented as percentages or frequencies. The Kolmogorov–Smirnov test was used to determine the normality of the distribution of continuous variables. Parametric and nonparametric variables were compared by using paired t tests and Wilcoxon tests, respectively. Categorical variables were compared by using the chi-square (χ2) test. The patient population was divided into two groups according to blood glucose levels. Results for which the p value was <0.05 were considered statistically significant.
3. Results
The study included 148 subjects (74 female and 74 male). The demographic, clinical and laboratory characteristics of the patients included are given in
Table 1.
When the normal FBG group was compared with the impaired FBG group, the mean age of the impaired FBG group was higher (33.2±9 vs. 38.5±10.1; p=0.001), and no significant difference was found in other demographic parameters (
Table 1). No significant difference was observed in other laboratory parameters (
Table 1). There were significant differences between the groups in several markers of LV diastolic function.
Considering all TTE parameters, the LA was larger (3.1±0.5 vs. 3.3±0.4; p=0.036), the LA volume was greater (39.7±16 vs. 48.1±17.8; p=0.006), the right atrium was larger (3.3±0.5 vs. 3.4±0.5; p=0.05), the IVRT was longer (82.7±19.9 vs. 93.4±22.6; p=0.005), the mitral E wave was shorter (0.8±0.2 vs. 0.7±0.2; p=0.037), and the septal e' was lower (10.8±2.1 vs. 9.6±2.2; p=0.001) in the impaired FBG group.
When the STE findings of both ventricles were compared, no significant difference was observed between the groups in right and LV strain imaging. A comparison of the STE findings of the groups is summarized in
Table 2.
Left atrium function differences between the groups
While there was a significant decrease in the LA reservoir (52.3±15 vs. 44.5±10.7; p=0.001) and conduit strain (36.9±11.7 vs. 28.4±9.7; p < 0.001) in the impaired FBG group, no significant difference was observed in contractile strain (15.4± 9.4 vs. 16.1±7.3; p=0.653). The findings from the LA speckle tracking imaging of the patients are shown in
Table 2.
4. Discussion
In this study, we aimed to evaluate the effect of impaired FBG, one of the most important parameters of metabolic syndrome, on LA strain, which is a reliable measure in evaluating LA functions.
The main findings of our study can be listed as follows: 1. The LAR and LACD strain values were found to be lower in the patient group with impaired FBG than in the normal FBG Group. 2. The left and right atrial diameters and LA volume were found to be larger in the group with impaired FBG. These results show that LAS values are more decreased in the group of patients with impaired FBG than in the normal FBG group.
Various studies have shown that echocardiographic LV diastolic function and strain imaging parameters are impaired in patients with DM compared to individuals without a diagnosis of DM [
11,
13], and the prevalence of LVDD in patients with DM is significantly higher than that in the general population [
14]. The aforementioned findings are of the utmost importance, as they are associated with one of the most critical risk factors for the development of atrial fibrillation and HFpEF [
15,
16]. Both of those conditions are associated with global and cardiovascular hospitalization and mortality [
17].
Similar to diabetes, the presence of LVDD has been reported in various studies that were conducted with people with a diagnosis of impaired FBG [
4,
18].
In a study conducted in 2971 middle-aged people, Milwidsky et al. [
4] observed that impaired FBG was associated with LVDD. In that study, the presence of LVDD was determined by 2D TTE and Doppler echocardiography findings, and strain imaging was not utilized. As a result of the study, it was demonstrated that people with impaired FBG were 43% more likely to have LVDD than people with normal FBG [
4]. Pareek et al. [
19], on the other hand, showed that echocardiographic LVDD was observed significantly more often in patients with impaired FBG who had no comorbidity at the beginning of the study as a result of a median follow-up of 8.3 years. Similarly, in that study, diastolic function was evaluated with 2D TTE and transmitral Doppler and tissue Doppler parameters [
19].
As seen in the studies above, LVDD in people with impaired FBG is evaluated with TTE and generally by transmitral Doppler and tissue Doppler imaging.
In the earliest stage of LVDD, changes in atrial functional parameters become particularly evident. Echocardiographic analyses of these parameters can help to diagnose and determine the degree of LVDD while the morphological parameters are still normal [
7,
20].
Anterior-posterior (AP) LA diameter or LA volume obtained by 2-dimensional (2D) echocardiography are the simplest markers of LA remodelling [
21]. Due to their simplicity and reproducibility, these markers are widely used in many patients. Because the LA is directly exposed to LV filling pressure during diastole, LA dilation is one of the strongest indicators of LVDD [
22].
In our study, LA diameter and volume were found to be significantly higher in patients with impaired FBG, in parallel with other studies. Diastolic dysfunction causes various changes in the functions of the LA apart from geometric changes in the LA. Echocardiographic evaluation of LA deformation is a relatively novel modality for the assessment of LA remodelling. There are 2 methods of assessment: tissue Doppler imaging and speckle-tracking imaging. Tissue Doppler is widely available and practical for routine echocardiography. The A′ velocity at the mitral annulus provides information about regional atrial systolic motion. Early systolic (S′) and early diastolic (E′) velocities correspond to the LA reservoir and conduit function, respectively. However, these velocity measures also bear some limitations related to angle dependency and tethering [
22]. LA deformation evaluated by 2D STE imaging is generally known for LA strain. It has various advantages over conventional echocardiography, such as its independence from angle alignment and loading conditions [
23]. Today, LAS measurements using STE are considered the most specific and sensitive method for the functional assessment of LA [
24]. LA strain was shown to be useful in the detection and grading of HFpEF.
LAS comprises three phases as follows: reservoir, conduit and contractile. Studies have shown that LAS imaging is a useful additional method for evaluating LV diastolic function [
25,
26]. A study by Cameli et al. [
25] demonstrated that LA GLS is better than the mean E/e' ratio in predicting LV end-diastolic pressure. In a study by Morris et al. [
26], it was shown that the addition of LA longitudinal peak strain to the LV volume index in evaluating LVDD in patients with preserved EF was better than the LAVI alone in determining diastolic dysfunction. In addition, it was shown that the risk of hospitalization for HF was increased in patients with abnormal LAS, even if the LAVI was normal in two years of follow-up [
26].
Similar to diabetes, prediabetes is also associated with HF and atrial fibrillation, which are determinants of cardiovascular mortality. In a prospective study involving 294,057 patients, high glucose levels, including the impaired FBG range, were found to be associated with the development of atrial fibrillation and HF during an average of 19.1 years of follow-up of patients with known diabetes mellitus, newly diagnosed diabetes or impaired fasting glucose [
27]. One of the possible mechanisms leading to this condition is increased fibrosis and atrial remodelling [
28]. The development of LVDD and HFpEF, elevated filling pressures and volume overload of the LA could be other potential drivers for the association between dysglycaemic conditions.
The limitations of our study include the single-centre nature of the study and the small number of patients.
5. Conclusions
In conclusion, the addition of LAS imaging to the routine TTE study in patients with impaired FBG but without a DM diagnosis may be helpful in demonstrating subclinical LVDD or identifying patients at risk for LVDD in this patient group. We believe that further studies are needed on this subject.
Author Contributions
Concept: GB, FAD, Design: EO, SU, OO, Materials: OO, GB, KOT, Data Collection And /Or Processing: GB, KOT, Analysis And/Or Interpretation: SU, LB,BO, Literature Review: SU, OO LB, BO, FOK Writer: FAD,EO, Critical Review LB, BO, FOK. All authors read and approved the final manuscript.
Acknowledgments
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Ethics approval and consent to participate
The study was approved by the local ethics committee of our own institution. Written informed consent was obtained from each patient or their relatives following a detailed explanation of the objectives and protocol of the study.
Consent for publication
Not applicable.
References
- Henning, R.J.; Duntas, L.; Kolovou, G.; Ussher, J.R.; Sutendra, G.; Jaswal, J.S.; Tkáč, I.; Gotthardová, I.; Jamaluddin, J.L.; Huri, H.Z.; et al. Type-2 diabetes mellitus and cardiovascular disease. Futur. Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes:Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Koro, C.E.; Kolatkar, N.S. The incidence of heart failure among nondiabetic patients with and without impaired fasting glucose. J. Diabetes its Complicat. 2009, 23, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Milwidsky, A.; Maor, E.; Kivity, S.; Berkovitch, A.; Ben Zekry, S.; Tenenbaum, A.; Fisman, E.Z.; Erez, A.; Segev, S.; Sidi, Y.; et al. Impaired fasting glucose and left ventricular diastolic dysfunction in middle-age adults: a retrospective cross-sectional analysis of 2971 subjects. Cardiovasc. Diabetol. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Martinis, F.; Pezzutto, F.; Sechi, L.A. Plasma Glucose Levels and Left Ventricular Diastolic Function in Nondiabetic Hypertensive Patients. Am. J. Hypertens. 2013, 26, 1353–1361. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Dini, F.L.; Henein, M.; Mondillo, S. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Hear. Fail. Rev. 2016, 21, 65–76. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Benfari, G.; Bisleri, G.; Maccherini, M.; Lisi, G.; Cameli, P.; Lisi, M.; Dokollari, A.; Carrucola, C.; et al. Left atrial strain as a pre-operative prognostic marker for patients with severe mitral regurgitation. Int. J. Cardiol. 2021, 324, 139–145. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Loiacono, F.; Sparla, S.; Iardino, E.; Mondillo, S. Left atrial strain: A useful index in atrial fibrillation. Int. J. Cardiol. 2016, 220, 208–213. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Shi, K.; Yang, M.-X.; Huang, S.; Yan, W.-F.; Qian, W.-L.; Li, Y.; Guo, Y.-K.; Yang, Z.-G. Effect of diabetes mellitus on the development of left ventricular contractile dysfunction in women with heart failure and preserved ejection fraction. Cardiovasc. Diabetol. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Georgievska-Ismail, L.; Zafirovska, P.; Hristovski, Z. Evaluation of the role of left atrial strain using two-dimensional speckle tracking echocardiography in patients with diabetes mellitus and heart failure with preserved left ventricular ejection fraction. Diabetes Vasc. Dis. Res. 2016, 13, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.K.; Thanigaraj, S.; Schechtman, K.B.; E Pérez, J. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am. J. Cardiol. 2004, 93, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Kimura, K.; Aoki, J.; Kobayashi, K.; Terasawa, Y.; Sakai, K.; Shibazaki, K. Prevalence of Atrial Fibrillation in Community-Dwelling Japanese Aged 40 Years or Older in Japan Analysis of 41,436 Non-Employee Residents in Kurashiki-City. Circ. J. 2008, 72, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Hillier, T.A.; Erbey, J.R.; Brown, J.B. Congestive Heart Failure in Type 2 Diabetes. Diabetes Care 2001, 24, 1614–1619. [Google Scholar] [CrossRef]
- Polovina, M.; Lund, L.H.; Đikić, D.; Petrović-Đorđević, I.; Krljanac, G.; Milinković, I.; Veljić, I.; Piepoli, M.F.; Rosano, G.M.; Ristić, A.D.; et al. Type 2 diabetes increases the long-term risk of heart failure and mortality in patients with atrial fibrillation. Eur. J. Hear. Fail. 2020, 22, 113–125. [Google Scholar] [CrossRef]
- Pareek, M.; Nielsen, M.L.; Gerke, O.; Leósdóttir, M.; Møller, J.E.; Hindersson, P.; Sehestedt, T.B.; Wachtell, K.; Nilsson, P.M.; Olsen, M.H. Worsening diastolic function is associated with elevated fasting plasma glucose and increased left ventricular mass in a supra-additive fashion in an elderly, healthy, Swedish population. Int. J. Cardiol. 2015, 184, 466–472. [Google Scholar] [CrossRef]
- Pareek, M.; Vaduganathan, M.; Bhatt, D.L.; Leósdóttir, M.; Olsen, M.H. Prognostic implications of fasting plasma glucose in subjects with echocardiographic abnormalities. Int. J. Cardiol. 2017, 241, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.J.; Tajik, A.J.; Nishimura, R.A.; Oh, J.K.; Khandheria, B.K.; Seward, J.B. Unlocking the Mysteries of Diastolic Function: Deciphering the Rosetta Stone 10 Years Later. J. Am. Coll. Cardiol. 2008, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Park, J.-H. Echocardiographic Measurement of Left Atrial Strain ― A Key Requirement in Clinical Practice ―. Circ. J. 2021, 86, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left Atrial Size and Function. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.J.; Teixeira, R.; Gonçalves, L.; Gersh, B.J. Left Atrial Mechanics: Echocardiographic Assessment and Clinical Implications. J. Am. Soc. Echocardiogr. 2014, 27, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: have we finally found the missing piece of the puzzle? Hear. Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef]
- Cameli, M.; Sparla, S.; Losito, M.; Righini, F.M.; Menci, D.; Lisi, M.; D'Ascenzi, F.; Focardi, M.; Favilli, R.; Pierli, C.; et al. Correlation of Left Atrial Strain and Doppler Measurements with Invasive Measurement of Left Ventricular End-Diastolic Pressure in Patients Stratified for Different Values of Ejection Fraction. Echocardiography 2016, 33, 398–405. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC: Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef]
- Lind, V.; Hammar, N.; Lundman, P.; Friberg, L.; Talbäck, M.; Walldius, G.; Norhammar, A. Impaired fasting glucose: a risk factor for atrial fibrillation and heart failure. Cardiovasc. Diabetol. 2021, 20, 1–9. [Google Scholar] [CrossRef]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).