Introduction
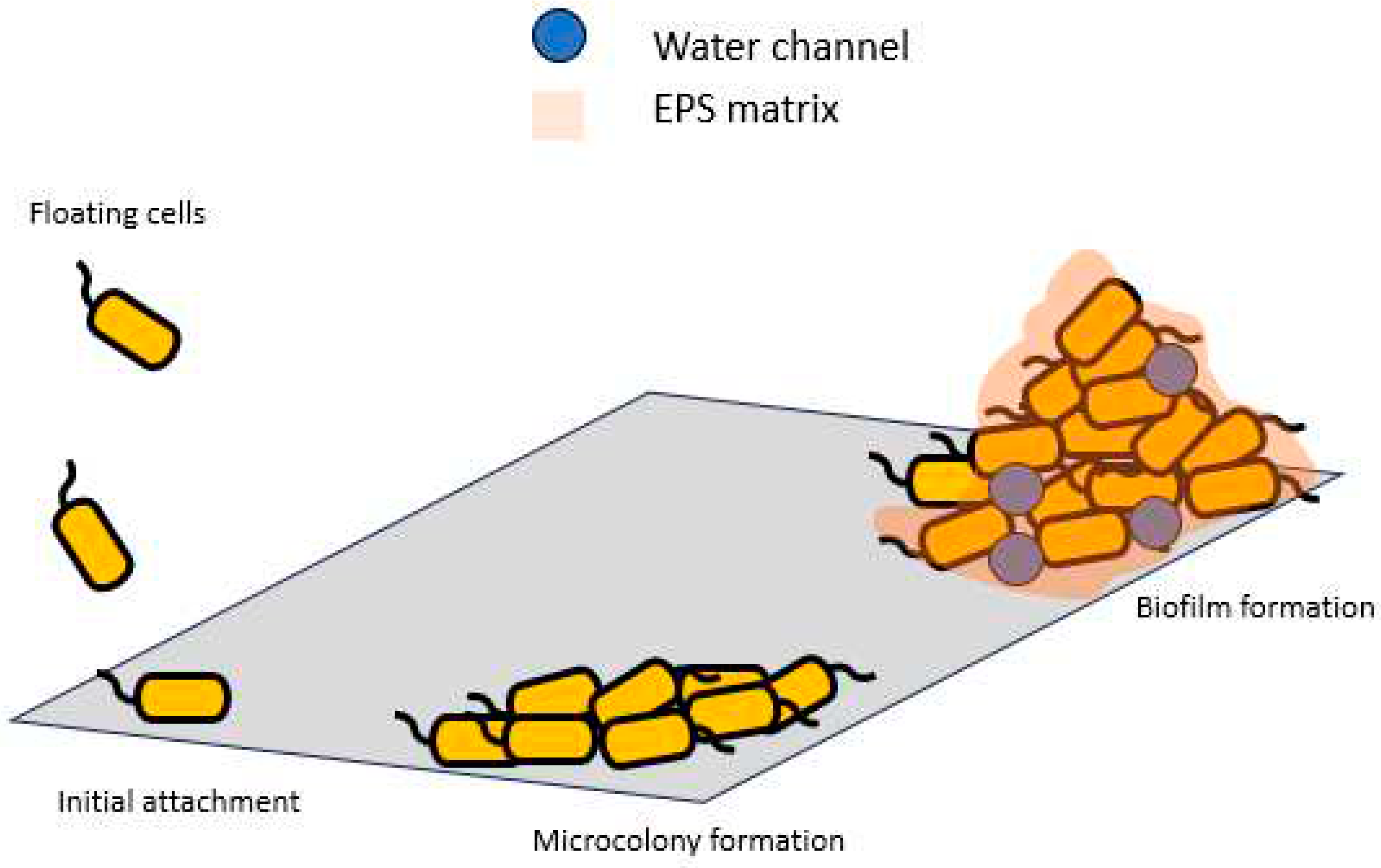
A biofilm is a complex community of microorganisms, such as bacteria, fungi, and protists, that adhere to each other and often to surfaces. These microorganisms are embedded within a slimy extracellular matrix composed of extracellular polymeric substances (EPSs) (Vieira-da-Silva & Castanho 2023). Biofilms are commonly found in various environments, including natural settings and industrial systems. Biofilms serve several functions, including protecting microorganisms from external threats, promoting their growth and survival, and enabling adaptation to changing environmental conditions. The EPS matrix provides a protective shield against antibiotics, immune responses, and physical forces. In the last few decades, the study of protein expression in microbial biofilms has dramatically increased due to the fact that biofilm proteins represent important drug and vaccine targets (Othman & Yahya 2019; Isa et al. 2022; Rashid et al. 2022). Life cycle of biofilm formation is shown in
Figure 1 below.
Microbial biofilms pose a significant challenge in various industrial settings. For instance, in the food industry, they can contaminate production equipment, surfaces, and food products themselves, leading to potential food safety issues, quality deterioration, and economic losses. To combat this problem effectively, it is essential to implement a comprehensive strategy that includes preventive measures, early detection, and targeted interventions. Important strategies to combat microbial biofilms in the various industries include:
1. Establishing Proper Cleaning and Sanitization Procedures
One of the most critical steps in preventing and combating microbial biofilms is the establishment and implementation of proper cleaning and sanitization procedures. A combination of mechanical (scrubbing, scraping) and chemical (detergents, sanitizers) methods should be used to remove and destroy biofilms effectively (Bonafé et al. 2023). The cleaning protocols should be tailored to the specific equipment and surfaces, considering factors such as surface characteristics, location, and susceptibility to biofilm formation.
2. Promoting Good Hygiene Practices
Good hygiene practices play a crucial role in reducing the risk of biofilm formation and contamination. This includes ensuring that employees follow proper hand hygiene protocols, wear appropriate protective clothing, and receive regular training on food safety and sanitation practices. Implementing a culture of cleanliness and accountability within the organization can significantly contribute to preventing and controlling biofilm-related issues.
3. Implementing Effective Monitoring Techniques
Early detection of biofilm formation is essential to initiate timely interventions and prevent spreading. Various monitoring techniques, such as visual inspections, adenosine triphosphate (ATP) bioluminescence (Fischer & Rundels 2023), and microbial swabbing, can be used to identify potential biofilm sites. Regular and systematic monitoring should be conducted throughout the food production facility to identify areas prone to biofilm formation and to verify the effectiveness of cleaning and sanitization procedures.
4. Utilizing Advanced Cleaning Technologies
Advancements in cleaning technologies have facilitated more effective eradication of biofilms. Techniques such as high-pressure cleaning, steam cleaning, and ultrasonic cleaning can provide better penetration into biofilm structures, increasing the likelihood of complete removal. Employing these technologies alongside traditional cleaning methods can enhance the efficacy of biofilm control in complex equipment and hard-to-reach areas.
5. Implementing Biofilm-specific Disinfection Measures
Biofilms can exhibit resistance to traditional disinfection methods due to the protective matrix they create. Implementing biofilm-specific disinfection measures, such as the use of enzymatic cleaners and targeted bactericides, can be effective in breaking down the biofilm structure and killing the protected microorganisms. These products are specifically formulated to penetrate and disrupt biofilms, enhancing their efficacy compared to conventional disinfectants.
6. Enhancing Surface Properties
Modifying the surface properties of equipment and food contact surfaces to prevent or hinder bacterial attachment can be an effective preventive strategy. This can be achieved through the use of antimicrobial coatings (Chen et al. 2022), surface roughening, or selecting materials with inherent antimicrobial properties. The goal is to create surfaces that are less favorable for microbial adhesion, making it difficult for biofilms to establish.
7. Implementing Effective Drainage Systems
Poorly designed or maintained drainage systems can become a breeding ground for biofilms, making it crucial to design, install, and maintain effective drainage systems. Ensuring that drainage systems are designed to promote efficient flow and prevent standing water can minimize the opportunities for biofilm formation.
8. Conducting Regular Audits and Risk Assessments
Regular audits and risk assessments should be conducted to identify potential biofilm-prone areas within the food production facility. This includes inspecting equipment and surfaces, reviewing cleaning and sanitation procedures, and assessing general hygiene practices. The findings from these audits can inform improvements in cleaning protocols, employee training, and the implementation of additional preventive measures.
How do Existing Disinfectant Products Control Microbial Biofilms?
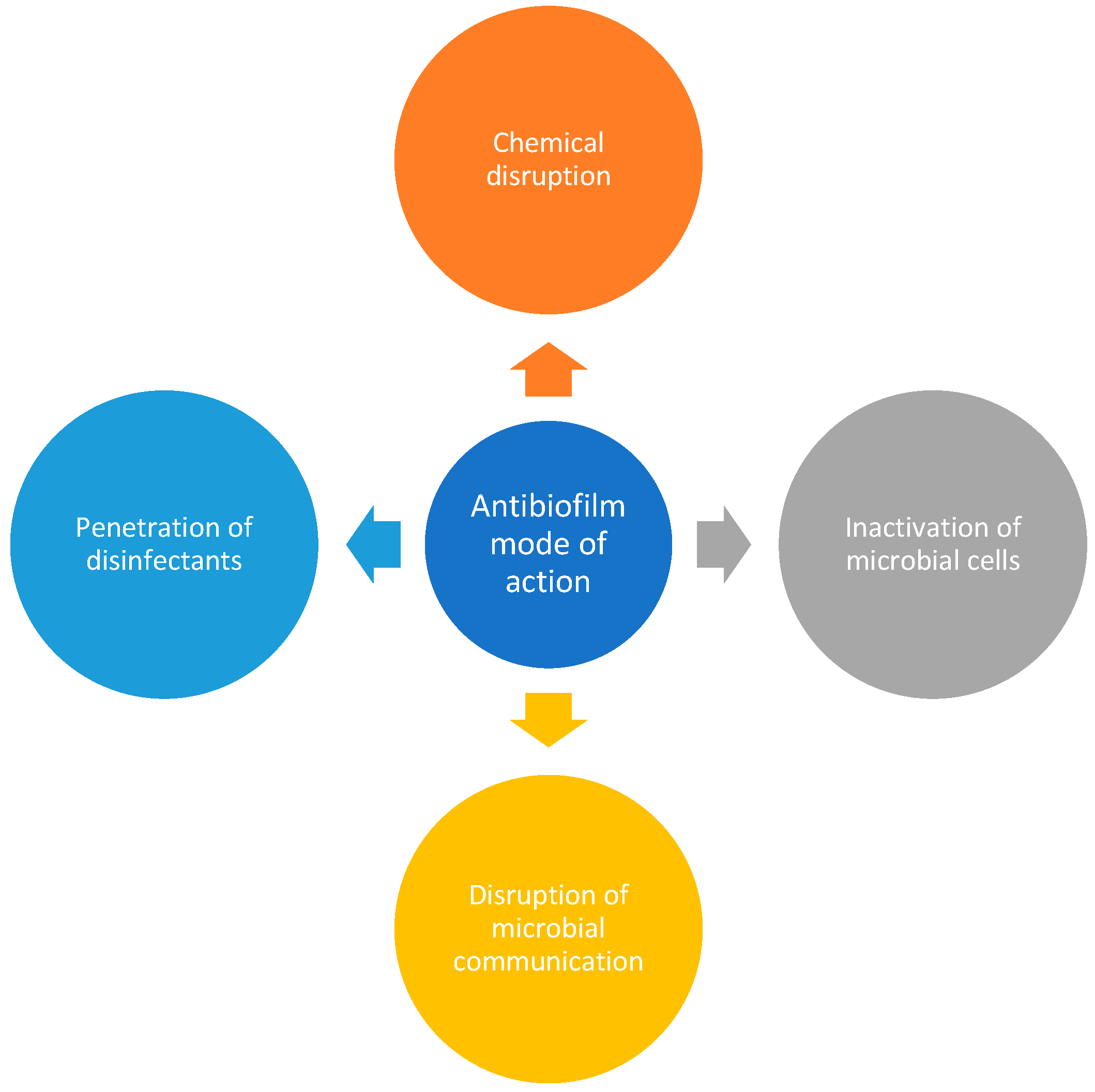
Chloroxylenol, sodium dodecyl-benzene sulfonate, benzalkonium chloride, and sodium hypochlorite are disinfectants commonly used for disinfecting surfaces and cleaning equipment. To combat biofilm-related issues effectively, understanding the mode of action of existing disinfectants is crucial. Understanding the different mechanisms by which disinfectants act against microbial biofilms, providing insights into their effectiveness. The main antibiofilm mode of actions are summarized in
Figure 2.
1. Chemical Disruption of Biofilm Matrix
One of the primary targets of disinfectants is the biofilm matrix that provides structural support and protection to the microorganisms residing within it (Yahya et al. 2018; Kamaruzzaman). Different classes of disinfectants, such as oxidizing agents (e.g., chlorine, hydrogen peroxide), surfactants, and enzymes, can disrupt the matrix by degrading its components. Oxidizing agents generate reactive oxygen species (ROS) that damage the matrix and the microorganisms. Surfactants work by reducing the surface tension of the matrix, facilitating penetration of the disinfectant. Enzymes, such as proteases and lipases, enzymatically degrade the extracellular polymeric substances (EPS) present in the matrix, leading to its disruption.
2. Inactivation of Microbial Cells
Disinfectants can directly target and inactivate the microbial cells within the biofilm. The mode of action varies depending on the type of disinfectant used. For instance, chemical disinfectants such as alcohols, halogens, and quaternary ammonium compounds (QACs) can disrupt the cell membrane (Kwaśniewska et al. 2020), leading to leakage of cellular contents, which ultimately leads to cell death. Other disinfectants like phenolics and heavy metals interfere with cellular processes, inhibiting vital enzymes or disrupting DNA replication, resulting in microbial inactivation.
3. Penetration and Distribution within Biofilm
Biofilms have intricate three-dimensional structures with channels and porous matrices, making it challenging for disinfectants to penetrate and reach all microorganisms. Disinfectants with low molecular weights and surfactant properties can penetrate biofilms more effectively. Additionally, physical methods such as ultrasonic waves, which create localized disturbances, can enhance the penetration of disinfectants. The distribution of disinfectants within biofilms is influenced by the biofilm's architecture, flow rates, and diffusion rates. Understanding these factors is crucial for selecting appropriate disinfectant concentrations and contact times.
4. Disruption of Microbial Communication
Quorum sensing, a bacterial cell density-dependent communication mechanism, plays a significant role in biofilm formation and maintenance. Some disinfectants, such as certain aldehydes and terpenes, can interfere with quorum sensing signals, disrupting microbial communication and inhibiting biofilm formation. By disrupting quorum sensing, these disinfectants hinder the cohesive activities of biofilm-forming microorganisms, weakening the biofilm structure and making it more susceptible to disinfection.
5. Combination Approaches
Combination approaches involving multiple disinfectants or disinfectants with different mechanisms of action are often employed to overcome the resistance mechanisms of biofilms. Synergistic interactions between disinfectants can enhance their efficacy against biofilms by targeting multiple aspects simultaneously. For instance, a combination of enzyme-based cleaners and oxidizing agents can effectively degrade the biofilm matrix and inactivate microbial cells within the biofilm.
6. Persistence and Residual Activity
The persistence and residual activity of disinfectants within biofilms are crucial factors for successful biofilm eradication. Some disinfectants are capable of penetrating the biofilm matrix and remaining active for an extended period, continuously exerting their antimicrobial effects. This sustained activity helps combat the regrowth of microorganisms and further biofilm formation.
7. Environmental Factors
The effectiveness of disinfectants against biofilms can also be influenced by various environmental factors. pH, temperature, levels of organic and inorganic matter, and water hardness can impact the disinfectant's ability to penetrate biofilms and exert its antimicrobial effects. Understanding the effect of these factors can help optimize disinfection protocols for specific biofilm environments.
How do Water Channels in Microbial Biofilms Contribute to Resistance Agains Disinfectants?
Microbial biofilms are complex and structured communities of microorganisms that adhere to surfaces and are embedded within a self-produced matrix. Within these biofilms, water channels play a crucial role in facilitating nutrient and waste exchange, maintaining hydration, and supporting cellular communication (Quan et al. 2022). These water channels also contribute to the development of antibiotic resistance within biofilms.
1. Formation of Water Channels in Biofilms
Water channels, also known as water-filled channels or water-conducting channels, provide pathways for the movement of water and solutes within biofilms. These channels are created by the arrangement of bacterial cells and the extracellular polymeric substances (EPS) matrix. EPS components, such as polysaccharides, proteins, and extracellular DNA, can form intricate networks that allow water to flow through them. The channels vary in size and shape, and their presence is essential for the overall biofilm architecture and function.
2. Nutrient and Waste Exchange
Water channels enable nutrient and waste exchange within biofilms, allowing for the transport of essential compounds to the cells and the removal of metabolic byproducts. Nutrients, such as sugars, amino acids, and ions, can diffuse through the water channels, providing a constant supply for the biofilm's microbial community. Similarly, waste products, including organic acids and toxins, can be expelled through the channels, preventing their accumulation and maintaining biofilm viability.
3. Oxygen and Redox Gradients
Water channels also contribute to the establishment of oxygen and redox gradients within biofilms. Oxygen availability decreases with increasing depth within a biofilm due to limited diffusion through the EPS matrix. Water channels provide pathways for the entry of oxygen from the environment into the deeper layers of the biofilm, supporting the growth of oxygen-dependent microorganisms. Oxygen gradients have implications for the distribution of microbial populations and the metabolic activities within the biofilm.
4. Cellular Communication
Cell-to-cell communication, known as quorum sensing, is crucial for biofilm development and the coordination of certain behaviors among microorganisms. Water channels play a significant role in facilitating the diffusion of signaling molecules, such as acyl homoserine lactones (AHLs), autoinducer-2 (AI-2), and peptides, which are involved in quorum sensing. This communication allows the microbial community within the biofilm to coordinate behaviors such as the production of EPS, virulence factors, and antibiotic resistance mechanisms.
5. Contribution to Disinfectant Resistance
Water channels within biofilms create a unique microenvironment that contributes to increased resistance against disinfectant (Kowalski et al. 2020). The presence of these channels aids in the diffusion and distribution of antibiotics throughout the biofilm, ensuring that all cells have access to sub-MIC (sub-minimum inhibitory concentration) levels of the disinfectant. However, the structured nature of biofilms, along with the hydrophobic properties of the EPS matrix, impedes the penetration of disinfectant into deeper biofilm layers. This reduced disinfectant penetration, combined with other resistance mechanisms, leads to the persistence of subpopulations of resistant microorganisms.
6. Phenotypic and Genotypic Adaptations
Water channels also contribute to the phenotypic and genotypic adaptations associated with disinfectant resistance. Within biofilms, subpopulations of microorganisms can occupy different microenvironments with varying nutrient availability, oxygen levels, and exposure to stressors, including antibiotics. This diversity of microenvironments promotes the emergence of resistant phenotypes through the upregulation of stress response genes, altered metabolic pathways, and changes in membrane permeability.
7. Enhanced Gene Transfer
Water channels may facilitate the transfer of genetic material, including resistance genes, between bacterial species within biofilms. Horizontal gene transfer mechanisms, such as conjugation, transformation, and transduction, rely on physical proximity and close contact between cells. The presence of water channels allows for increased cell-to-cell interactions, promoting the exchange of genetic material and the spread of disinfectant resistance determinants among different species within the biofilm.
Conclusion
Water channels play a critical role in the formation and function of microbial biofilms. While essential for nutrient exchange, waste removal, oxygen gradients, and cellular communication, these channels also contribute to antibiotic resistance within biofilms. The presence of water channels allows for localized microenvironments, reduced antibiotic penetration, enhanced gene transfer, and the expression of biofilm-specific resistance mechanisms. Understanding the role of water channels in antibiotic resistance helps shed light on the complexity of biofilm-associated infections and underscores the need for novel approaches to combat these resilient microbial communities. Further research into the mechanisms and regulation of water channels in biofilms will contribute to the development of more effective strategies to mitigate biofilm-related antibiotic resistance.
References
- Bonafé, A. C. F., Oliveira, D. F. L. M., Fernandes, E. E., Garcia, M. T., Dias, I. P. S. S., Bressane, A., ... & de Mello Rode, S. (2023). Microbiological evaluation in invisible aligner chemical cleaning methods against Candida albicans and Streptococcus mutans. American Journal of Orthodontics and Dentofacial Orthopedics, 164(2), e43-e50. [CrossRef]
- Brindhadevi, K., LewisOscar, F., Mylonakis, E., Shanmugam, S., Verma, T. N., & Pugazhendhi, A. (2020). Biofilm and Quorum sensing mediated pathogenicity in Pseudomonas aeruginosa. Process Biochemistry, 96, 49-57. [CrossRef]
- Chen, C., Li, Z., Li, X., Kuang, C., Liu, X., Song, Z., ... & Shan, Y. (2022). Dual-functional antimicrobial coating based on the combination of zwitterionic and quaternary ammonium cation from rosin acid. Composites Part B: Engineering, 232, 109623. [CrossRef]
- Fisher, J. J., & Rundels, J. J. (2023). How clean is the library? Using ATP bioluminescence technology to identify surface contamination. The Journal of Academic Librarianship, 49(3), 102706. [CrossRef]
- Isa, S. F. M., Hamid, U. M. A., & Yahya, M. F. Z. R. (2022). Treatment with the combined antimicrobials triggers proteomic changes in P. aeruginosa-C. albicans polyspecies biofilms. ScienceAsia, 48(2). [CrossRef]
- Kamaruzzaman, A. N. A., Mulok, T. E. T. Z., Nor, N. H. M., & Yahya, M. F. Z. R. (2022). FTIR spectral changes in Candida albicans biofilm following exposure to antifungals. Malaysian Applied Biology, 51(4), 57-66. [CrossRef]
- Kowalski, C. H., Morelli, K. A., Schultz, D., Nadell, C. D., & Cramer, R. A. (2020). Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proceedings of the National Academy of Sciences, 117(36), 22473-22483. [CrossRef]
- Kwaśniewska, D., Chen, Y. L., & Wieczorek, D. (2020). Biological activity of quaternary ammonium salts and their derivatives. Pathogens, 9(6), 459. [CrossRef]
- Othman, N. A., & Yahya, M. F. Z. R. (2019). In silico analysis of essential and non-homologous proteins in Salmonella typhimurium biofilm. In Journal of physics: Conference series (Vol. 1349, No. 1, p. 012133). IOP Publishing. [CrossRef]
- Quan, K., Hou, J., Zhang, Z., Ren, Y., Peterson, B. W., Flemming, H. C., ... & van der Mei, H. C. (2022). Water in bacterial biofilms: pores and channels, storage and transport functions. Critical reviews in microbiology, 48(3), 283-302. [CrossRef]
- Rashid, S. A. A., Yaacob, M. F., Raihanah, N., Anuar, T., Johari, N., Kamaruzzaman, A. N. A., ... & Yahya, M. F. Z. R. (2022). A combination of in silico subtractive and reverse vaccinology approaches reveals potential vaccine targets in Corynebacterium pseudotuberculosis. Journal of Sustainability Science and Management, 17(1), 99-109.
- Vieira-da-Silva, B., & Castanho, M. A. (2023). The structure and matrix dynamics of bacterial biofilms as revealed by antimicrobial peptides' diffusion. Journal of Peptide Science, e3470. [CrossRef]
- Yahya, M. F. Z. R., Alias, Z., & Karsani, S. A. (2018). Antibiofilm activity and mode of action of DMSO alone and its combination with afatinib against Gram-negative pathogens. Folia microbiologica, 63, 23-30. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).