1. Introduction
In daily life, individuals often encounter situations where they need to simultaneously perform additional tasks during walking, known as dual-task (DT) walking. These additional tasks can include cognitive activities such as problem-solving. Walking itself, also known as single-task (ST) walking, requires the integration and coordination of various motor and cognitive processes, including attention to the environment and neuro-biomechanical control of body segments during locomotion [
1]. However, when additional cognitive activities are introduced during walking, they interfere with the execution of fundamental balance control, resulting in what is known as DT interference [
2,
3,
4]. This interference presents further challenges to an individual's walking performance and may increase the risk of injuries such as falls [
5]. For this reason, researchers have focused on investigating the extent to which balance control is disrupted during DT walking compared to ST walking [
6].
Numerous studies have examined spatiotemporal gait parameters during DT and ST walking, consistently revealing slower gait speed, shorter stride length, and greater gait variability (i.e., increased stride-to-stride fluctuations in spatiotemporal gait parameters) during DT walking. These findings remain consistent across various age groups and clinical conditions [
7,
8,
9]. While fewer studies have investigated lower-extremity joint kinematics during DT walking, recent research by Piche et al. reported differences in angular velocities at the hip and knee between DT and ST walking in older adults [
10]. Additionally, Ko et al. found that older adults unable to perform DT walking exhibited reduced ranges of motion in the knee and ankle compared to their counterparts capable of performing DTs [
11]. These findings suggest disruptions in lower-extremity movement during DT walking, potentially contributing to differences in spatiotemporal gait parameters. Although results from these reports may be slightly mixed, they provide implications that there may be some disruptions in the lower-extremity movement during DT walking compared to ST walking, which may have resulted in differences in spatiotemporal gait parameters. This further suggests that lower-extremity muscle activity may have contributed as more fundamental neuromuscular causes to disrupt the lower-extremity movement [
12], and yet, studies investigating lower-extremity muscle activity during DT walking compared to ST walking are scarce, with only a recent study existing [
13].
Furthermore, the effects of DT interference on neuromuscular control may have a more pronounced impact on locomotion tasks requiring substantial balance control, such as gait initiation (GI), compared to rhythmic and repetitive phases like steady-state (SS) gait [
14,
15,
16,
17]. However, to the best of our knowledge, no study has yet compared lower-extremity muscle activity during DT GI and ST GI. Therefore, this study aims to examine the effects of DT interference on lower-extremity muscle activity during both GI and SS gait in healthy young adults. We hypothesize that healthy young adults will have decreased lower-extremity muscle activity in both GI and SS gait during DT walking compared to ST walking.
2. Materials and Methods
2.1. Participants
Twenty-one healthy young adult volunteers from the university community participated in this study after providing signed informed consent, which was approved by the Institutional Review Board at the University of Texas at Dallas. Inclusion criteria for participants included English proficiency, age between 18 and 64 years, the ability to walk without any walking aids, good general health, and a body mass index (BMI) ranging from 18.5 to 29.9 kg/m². Exclusion criteria encompassed a history of musculoskeletal, neurological, or cardiovascular disease.
2.2. Experimental Protocol
After obtaining consent, we collected data on participants’ demographic information (
Table 1). We then administered the Montreal Cognitive Assessment (MOCA), the International Physical Activity Questionnaire (IPAQ) to evaluate participants’ cognitive status and physical activity levels, respectively [
18,
19].
We asked participants to change into tight-fitting clothing (short pants and short sleeves). We then attached wireless surface electromyography (EMG) sensors (16-channel Delsys Trigno, Delsys, Natick, MA, USA) bilaterally on the lower lmbs. We followed the non-invasive assessment of muscles (SENIAM) guidelines for the EMG attachment [
20]. Briefly, the participant’s skin was cleaned using isopropyl alcohol to limit impedance. Once applied, the skin was allowed time to dry. Following the skin preparation, the EMG sensors were cleaned with isopropyl alcohol and allowed to dry before placement. The associated thigh segment lower limb muscles (hamstring and quadricep muscles) were the vastus lateralis (VL) and biceps femoris (BF). The associated shank segment (lower knee and calf muscles) was tibialis anterior (TA) and gastrocnemius.
Once all EMGs were attached, data from the maximum voluntary contraction (MVC) was collected by two consecutive trials for each muscle as reference value to normalize EMG data from gait trials. For the MVC trials, participants were asked to sit at a 90-degree angle between the seat and lower limb joints for each muscle configuration [
21]. During each trial, the participants were given the following queues: to move the limb in the contracting direction (build up) from 0 to 3 sec, give as much force as possible (max) from 4 to 8 sec, and stop the movement (relax) from 8 to 10 sec. All EMG data (MVC trials and gait trials) were collected at a sampling of 2000 Hz.
In addition to the EMG sensors, we used 10-camera 3D motion capture system (Vicon, Oxford, UK) with a sampling rate of 100 Hz to identify GI and SS. For the motion capture, we placed the 74 reflective markers on each participant’s full body: the clavicular notch (jugular notch), xiphisternum, along the spine C7 vertebrae, along the spine T10 vertebrae, apex of acromioclavicular joint bilaterally (including anterior and posterior), anterior superior iliac spine bilaterally, posterior superior iliac spine bilaterally, medial and lateral condyle of the femur, medial and lateral malleolus, calcaneus bilaterally, proximally to the medial and lateral epicondyle, center of the first and fifth metatarsals, apex of proximal phalanx 1. In addition, there were clustered markers placed on upper arm, forearm, thigh, and tibia to track segments. The medial markers were removed after the static and bodyweight trials [
22].
To track markers used in the motion trials, a static trial was conducted prior. The participant was instructed to stand in anatomical pose. Once completed, a body weight trial was taken to collect the participants’ weight from a single force plate (Kistler Instruments Ltd, Farnborough, UK).
After the static and body weight trials, the participant was given up to 5 minutes to understand the rules of the procedure for the tasks they would perform. During this time, the participants were able to get acquainted with the 10-mteter walkway by walking at their speed of preference. Once familiarized, they were asked to perform a body weight trial to collect the participants weight while standing on the force plate. Subsequently, the motion trials with the ST and DT condition were performed consecutively in sets of three repetitions each.
For the ST condition, the participants were queued to begin walking from the start of the 10-meter walkway, while standing on top of the two force plates, to the end at their selected speed with no additional task. For the DT condition, the participant repeated the ST walk trial, meanwhile verbally announcing the subtracted value given by the assessors originally queued numerical, as done previously [
3,
4]. For example, we gave instructions like “subtract by 3 from 110 during walking”.
2.3. Data Analysis
We used Visual3D (C-motion, Germantown, MD, USA) to process marker and ground reaction force data. These data were used to identify GI and SS gait. In brief, SS gait was estimated using markers placed at the foot, toe, heel, and sacrum, following a previously published protocol [
23].
For GI analysis, three parameters were considered. First, onset, the duration of time until the vertical ground reaction force deviates above the mean of three standard deviations. This marks the initiation of gait movement. Second, weight transition, normally referred to as weight acceptance [
23]. This represents the time taken from the initial contact of the stepping limb with the force plate until the vertical ground reaction force is fully shifted to that plate. This is the instance when the body transfers its weight from the initial stance limb to the stepping limb. Third, offset, the duration of time during which the stepping limb is swinging, and the standing limb is reaching the instance of toe-off, showing significant reductions in force plate displacement, while preparing to take the next consecutive step. This concludes the process of GI. This data was obtained from motion capture and force plates, following previous methods described to ensure consistency and comparability of data analysis [
23].
In SS gait, we analyzed mean muscle activation by taking EMG signal data from the gait cycles in the motion trials. These gait cycles were created by Visual3D pipelines that calculated the foot strikes to toe-offs for each limb. In GI, we calculated the mean muscle activation and co-contraction from EMG signal data from the onset to the offset phases of the motion trial.
EMG data from GI and SS gait cycles were filtered in Visual3D. A band-pass filter (10-350 Hz) was applied to EMG signals collected from the MVC and motion trials [
24]. Then, a fourth order filter with a cut-off frequency of 10 Hz was applied. After, the EMG signals were full wave rectified. A linear envelope was applied by a 1 ms moving average window. EMG data was smoothed by normalizing time to 1000 data point samples.
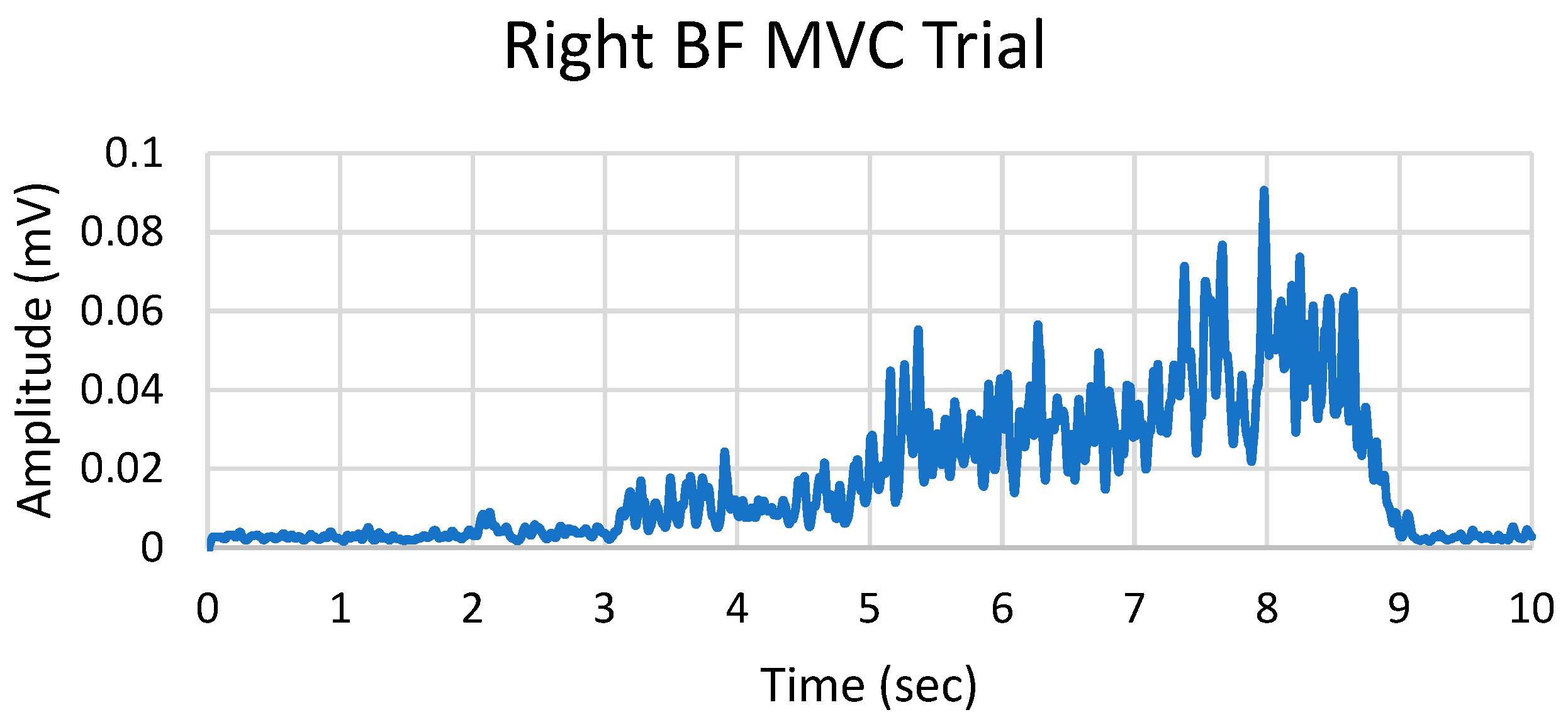
The EMG data was processed in Matlab (Mathworks, Inc, Natick, USA). The peak EMG signal value was extracted from the MVC trials. From the MVC trials, the highest peak EMG signal value was extracted. To consider the MVC trial for later reference in data analysis, a peak value was taken within a 10 sec time frame (
Figure 1). The peak EMG mean value was taken from three motion trials from the muscles investigated in the thigh and shank.
Co-contraction in GI (1) was calculated based on the activity between two muscles. For example, the muscle activity in the BF and VL is calculated as follow:
To calculate the percentage of coactivation, a custom Matlab code was utilized, which divides the lower muscle activation by the higher muscle activation and then multiplies the result by the combined high and low activities, all multiplied by 100 [
25].
2.4. Statistical Analysis
All participant data was statistically processed using SPSS version 29.0 IBM software. A paired t-test was used for comparing the muscle activation and coactivation between single and DT conditions. The means and standard deviation values were summarized and included in subject demographics. A Shapiro-Wilks test was done to determine the normality of data samples. For non-normal data, a transform was conducted through a logarithmic calculation. After, a paired t-test was conducted to obtain the two-tailed value. The Kruskal-Wallis test was done for non-normal data. The sample significance was observed between the groups (p < 0.05). We calculated the group effect size with Cohen’s d (d). The group effect size represents d < 0.2 as a small effect, 0.2 ≤ d < 0.5 as a medium effect, then 0.5 ≤ d < 0.8 as large effect.
3. Results
3.1. Participants
Analysis of 21 healthy young adults was conducted. Fourteen males and seven females participated in this study (
Table 1). The subject demographic represents the sample size (n), age (yrs.), ethnicity (caucasian, african american, asian american, american indian, asian pacific island, hispanic, mixed ethnicity), race (white, black, asian, mixed race), height (meters), weight (kg), BMI (kg/m²), MOCA (30 total points), IPAQ (time expended partaking in different activities).
3.2. Gait Initation
For GI, EMG data from 15 participants were analyzed due to protocol violation and technical issues. Values for the mean muscle activity and co-contraction between muscles relative to the foot stance and swing positions are provided in
Table 2 and
Table 3. The significant difference in dual task and ST conditions during the GI phases are given:
Mean Muscle Activity:
The significant effect values were found in GI phase events in mean muscle activation. The onset phase had medium group effect, Stance BF (p = 0.071, d = 0.50). The weight transition phase had medium group effect, Stance G (p = 0.088, d = 0.49). The offset phase significant values were, Stance BF (p = 0.038, d = 0.59) and Stance TA (p < .001, d = 1.1). The full GI phase had significant values, Stance TA (p = .003, d = 0.91)
Mean Muscle Coactivity:
The significant effect values were found in GI phase events in mean muscle coactivation. The onset phase had no significant values to report. The weight transition phase, Swing BF/VL (p = 0.14, d = 0.73); Stance BF/VL (p = .004, d = 0.88); and Stance G/TA (p = .043, d = 0.60). The offset phase significant values were, Stance G/TA (p < .001, d = 1.1). The full GI had significant values in Stance G/TA (p = .006, d = 0.84).
3.3. Steady-State Gait
In SS gait, the values for mean muscle activity are provided in
Table 4. For SS gait, EMG data from all 21 participants have been analyzed. These values were collected from EMG signals from left and right leg muscles. The significant difference in dual and singe task conditions are given in the stance phase:
Mean Muscle Activity:
In this phase, each muscle had significant differences between task conditions, however the gastrocnemius did not show any significant difference. The R BF had a (p = .003, d = 0.16) and L BF (p < .001, d = 0.1). The gastrocnemius exhibited no significant different values for either side anatomically. The R TA had a small group effect (p < .001, d = 0.11) and L TA had a large group effect (p < .001, d = 0.91). The R VL had a small group effect (p < .001, d = 0.11) and L VL had a small group effect (p = .001, d = 0.13).
4. Discussion
The purpose behind the study was to investigate the muscle activity changes involved during two different task conditions, DT and ST, while performing SS gait and GI. The hypothesis states that in healthy young adults, there will be reduced muscle activity in the lower extremities both during SS gait and GI when performing dual task walking compared to ST walking. The study outcomes confirm there are muscle activity changes caused by the dual task effects. Regarding muscle activation in lower muscles during SS gait, there were statistically significant decreases for all muscles except the gastrocnemius in dual task condition. Regarding muscle activation during GI on lower muscles, there were differences between trial groups during each phase of GI. The overall GI phase showed decreases under the dual task state. The coactivation for lower muscles in GI phase showed to increase for Stance BF/VL and Swing G/TA. These altered muscle patterns can be considered a potential indicator of a decline in aging adult cognitive aptitude to smoothly perform motor function.
GI muscle activation was assessed by phases from onset, weight transition, offset, and full GI. For onset muscle activation, Stance BF had a medium difference between the two groups (d = 0.50). In weight transition muscle activation, the Stance G had a medium difference between the two groups (d = 0.49). In offset muscle activation, Stance BF (p = 0.038, d = 0.59) and Stance TA (p < .001, d = 1.1) had significant changes. In addition, the offset phase Stance G had medium difference between two groups (d = 0.52). The full GI shows significant changes in Stance TA (p = 0.003, d = 0.91).
GI coactivation was quantified in phases from onset, weight transition, offset, and full GI. For onset muscle coactivation, there were no significant changes. In weight transition muscle coactivation, Stance BF/VL (p = .004, d = 0.88) and Stance Gastrocnemius /TA (p = .043, d = 0.60). In addition, Swing BF/VL has a large difference between the two groups (Group Effect = 0.73). In offset muscle coactivation, Stance Gastrocnemius /TA had a (p < .001, Group Effect = 1.1). The full GI shows Stance Gastrocnemius/ TA had a (p = .006, Group Effect = 0.84).
SS gait muscle activation was reduced in lower muscles in the stance phase of the gait cycle. The BF (Left, p = .003; Right, p < .001), TA (Left, p < .001; Right, p <.001), and VL (Left, p = .001; Right, p < .001) were significantly different between the ST and ST. In the L TA, there was a large difference between the two groups (d = 0.91). The gastrocnemius muscle had no significant change on either side of the limb.
4.1 Gait Initiation Phases
The GI phases were divided into three sections from the full GI gait cycle, which provided a closer observation of change in muscle activation. We have found significant changes in the offset phase of GI, compared to onset and weight transition [
26]. The TA is known to generate forces associated with ground push-off or toe-off during this offset phase [
26]. Our significant findings were consistent with Stance TA muscle activity for normal older adults performing GI [
26]. Though, we did not find significance in the gastrocnemius muscle activity, which [
26] study reported. The Stance limb for each muscle showed higher muscle activation than Swing limb, setting a similar response seen in older adults [
26], which may be understood as a normal healthy type of body response in GI. Alternatively, the Stance BF muscle activation became significant in offset phase, which could indicate a center of mass load response from the prior weight shift occurring from the weight transition phase [
27]. A study found that young and older adults use the same motor programming to perform GI [
28]. Previous studies show there are somewhat similar muscle firing patterns in old and young adults, though older adults tend to inhibit their gastrocnemius muscle activation. Thus, older adults possibly rely on TA activation when controlling forward acceleration of posture center of pressure [
25,
28].
During the DT, the mean muscle activation was reduced in all phases, including the full GI. For onset phase, muscles in both stance and swing decreased, except for Swing VL. For weight transition, there was less activation in Swing BF, Stance Gastrocnemius, Swing TA, and Stance TA. For offset phase, the Swing BF, Stance BF, Swing Gastrocnemius, Stance Gastrocnemius, Swing TA, Stance TA, and Stance VL showed decreased activation. Khanmohammadi et al. reported diminished levels of muscle activity in weight transitioning phase was caused by a center of pressure shift, done by older adults switching their stance limb to swing limb [
25]. The muscles associated with the shift were VL and TA. Currently, there is a gap in literature sources discussing both GI and DT muscle activation.
In coactivation, the coordinating muscle contractions between BF and VL or TA and gastrocnemius were investigated, which share an agonist and antagonist muscle relationship. There were several GI phases that provided significant findings for muscle coactivation. The weight transition and offset phases, which contributed to the significance found during the full GI phase. The weight transition and offset phases showed significance on the Stance limb of the gastrocnemius and TA contractions, which was also seen as significant in a study characterizing muscle activity patterns in older and younger adults coactivation [
25]. In this study they reported locomotor, which we have referred to as “offset phase”. Here, the study linked increased coactivity as being involved with a compensation strategy that the older adults employed to maintain posture that was in response to reduced muscular power from involuntary contractions [
25].
Considering DT, the muscle coactivity across GI phases had increased. For onset phase, Swing BF/VL increased for coactivation. For weight transition, Swing BF/VL increased. For offset phase, Swing Gastrocnemius /TA increased. Saraiva et al. reported young adults reallocated their cognitive resources while performing a standing task [
29]. This could imply that DT presents a cognitive load that interferes with motor function response. Currently, there is a need for further study in coactivation and DT during GI, to provide clearer context to reasoning behind the associated mechanisms.
4.2 Steady-State Gait
In stance phase of SS gait all muscles involved during the DT exhibited lower man muscle activation (
Table 4). A past study comparing young and older adults has connected these populations by the gap in cognitive response to execute motor movement [
30]. Dual tasking diverts signals that are involved with performing motor function in the somatosensory system, to active thought, which is deemed to be demanding causing spatial interference [
30,
31]. In healthy young adults, BF, TA, and VL have been primary forward propelling muscles used in step-by-step, cyclic walking.
5. Conclusions
In conclusion, our findings suggest that DT leads to decreased muscle activity in both SS gait and GI. Muscles such as BF, VL and TA are required to generate smooth gait movements in SS gait. On the other hand, the TA, gastrocnemius and BF are required for stable posture during GI. Overall, there is a consensus in the field that additional research is essential to narrow the potential causes of falls in older adults. Some ideas considered were rehabilitation balance training exercises, investigating joint kinematics, and further examining neuromotor interference [
25,
28,
32].
Author Contributions
Conceptualization, G.K., K.W. and A.S.; methodology, K.W., A.S. and K.M.; software, K.W. A.S. and K.M.; validation, K.W., A.S. and K.M.; formal analysis, K.W. and K.M.; investigation, K.W., A.S. and K.M.; resources, G.K.; data curation, K.W., A.S. and K.M.; writing—original draft preparation, K.W.; writing—review and editing, K.W., A.S., K.M., L.G., D.W., G.K.; visualization, K.W. and K.M.; supervision, G.K.; project administration, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study received partial support from the Shirley Ryan AbilityLab C-STAR Collaborative Mentorship Funding; grant number P2C HD101899. However, the funding sources had no role in study design, data collection and analysis, and the presentation of the results.
Institutional Review Board Statement
This study was approved by The University of Texas at Dallas (UTD) IRB-22-569. This study was done in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The presented data is available upon request and approval of the corresponding author.
Acknowledgments
We thank Arianna Monteiro, Kaye Mabbun, Sandra Cuenca, Miguel Barcellano, Parker McCurdy, and Bisrat Assefa for their assistance in data collection and all participants that gave their time for this study. We also thank Aakriti Jaiswal, and Snehal Mazumder for their help in the analysis of data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yogev-Seligmann, G.; Hausdorff, J. M.; Giladi, N. The role of executive function and attention in gait. Movement disorders: official journal of the Movement Disorder Society 2008, 23, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Bayot, M.; Dujardin, K.; Tard, C.; Defebvre, L.; Bonnet, C. T.; Allart, E.; Delval, A. , The interaction between cognition and motor control: A theoretical framework for dual-task interference effects on posture, gait initiation, gait and turning. Neurophysiologie Clinique 2018, 48, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Kang, G. E.; Zhou, H.; Varghese, V.; Najafi, B. , Characteristics of the gait initiation phase in older adults with diabetic peripheral neuropathy compared to control older adults. Clinical Biomechanics 2020, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kang, G. E.; Yang, J.; Najafi, B. , Does the presence of cognitive impairment exacerbate the risk of falls in people with peripheral neuropathy? An application of body-worn inertial sensors to measure gait variability. Sensors 2020, 20, 1328. [Google Scholar] [CrossRef]
- Muir-Hunter, S.; Wittwer, J. , Dual-task testing to predict falls in community-dwelling older adults: a systematic review. Physiotherapy 2016, 102, 29–40. [Google Scholar] [CrossRef]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: a review of an emerging area of research. Gait & posture 2002, 16, 1–14. [Google Scholar]
- Smith, E.; Cusack, T.; Blake, C. The effect of a dual task on gait speed in community dwelling older adults: A systematic review and meta-analysis. Gait & posture 2016, 44, 250–258. [Google Scholar]
- Plummer, P.; Apple, S.; Dowd, C.; Keith, E. Texting and walking: Effect of environmental setting and task prioritization on dual-task interference in healthy young adults. Gait & posture 2015, 41, 46–51. [Google Scholar]
- Kelly, V. E.; Eusterbrock, A. J.; Shumway-Cook, A. A review of dual-task walking deficits in people with Parkinson's disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinson’s disease 2012, 2012. [Google Scholar] [CrossRef]
- Piche, E.; Gerus, P.; Chorin, F.; Jaafar, A.; Guerin, O.; Zory, R. The effect of different dual tasks conditions on gait kinematics and spatio-temporal walking parameters in older adults. Gait & Posture 2022, 95, 63–69. [Google Scholar]
- Ko, S.-u.; Jerome, G. J.; Simonsick, E. M.; Studenski, S.; Hausdorff, J. M.; Ferrucci, L. Differential associations between dual-task walking abilities and usual gait patterns in healthy older adults—Results from the Baltimore Longitudinal Study of Aging. Gait & posture 2018, 63, 63–67. [Google Scholar]
- Hallal, C. Z.; Marques, N. R.; Spinoso, D. H.; Vieira, E. R.; Gonçalves, M. , Electromyographic patterns of lower limb muscles during apprehensive gait in younger and older female adults. Journal of Electromyography and Kinesiology 2013, 23, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Castro, M. A.; Vilas-Boas, J. P. , Influence of cognitive and motor tasks using smartphone during gait: EMG and gait performance analysis–Dual-task study. Human Movement Science 2023, 89, 103097. [Google Scholar] [CrossRef] [PubMed]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.-L. , Balance control during gait initiation: State-of-the-art and research perspectives. World journal of orthopedics 2017, 8, 815. [Google Scholar] [CrossRef]
- Kang, G. E.; Gross, M. M. , Emotional influences on sit-to-walk in healthy young adults. Human movement science 2015, 40, 341–351. [Google Scholar] [CrossRef]
- Kang, G. E.; Gross, M. M. , The effect of emotion on movement smoothness during gait in healthy young adults. Journal of biomechanics 2016, 49, 4022–4027. [Google Scholar] [CrossRef]
- Kang, G. E.; Mickey, B. J.; Krembs, B. S.; McInnis, M. G.; Gross, M. M. , The effect of mood phases on balance control in bipolar disorder. Journal of Biomechanics 2019, 82, 266–270. [Google Scholar] [CrossRef]
- Nasreddine, Z. S.; Phillips, N. A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J. L.; Chertkow, H. , The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Craig, C. L.; Marshall, A. L.; Sjöström, M.; Bauman, A. E.; Booth, M. L.; Ainsworth, B. E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J. F. International physical activity questionnaire: 12-country reliability and validity. Medicine & science in sports & exercise 2003, 35, 1381–1395. [Google Scholar]
- Hermens, H. J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. , Development of recommendations for SEMG sensors and sensor placement procedures. Journal of electromyography and Kinesiology 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Heintz, S.; Gutierrez-Farewik, E. M. Static optimization of muscle forces during gait in comparison to EMG-to-force processing approach. Gait & posture 2007, 26, 279–288. [Google Scholar]
- Kang, G. E.; Mickey, B. J.; McInnis, M. G.; Krembs, B. S.; Gross, M. M. , Motor behavior characteristics in various phases of bipolar disorder revealed through biomechanical analysis: Quantitative measures of activity and energy variables during gait and sit-to-walk. Psychiatry research 2018, 269, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zeni Jr, J.; Richards, J.; Higginson, J. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait & posture 2008, 27, 710–714. [Google Scholar]
- Acuña, S. A.; Francis, C. A.; Franz, J. R.; Thelen, D. G. , The effects of cognitive load and optical flow on antagonist leg muscle coactivation during walking for young and older adults. Journal of Electromyography and Kinesiology 2019, 44, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, R.; Talebian, S.; Hadian, M. R.; Olyaei, G.; Bagheri, H. Characteristic muscle activity patterns during gait initiation in the healthy younger and older adults. Gait & posture 2016, 43, 148–153. [Google Scholar]
- Mickelborough, J.; Van Der Linden, M.; Tallis, R.; Ennos, A. Muscle activity during gait initiation in normal elderly people. Gait & posture 2004, 19, 50–57. [Google Scholar]
- Hass, C. J.; Gregor, R. J.; Waddell, D. E.; Oliver, A.; Smith, D. W.; Fleming, R. P.; Wolf, S. L. , The influence of Tai Chi training on the center of pressure trajectory during gait initiation in older adults. Archives of physical medicine and rehabilitation 2004, 85, 1593–1598. [Google Scholar] [CrossRef]
- Polcyn, A. F.; Lipsitz, L. A.; Kerrigan, D. C.; Collins, J. J. , Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Archives of physical medicine and rehabilitation 1998, 79, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Castro, M. A.; Vilas-Boas, J. P. Muscular and Prefrontal Cortex Activity during Dual-Task Performing in Young Adults. European Journal of Investigation in Health, Psychology and Education 2023, 13, 736–747. [Google Scholar] [CrossRef]
- Horata, E. T.; Kundakci, Y. E. Comparison of gait parameters under single-and dual-task conditions between children with specific learning disorder and typically developing children. Gait & Posture 2022, 98, 128–133. [Google Scholar]
- Abbud, G. d. A.; Li, K.; DeMont, R. Attentional requirements of walking according to the gait phase and onset of auditory stimuli. Gait & posture 2009, 30, 227–232. [Google Scholar]
- Henriksson, M.; Hirschfeld, H. Physically active older adults display alterations in gait initiation. Gait & posture 2005, 21, 289–296. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).