Introduction
Post-traumatic stress disorder (PTSD) may develop in select individuals after exposure to extreme traumatic events and has been associated with “hyperarousal” and increased sympathetic output. (1-6) It has been estimated that more than 7 million Americans are diagnosed with PTSD every year. (7) In addition to pharmacotherapy and psychological interventions, treatment for PTSD has included various methods of relaxation including yoga and deep breathing exercises. (2-5,8) Nevertheless, PTSD remains a major cause of quality-of-life impairment for patients and a significant societal burden in terms of medical costs (3-7). Peripheral somatosensory stimulation (PSS) therapy is a non-invasive technique which may be beneficial to patients with a variety of neurological disorders. (9-13) The current trial was undertaken to evaluate the potential benefit of such PSS therapy in patients with PTSD.
Methods
Study Description
NeuroGlove is a non-invasive device that provides PSS stimulation in the form of pneumatic puffs of air directed at the volar surface of the distal forearm, the palm, and the fingers. This study was designed as a prospective, single center trial enrolling six patients to explore the effect of PSS therapy on symptoms and quality-of-life measures in patients with PTSD. Men and women between the ages of 18 and 85 years with an active diagnosis of PTSD who were able to provide informed consent were considered eligible for trial enrollment. Patients who were unable to comprehend or follow instructions or unable to use the device due to physical limitations of the upper extremity including fracture, joint deformity, severe spasticity/contracture, or skin breakdown were excluded from participation.
Device Use
Subjects were instructed to use the device at home for 1 hour of therapy per day (30 minutes using each hand) for 4 weeks. Subjects were directed to synchronize their breathing to the firing (on/off cycle) of the machine to encourage relaxation during device use. At the conclusion of the trial, compliance was determined based on patient reporting and using an internal computerized system that allowed the investigators to track device use during the course of the trial.
Survey 1: Patient Satisfaction
The primary objective of Survey 1 (Table 1) was to evaluate overall patient satisfaction scores on a weekly basis during the trial. Each question was answered using the following ordinal scoring system: “Strongly Disagree” = 1, “Disagree” = 2, “Neutral” = 3, “Agree” = 4, and “Strongly Agree” = 5. Simple descriptive statistics were calculated for individual scores at each timepoint, including median and interquartile ranges (IQR). A composite score of all questions using the pooled median value of patient-specific survey questions was also generated and compared between patient visits. Additionally, for in-text summaries, scores were trichotomized as ≥4 (positive), 3 (neutral), and <2 (negative). For trichotomized scores, counts and percentage of total were calculated. Friedman’s test was used to determine if ordinal response scores significantly changed over time from week 1 to week 4. Box and whisker plots were generated in order to visually show overall and patient-specific results across timepoints.
Table 1.
Questions from Survey 1.
Table 1.
Questions from Survey 1.
| Question |
Description |
| Q1 |
My Memory is better |
| Q2 |
I’m more relaxed |
| Q3 |
My thoughts are more positive |
| Q4 |
It’s easier to concentrate |
| Q5 |
I’m less irritable |
| Q6 |
I feel better about myself |
| Q7 |
My thinking ability is better |
| Q8 |
I’m using less alcohol and/or drugs |
| Q9 |
I look forward to and enjoy using the device |
| Q10 |
My PTSD Symptoms are reduced |
| Q11 |
I would like to keep the device |
Survey 2: Severity of PTSD symptoms
The primary objective Survey 2 (Table 2) was to evaluate change from baseline severity of posttraumatic stress symptoms. Survey 2 was based on the National Stressful Events Survey PTSD Short Scale (NSESSS) and was administered at baseline and at week 4 after consistent use of the device. Each question was answered using the following ordinal scoring system: “Not At All” = 0, “A Little Bit” = 1, “Moderately” = 2, “Quite a Bit” = 3, and “Extremely” = 4.
Table 2.
Questions from Survey 2.
Table 2.
Questions from Survey 2.
| Question |
Description |
| Q1 |
Having "flashbacks", that is, you suddenly acted or felt as if a stressful experience from the past was happening all over again (for example, you re-experienced parts of a stressful experience by seeing, hearing, smelling, or physically feeling parts of the experience)? |
| Q2 |
Feeling very emotionally upset when something reminded you of a stressful experience? |
| Q3 |
Trying to avoid thoughts, feelings, or physical sensations that reminded you of a stressful experience? |
| Q4 |
Thinking that a stressful event happened because you or someone else (who didn't directly harm you) did something wrong or didn't do everything possible to prevent it, or because of something about you? |
| Q5 |
Having a very negative emotional state (for example, you were experiencing lots of fear, anger, guilt, shame, or horror) after a stressful experience? |
| Q6 |
Losing interest in activities you used to enjoy before having a stressful experience? |
| Q7 |
Being "super alert," on guard, or constantly on the lookout for danger? |
| Q8 |
Feeling jumpy or easily startled when you hear an unexpected noise? |
| Q9 |
Being extremely irritable or angry to the point where you yelled at other people, got into fights, or destroyed things? |
We assessed changes in ordinal response scores from patient-specific matched pairs using Wilcoxon’s signed-rank test to determine if there were significant improvements from baseline to week 4. A composite score of all questions using the pooled median value of patient-specific survey questions was also generated and compared between baseline and week 4. Effect sizes from Wilcoxon’s signed rank tests were reported as the median of differences alongside approximates of the 95% confidence interval. Since this nonparametric test works with ranks, it is typically not possible to derive a confidence interval with exactly 95% confidence; instead, the closest approximate was calculated, corresponding to true CIs calculated with 96.88% confidence; for simplicity these are reported as 95% CIs in text.
To formally analyze overall cumulative probability of improved scores across measurement times, we employed a Cumulative Link Mixed Model (CLMM) with a logit link function. The model was specified with the following formula using the clmm() function in the ‘ordinal’ package for R: Score ~ Visit + (1 | Subject), where, ‘Score’ represents individual ordinal-scale responses, ‘Visit’ is the predictor variable of interest (baseline or week 4), and ‘(1 | Subject)’ indicates the inclusion of random intercepts for individual subjects to account for within-subject variability. Laplace approximation was employed to estimate the model parameters due to its suitability for handling ordinal response data. Predicted probabilities and 95% CIs for each score at a given timepoint were extracted from the model. Overall effect sizes from the CLMM model are reported as cumulative odds ratios.
Simple descriptive statistics were also calculated, including median and IQRs. Additionally, for in-text summaries, scores were dichotomized as ≤1 (positive) or >1 (negative). Box and whisker plots were generated in order to visually show overall and patient-specific results across timepoints.
Software
All analyses were conducted in RStudio (2023.06.2 Build 561), running on R version 4.2.2. CLMM analyses were performed using the ‘ordinal’ package (version 2022.16). Figures were generated using the ‘ggplot2’ package (version 3.4.0). All data were analyzed by an independent statistician at the conclusion of the trial.
Results
Six patients with a formal diagnosis and active symptoms of PTSD were consented and enrolled in the trial. All patients were men, and all patients completed the trial. Compliance with device use was greater than 95% based on self-reporting and internal control checks at the conclusion of the trial. No patient reported an adverse event related to use of the device. All patients reported enjoying using the device and wished to keep the device at the conclusion of the trial. One patient was bothered by the noise level associated with device use, but othes found the background noise calming.
Survey 1
By week 4, all six patients (100%) had positive scores (scores 4 and 5) for feelings of relaxation (Q2), positive thoughts (Q3), enjoying using the device (Q9), and wanting to keep the device (Q11). Regarding feeling better about oneself (Q6) and reduction in PTSD symptoms (Q10), 5 out 6 patients (83%) showed positive scores. Regarding memory (Q1), concentration (Q4), and irritability (Q5), 4 patients (67%) had positive scores. Half of the patients had improved thinking ability (Q7). Patients exhibited the lowest scores regarding using less alcohol and/or drugs (Q8), with 2 out 6 (33%) having positive scores, and the remaining had neutral scores. Of note, no individual patient reported scores of 1 or 2 for any of the questions by week 4, suggesting that no patients disagreed with improved satisfaction scores, and either felt improved or at least neutral.
The pooled median score by week 4 was 4 (IQR: 4-4), and ranged from 3 to 5, suggesting a typically positive outcome and significantly improved overall score over time (p < 0.001). (Table 3). The question that had the largest upward trend from week 1 was regarding improved memory (Q1), showing a statistically significant improvement by week 4 (p < 0.001). Other individual questions did not demonstrate significant time-dependent improvements. Patients had the lowest upward trend regarding thinking ability (Q7) and use of drugs/alcohol (Q8), exhibiting slight, albeit not significant, reductions in score on week 4 compared to week 1.
Table 3.
Summary of Survey 1 results across timepoints.
Table 3.
Summary of Survey 1 results across timepoints.
| Survey Question |
Week 1
Median
(IQR) |
Week 2
Median
(IQR) |
Week 3
Median
(IQR) |
Week 4
Median
(IQR) |
P-value for time-dependent trend of improvement |
| Q1 |
3
(2 – 3) |
3
(2.75 – 3) |
3.5
(3 – 4) |
4
(3 – 4) |
0.007 |
| Q2 |
4
(3.5 – 4) |
4.5
(3.75 – 5) |
4
(4 – 4) |
4
(4 – 5) |
0.266 |
| Q3 |
3.5
(3 – 4) |
4
(4 – 4) |
4
(3 – 4) |
4
(4 – 4) |
0.125 |
| Q4 |
3
(3 – 4) |
3
(3 – 4) |
3
(3 – 4) |
4
(3 – 4.25) |
0.561 |
| Q5 |
3.5
(2.75 – 4) |
4
(3 – 4) |
3.5
(3 – 4) |
4
(3 – 4.25) |
0.438 |
| Q6 |
3
(3 – 4) |
3
(3 – 4) |
4
(3.75 – 4) |
4
(3.75 – 4.25) |
0.146 |
| Q7 |
4
(3 – 4) |
3
(3 – 4) |
3
(3 – 4) |
3.5
(3 – 4) |
0.719 |
| Q8 |
3.5
(3 – 4) |
3
(3 – 3.25) |
3
(3 – 4) |
3
(3 – 4) |
0.917 |
| Q9 |
4
(3.5 – 4.25) |
5
(3.5 – 5) |
4
(3.75 – 4.25) |
4
(4 – 5) |
0.305 |
| Q10 |
3
(2.75 – 4) |
4
(4 – 4) |
4
(3.75 – 4) |
4
(3.75 – 4) |
0.133 |
| Q11 |
4
(3.75 – 4) |
4
(4 – 5) |
4
(4 – 4.25) |
4
(4 – 5) |
0.333 |
| Overall* |
4
(3 – 4)
|
4
(3 – 4)
|
4
(3 – 4)
|
4
(4 – 4)
|
<0.001 |
Survey 2
By week 4, all six patients (100%) had positive scores (0 and 1) for having flashbacks (Q1) and negative emotional state (Q5). Regarding stressful events (Q4), losing interest in activities (Q6), being super alert (Q7), being easily startled (Q8), and being irritable or angry (Q9), 5 out 6 (83%) had positive scores, while 1 patient had moderate severity. No individual patient reported adverse scores of 3 or 4, suggesting no severe symptoms by week 4 (Table 4).
Table 4.
Summary of Survey 2 questions at baseline and at week 4.
Table 4.
Summary of Survey 2 questions at baseline and at week 4.
| Survey Question |
Baseline
Median (IQR) |
Week 4
Median (IQR) |
Median of differences
[95% CI] |
p-value |
| Q1 |
2
(0.75 – 2.25) |
0
(0 – 1) |
-1
[-3; 0] |
0.063 |
| Q2 |
3
(1.75 – 4) |
0
(0.75 – 2) |
-1.5
[-3; -1] |
0.031 |
| Q3 |
4
(1.75 – 4) |
1
(0.75 – 1.5) |
-2.5
[-3; 0] |
0.063 |
| Q4 |
2
(0 – 2.5) |
0
(0 – 1.25) |
-1
[-4; 1] |
0.250 |
| Q5 |
4
(2.5 – 4) |
0.25
(0 – 1) |
-3.25
[-4; 0] |
0.063 |
| Q6 |
3.5
(0.75 – 4) |
0.5
(0 – 1.25) |
-2
[-4; 0] |
0.063 |
| Q7 |
4
(3.75 – 4) |
1
(0.75 – 1.25) |
-3
[-3; -2] |
0.031 |
| Q8 |
4
(2.5 – 4) |
1
(0.75 – 1.25) |
-2.5
[-3; -1] |
0.031 |
| Q9 |
2.5
(0.75 – 3.25) |
0.5
(0 – 1.25) |
-2
[-2; 0] |
0.063 |
| Overall* |
3
(2 – 4)
|
1
(0 – 1)
|
-2
[-3; -2]
|
<0.001 |
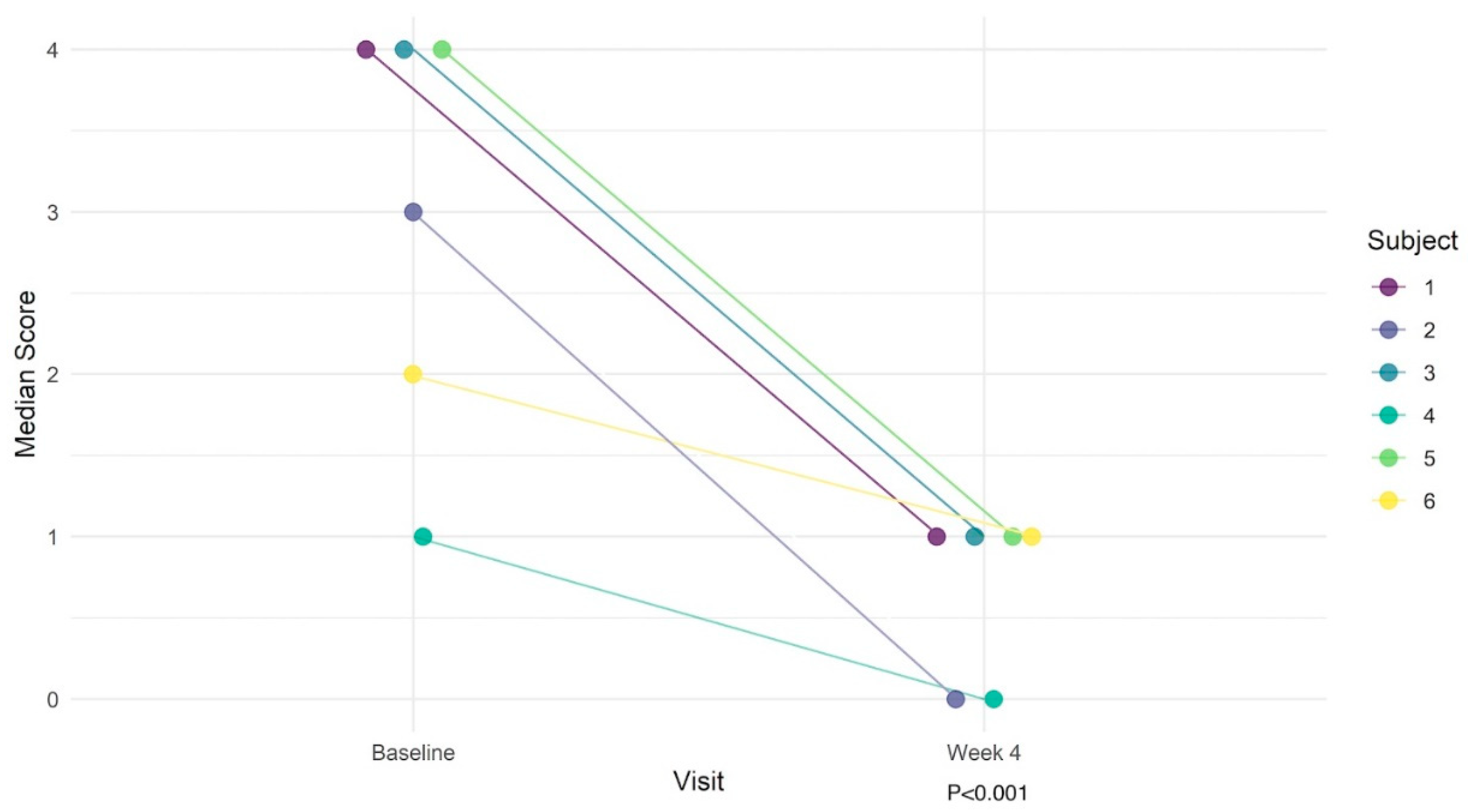
Of the individual survey questions, patients had statistically significant improvements regarding feelings of being emotionally upset (Q2: MD = -1.5, p = 0.031) and being overly alert (Q7: MD = -3, p = 0.031; Table 5). Although not statistically significant for all individual questions, all patients uniformly had numerically improved scores by week 4. The composite score was substantially improved from baseline, with an overall median score of 3 (IQR: 2 – 4) at baseline to 1 (IQR: 0 – 1) by week 4 (MD = -2 [95% CI: -3; -2], p < 0.001). Overall, the predicted probability of obtaining the best outcome (score=0) was 2% at baseline vs. 43% by week 4. Conversely, the predicted probability of obtaining the worst outcome (score=4) was 39% at baseline vs. 2% at week 4. The overall cumulative odds ratio was 38.2 (p < 0.001), suggesting that on average, the odds of moving from one score to a lower (improved) score at week 4 compared to the baseline are 38.2 times higher. The significant decrease in symptoms based on Survey 2 over time is illustrated in Figure 1.
Table 5.
Cumulative link mixed model results of overall improvement in PTSD symptoms.
Table 5.
Cumulative link mixed model results of overall improvement in PTSD symptoms.
| Survey Score |
Baseline Probability
[95% CI] |
Week 4 probability
[95% CI] |
Cumulative odds ratio
[95% CI] |
P-value |
| Score 0 |
2% [0 – 5%] |
43% [13 – 74%] |
38.2
[13.8 – 105.5] |
<0.001 |
| Score 1 |
13% [0 – 27%] |
43% [24 – 63%] |
| Score 2 |
28% [10 – 47%] |
10% [0 – 21%] |
| Score 3 |
18% [6 – 29%] |
2% [0 – 4%] |
| Score 4 |
39% [9 – 68%] |
2% [0 – 4%] |
Figure 1.
Illustration of changes in PTSD symptom severity in 6 patients over the course of the study.
Figure 1.
Illustration of changes in PTSD symptom severity in 6 patients over the course of the study.
Discussion
PTSD is a prevalent and potentially disabling condition that appears to represent a form of anxiety disorder associated with hypervigilance and increased sympathetic output. (1-7) Although the mainstay of treatment for PTSD was originally pharmacologic, an increasing emphasis has been placed on psychological interventions and various relaxation techniques such as yoga and deep breathing exercises. (3-8) Despite ongoing research into newer treatment options, PTSD remains a major cause of individual disability, quality-of-life impairment, and significant medical and societal costs (3-4,6).
A variety of physiological alterations within the brain have been associated with PTSD, and increasing evidence suggests that impaired sensory processing may play a critical role in the development and pathophysiology of this disorder. As early as 1972, Ayres suggested that sensory processing forms a significant basis for an individual’s physiological state (14). Haricharichan et al postulated that alterations in the neural pathways important for processing sensory input have a cascading effect on the ability to perform higher cognitive functions implying that abnormal sensory processing may be contributory and associated with PTSD (15). In addition, it has been shown that individuals with PTSD have decreased prefrontal cortex activation resulting in impaired sensory integration and emotional regulation (2,16-18). Engel-Yeger et al identified reproducible patterns of sensory hypersensitivity in patients suffering from PTSD (19). Based upon these findings, we hypothesized that impaired sensory perception and processing, which may play a role in PTSD, might also represent a potential treatment avenue using PSS therapy in this patient population.
It has been shown that PSS significantly improves neurological outcomes following ischemic injury in rodent models of stroke (20-22). Potential suggested mechanisms for this benefit include improved regional cerebral perfusion through the recruitment of local collateral blood supply and/or delayed neuronal reorganization allowing for better neural “learning” and functional recovery (10). Preliminary clinical experience in stroke patients has suggested that such peripheral sensory stimulation can improve recovery and rehabilitation in stroke survivors (9-10,23-33). Interestingly, similar benefits have been demonstrated in patients with Parkinson’s disease, and PSS has also shown promise following traumatic brain injury and in inflammatory, auto-immune conditions such as multiple sclerosis (34-39).
In this study, we encountered a significant early response to PSS treatment as evidenced by the improvement in symptoms just one week after initiating therapy. This benefit appeared to be sustained and to further increase over the course of the study. Interestingly, multiple patients reported that when they were challenged by a stress-inducing event, they used the device to help them achieve a calmer state and mitigate their symptoms.
Our survey results suggest promising preliminary evidence of improved PTSD scores and high user satisfaction after 4 weeks of PSS therapy. Regarding Survey 1, patients seemed most improved regarding feelings of relaxation, positive thoughts, enjoying using the device, and wanting to keep the device. The pooled median scores on Survey 1 demonstrated significant and time-dependent overall improvement. No patients demonstrated negative survey responses by the week 4 survey. Regarding Survey 2, patients demonstrated unanimous improvement by week 4, with composite scores substantially improved from baseline. By week 4, all patients agreed that they enjoyed the treatments and wanted to keep their device.
Limitations
The main limitation of our study is the small sample size evaluated in this trial. Data are also limited to self-reported survey questions and may, therefore, fail to capture other clinically important outcomes. In addition, the exact mechanism by which patients in this study benefitted from device use is unclear. This is a small trial with a small sample size representing a preliminary investigation of the potential benefit of PSS in patients with PTSD.
Conclusions
We describe the results of a clinical trial evaluating the impact of one month of treatment with PSS on symptoms in patients with a diagnosis of PTSD. All patients completed the trial, and all appeared to benefit from the therapy. The improvement in symptomatology was apparent at one week of device use and was sustained and typically increased through the course of the trial. A significant reduction in symptoms was achieved in all patients when comparing baseline (pre-trial) and 4-week (post-trial) assessment using the NSESSS Scale. We suggest that further investigation into the potential use of PSS in the treatment of patients with PTSD is warranted.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Western (WCG) Institutional Review Board (protocol code: 20233103, date of approval: 7/19/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Disclosure
Dr. Eric Nussbaum is a shareholder in NeuroGlove, LLC
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-R. 4. Washington DC: 2000. Revised.
- Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, Hobfoll SE, Koenen KC, Neylan TC, Hyman SE. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015 Oct 08;1:15057.
- Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. 2011;13(3):263-78. [PMC free article] [PubMed]. [CrossRef]
- Bryant RA, Friedman MJ, Spiegel D, Ursano R, Strain J. A review of acute stress disorder in DSM-5. Depress Anxiety. 2011 Sep;28(9):802-17.
- Qi W, Gevonden M, Shalev A. Prevention of Post-Traumatic Stress Disorder After Trauma: Current Evidence and Future Directions. Curr Psychiatry Rep. 2016 Feb;18(2):20. [CrossRef]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007 Dec;69(9):935–943. [CrossRef]
- National Institute of Mental Health. [March 6, 2011];The Numbers Count: Mental Disorders in America.
- Descilo T, Vedamurtachar A, Gerbarg PL, et al. Effects of a yoga breath intervention alone and in combination with an exposure therapy for post-traumatic stress disorder and depression in survivors of the 2004 South-East Asia tsunami. Acta Psychiatr Scand. 2010 Apr;121(4):289–300. [CrossRef]
- Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Archives of Physical Medicine and Rehabilitation, 2007;88(11), 1369–1376. [CrossRef]
- Nussbaum ES, Janjua TM, Lowary J, Defillo A, Myers M, Nussbaum LA: Peripheral sensory stimulation of the hand in the treatment of stroke. A preliminary study of safety and effectiveness. J Neurol Disord 11(1):2023, in press.
- Nussbaum HA, Nussbaum ES: Peripheral Sensory Stimulation for Neurological Disorders. A Novel, Non-invasive Therapeutic Option. Review Article. J Neurosci and Neurol Surgery 13:267-271, 2023. [CrossRef]
- Kristina JP, Kromer JA, Cook AJ, Hornbeck T, Lim EA, Mortimer BJ, Fogarty A, Han SS, Dhall R, Halpern C, Tass PA.: Coordinated reset vibrotactile stimulation induces sustained cumuluative benefits in parkinson’s disease. Front Physiol 2021; 12: 624317.
- Lucente G, Valls-Sole J, Murillo N, Rothwell J, Coll J, Davalos A, Kumru H: Noninvasive Brain Stimulation and Noninvasive Peripheral Stimulation for Neglect Syndrome Following Acquired Brain Injury. Neuromodulation: Technology at the Neural Interface 23(3)2020, 312-323, . [CrossRef]
- Ayres AJ: Treatment of sensory integrative dysfunction A J Occ Ther 1440, 1972. [CrossRef]
- Harricharan S, McKinnon MC, Lanius RA: How processing sensory information from te internal and external worlds shape the perception and engagement with the world in the aftermath of trauma: Implications for PTSD. Front Neurosci 15:2021. [CrossRef]
- Etkin A, Wager TD: Functional neuroimaging of anxiety. A metanalysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatr 164:1476-88, 2007. [CrossRef]
- Nicholson AA, Friston KJ, Zeidman P, Harricharan S, McKinnon MC, Densmore M, et al: Dynamic causal modeling in PTSD and its dissociative subtype: bottom-up vs top-down processing within fear and emotional regulation circuitry. Human Brain Mapp 38:5551-61, 2017. [CrossRef]
- Shin LM, Rauch SL, Pittman RK: Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann NY Acadd Sci 1071:67-79, 2006. [CrossRef]
- Engel-Yager B, Palgy-Levin D, Lev-Wiesel R: The sensory profile of people with PTSD. Occup Ther Ment Health 29:266-78, 2013. [CrossRef]
- Lay CC, Jacobs N, Hancock AM, Zhou Y, Frostig RD. Early stimulation treatment provides complete sensory-induced protection from ischemic stroke under isoflurane anesthesia. The European Journal of Neuroscience. 2013;38: 2445-52. PMID 23586641 . [CrossRef]
- Frostig RD, Lay CC, Davis MF: A rat’s whiskers point the way toward a novel stimulus-dependent protective stroke therapy. The Neuroscientist, 2012. [CrossRef]
- Hancock AM, Lay CC, Davis MF, Frostig RD. Sensory Stimulation-Based Complete Protection from Ischemic Stroke Remains Stable at 4 Months Post-Occlusion of MCA. Journal of Neurological Disorders 2013; 1: 135. PMID 24634892. [CrossRef]
- Conforto AB, Cohen LG, Dos Santos RL, Scaff M, Marie SKN. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. Journal of Neurology, 2007;254(3), 333–339. [CrossRef]
- Conforto AB, dos Anjos SM, Bernardo WM, Silva AA, d Conti J, Machado AG, Cohen LG. Repetitive peripheral sensory stimulation and upper limb performance in stroke: A systematic review and meta-analysis. Neurorehabilitation and Neural Repair 2018; 32(10), 863–871. [CrossRef]
- Conrad MO, Scheidt RA, Schmidt BD. Effects of wrist tendon vibration on targeted upper-arm movements in poststroke hemiparesis. Neurorehab Neurol Repair 2011; 25:61-70. [CrossRef]
- Maeda M, Mutai H, Toya Y, Maekawa Y, Hitai T, Katai S. Effects of peripheral nerve stimulation on paralysed upper limb functional recovery in chronic stroke patients undergoing low-frequency repetitive transcranial magnetic stimulation and occupational therapy: A pilot study. HK J Occ Ther 2020;33:31-11. https://pubmed.ncbi.nlm.nih.gov/33815018/. [CrossRef]
- Nasrallah FA, Mohamed AZ, Hong KY, Hwa SL, Yeow C, Lim JH. Effect of proprioceptive stimulation using a soft robotic glove on motor activation and brain connectivity in stroke survivors. J Neural Eng 2021;18:1. [CrossRef]
- Seo NJ, Enders LR, Fortune A, Cain S, Vatinno AA, Schuster E, Ramakrishnan V, Feng W. Phase I safety trial: Extended daily peripheral sensory stimulation using a wrist-worn vibrator in stroke survivors. Translational Stroke Research, 2019;11(2), 204–213. [CrossRef]
- Seo NJ, Woodbury ML, Bonilha L, Ramakrishnan V, Kautz SA, Downey RJ, Dellenbach BHS, Lauer AW, Roark CM, Landers LE, Phillips SK, Vatinno AA. Therabracelet stimulation during task-practice therapy to improve upper extremity function after stroke: A pilot randomized controlled study. Physical Therapy,2019; 99(3), 319–328. [CrossRef]
- Vatinno AA, Hall L, Cox H, et al: Using subthreshold vibratory stimulation during poststroke rehabilitation therapy. A case series. OTJR 2021; 42: . [CrossRef]
- Carrico C, Chelette KC, Westgate PM, Salmon-Powell E, Nichols L, Sawaki L. A randomized trial of peripheral nerve stimulation to enhance modified constraint-induced therapy after stroke. Am J Phys Med Rehabil 2016;95:397-406. [CrossRef]
- Carrico C, Chelette KC, Westgate PM, Salmon-Powell E, Nichols L, Fleischer A, Sawaki L. Nerve stimulation enhances task-oriented training in chronic, severe motor deficit after stroke: A randomized trial. Stroke 2016; 47, 1879–1884. [CrossRef]
- Serrada I, Hordacre B, Hillier SL. Does Sensory Retraining Improve Sensation and Sensorimotor Function Following Stroke: A Systematic Review and Meta-analysis. Front Neurosci 2019; 13:402-15. https://pubmed.ncbi.nlm.nih.gov/31114472/. [CrossRef]
- K.J. Pfeifer, A.J. Cook, J.K. Yankulova, B.J.P. Mortimer, E. Erickson-DiRenzo, R. Dhall, L. Montaser-Kouhsari, P.A. Tass: Clinical efficacy and dosing of vibrotactile coordinated reset stimulation in motor and non-motor symptoms of Parkinson’s disease: a study protocol. Frontiers in Neurology 12:758481 (2021).
- Tass PA: Vibrotactile Coordinated Reset Stimulation for the Treatment of Parkinson’s Disease. Neural Regeneration Research. 17(7):1495-1497 (2021).
- Tramontano M, Morone G, De Angelis S, Conti L, Galeoto G, Grasso MG. Sensor-based technology for upper limb rehabilitation in patients with multiple sclerosis: A randomized controlled trial. Restor Neurol Neurosci 2020;38:333-41. https://pubmed.ncbi.nlm.nih.gov/32925119/. [CrossRef]
- Hall KD, Lifschitz J. Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Res 2010; 1323:161-173. [CrossRef]
- Lew HL, Weihing J, Myers PJ, Pogoda TK, Goodrich GL. Dual sensory impairment (DSI) in traumatic brain injury (TBI)--An emerging interdisciplinary challenge. Neurorehabilitation 2010; 26:213-222. [CrossRef]
- Zheng Z, Dong X, Li Y, Gao W, Jiang R, Yue S, Zhou Z, Zhang J: Electrical stimulation improved cognitive deficits associated with traumatic brain injury in rats. Brain and Behavior 2017; 7:e00667. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).