Submitted:
21 September 2023
Posted:
22 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Product
2.2. Evaluation of Antioxidant Activity
2.2.1. DPPH Radical Scavenging Activity
2.2.2. ABTS Radical Scavenging Assay
2.3. Caenorhabditis Elegans Strains
2.3.1. Reproduction Assay
2.3.2. Pharyngeal Pumping Rate
2.3.3. Growth Alteration Assay
2.3.4. Locomotion Analysis Assay
2.3.5. Lifespan Assessment
2.4. Statistical Analysis
3. Results
3.1. Radical Scavenging Assessment
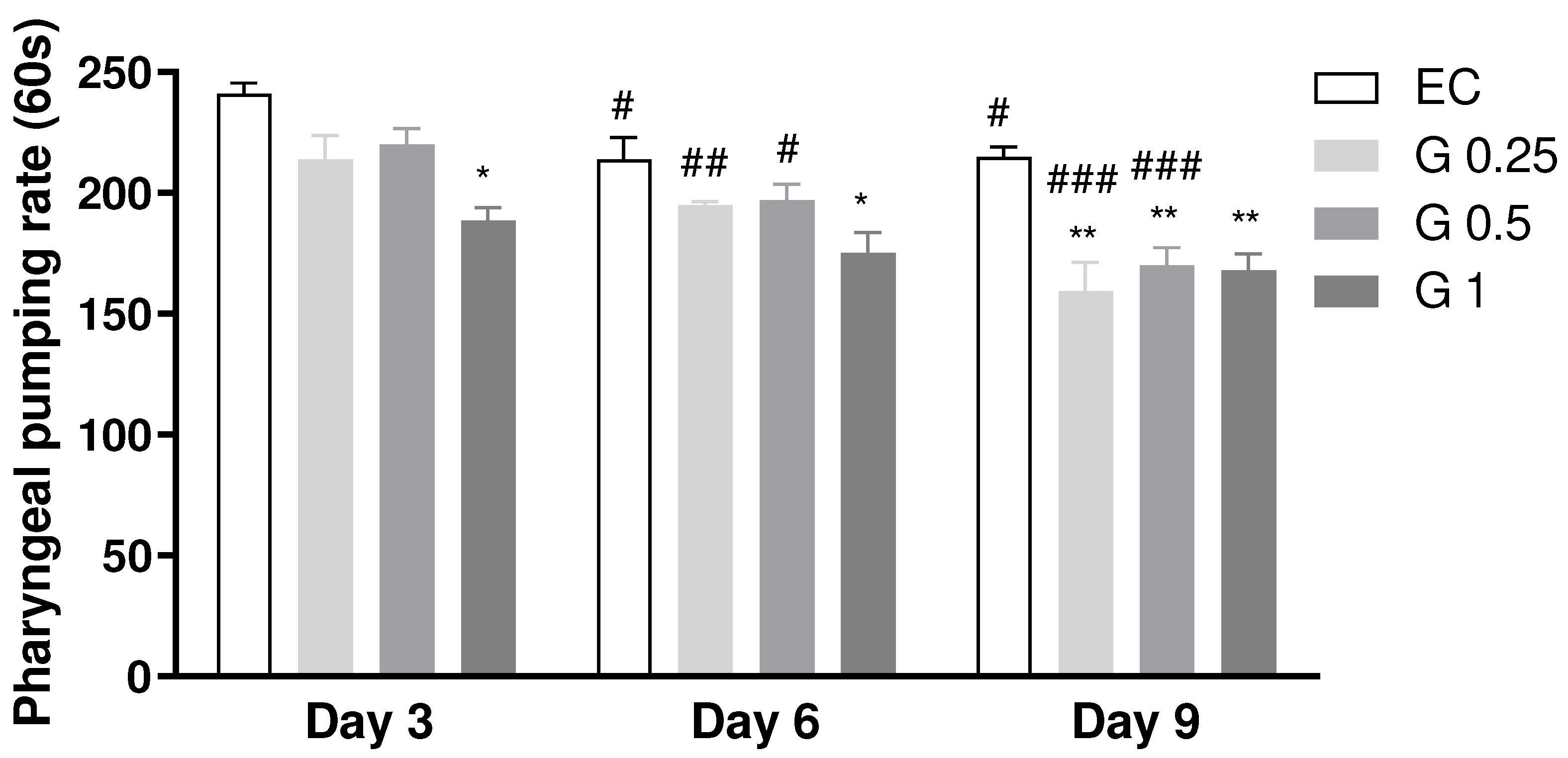
3.2. Assessment of Pharyngeal Pumping Rate
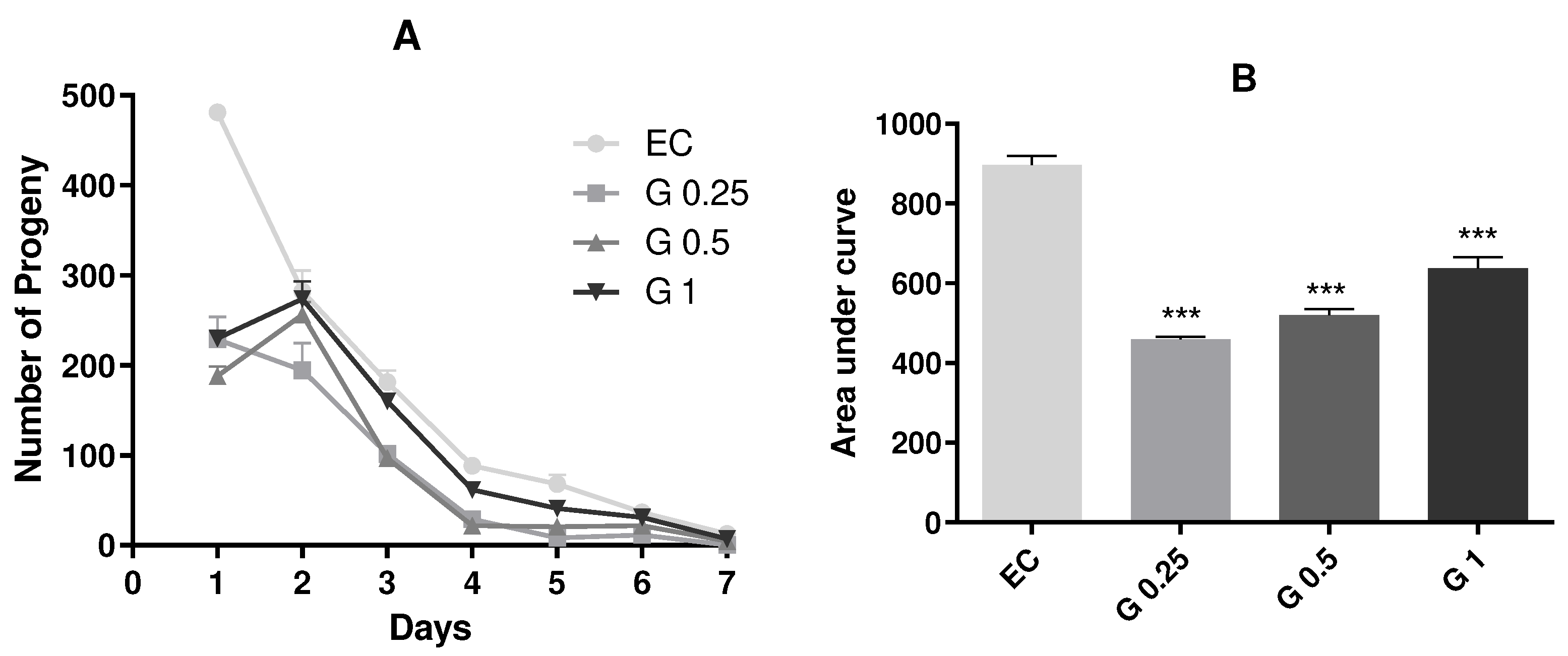
3.3. Reproduction Assessment
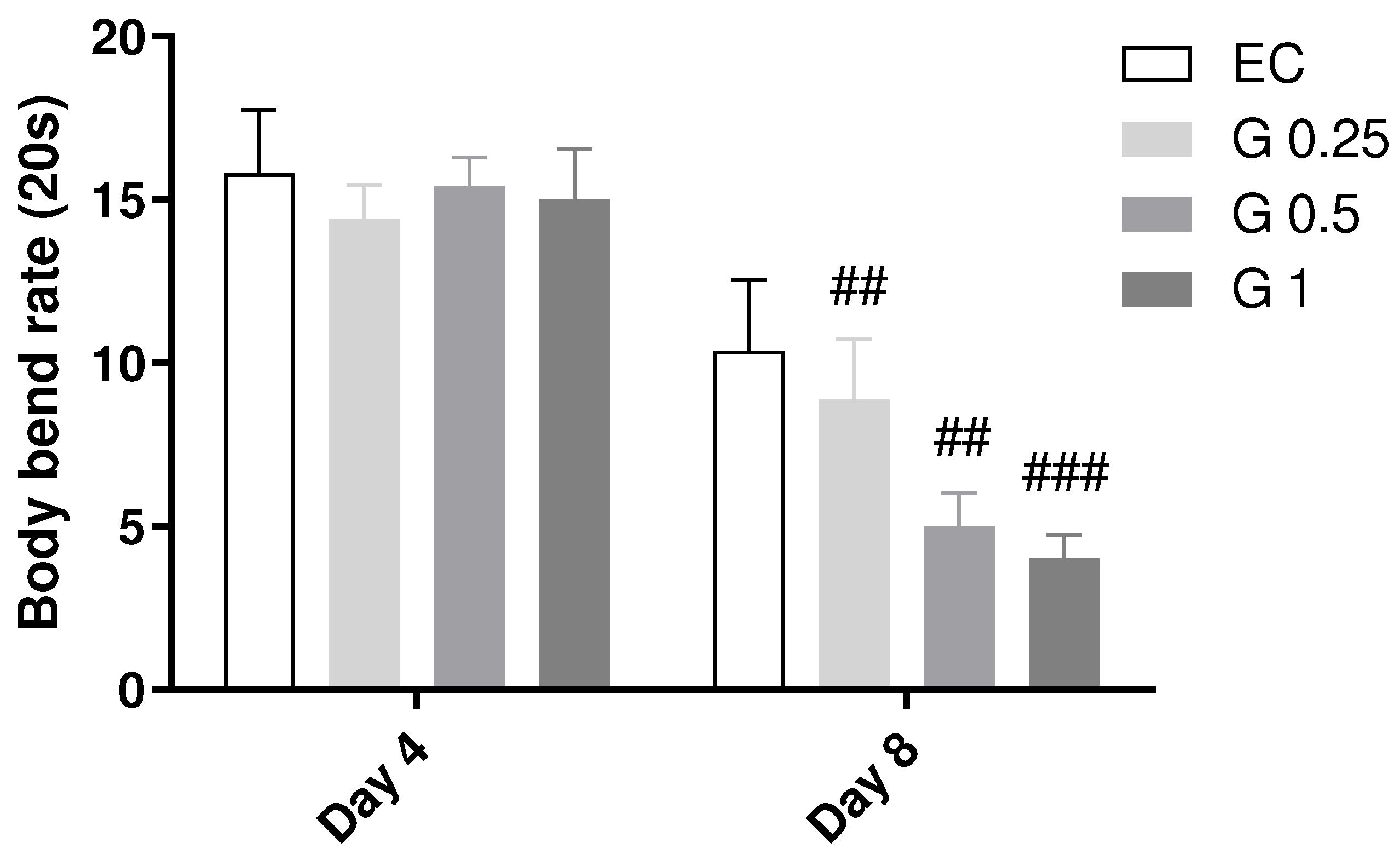
3.4. Locomotion Assessment
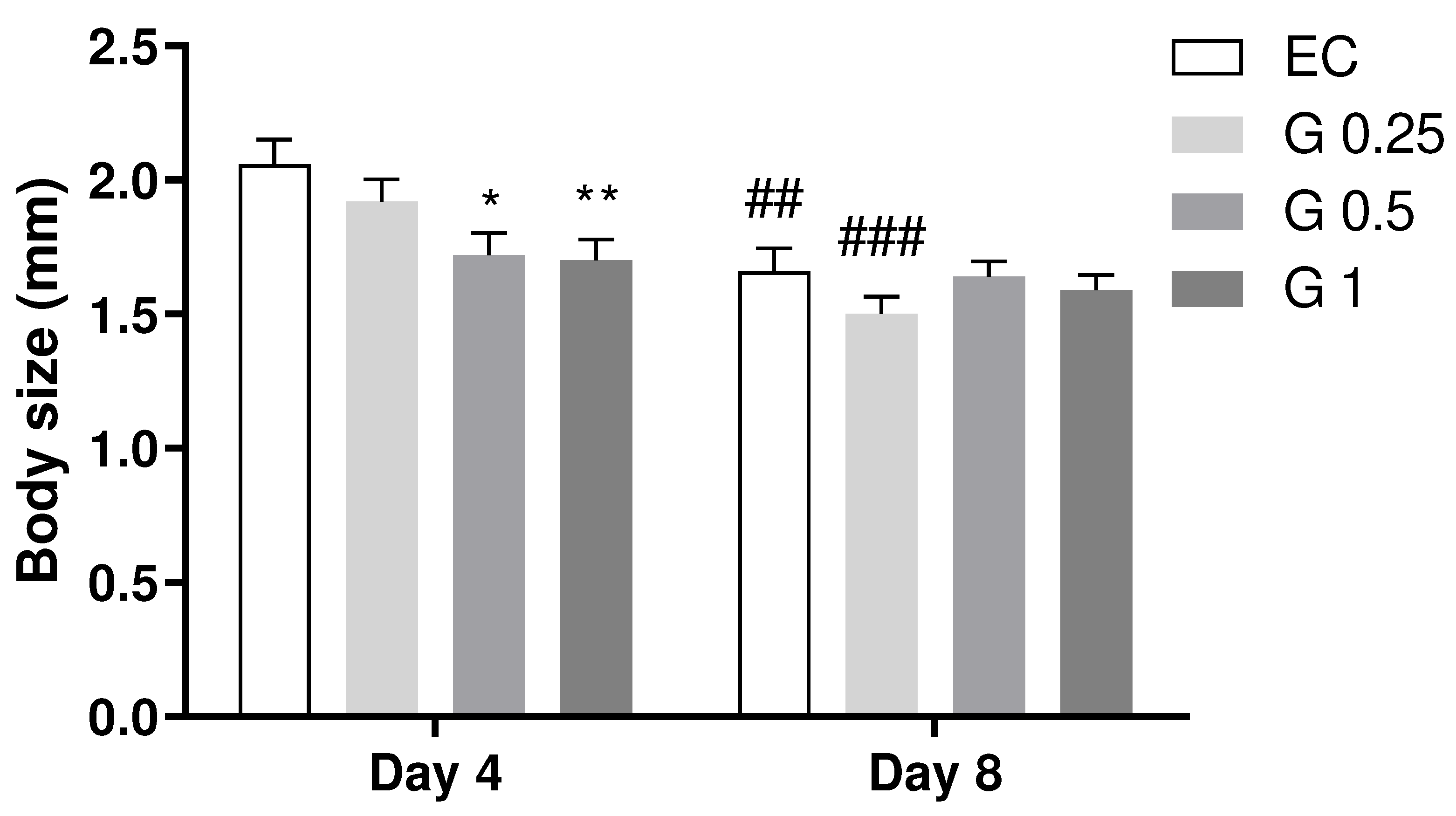
3.5. Size Evaluation
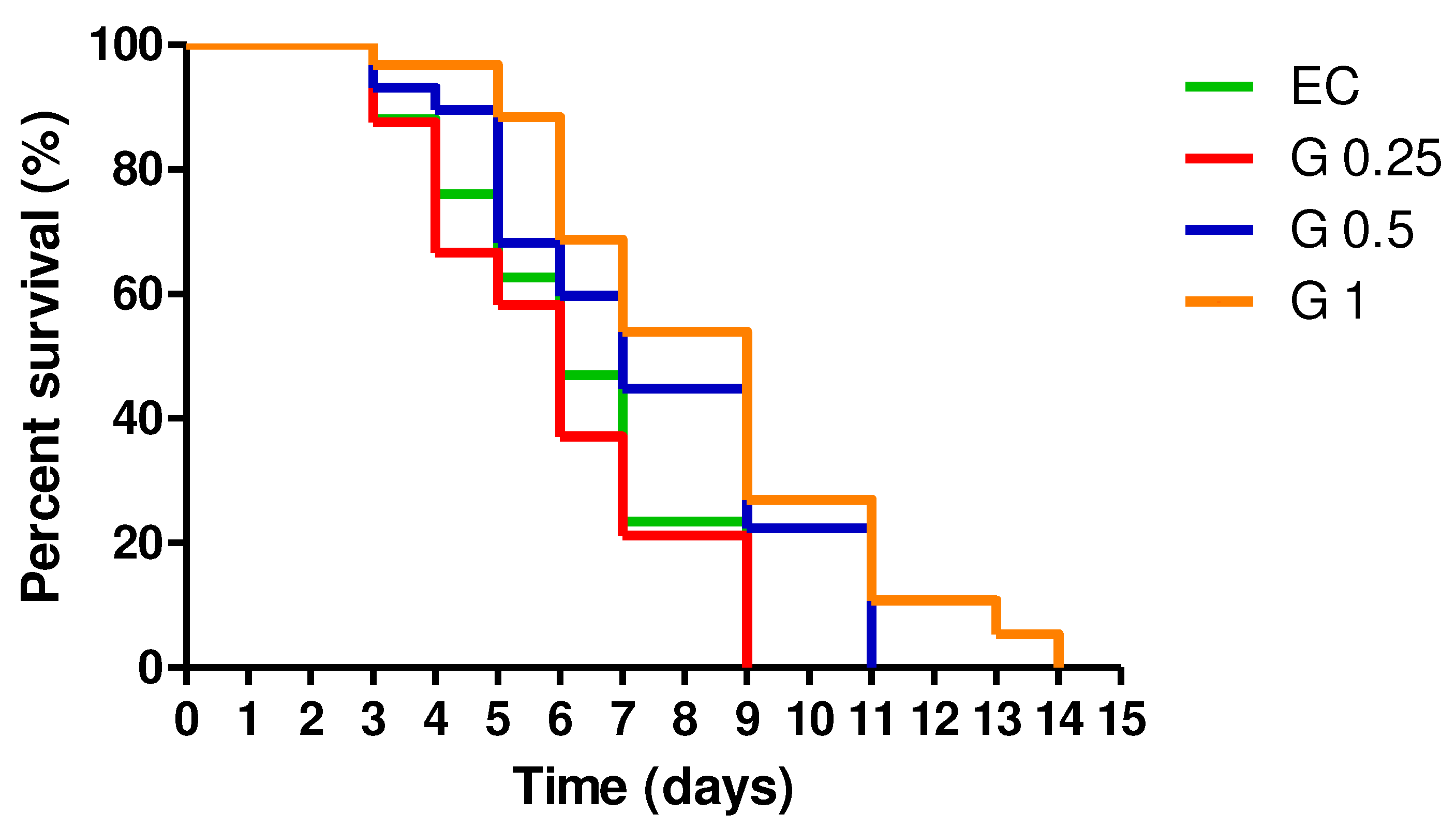
3.6. Lifespan Evaluation
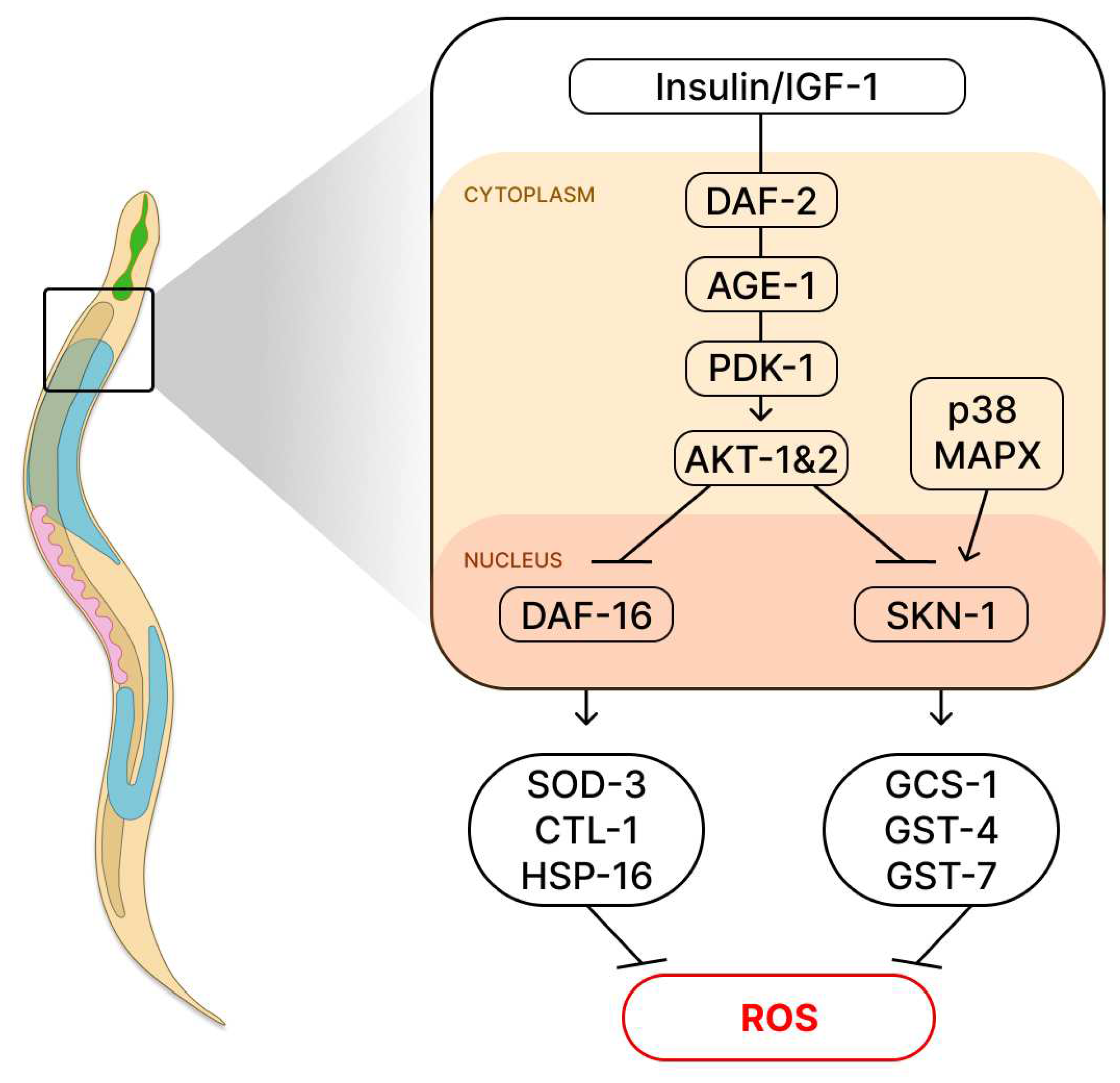
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Briga, M.; Verhulst, S. What Can Long-Lived Mutants Tell Us about Mechanisms Causing Aging and Lifespan Variation in Natural Environments? Exp Gerontol 2015, 71, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Padwad, Y. Perspectives of the Potential Implications of Polyphenols in Influencing the Interrelationship between Oxi-Inflammatory Stress, Cellular Senescence and Immunosenescence during Aging. Trends Food Sci Technol 2020, 98, 41–52. [Google Scholar] [CrossRef]
- de Freitas Rodrigues, C.; Ramos Boldori, J.; Valandro Soares, M.; Somacal, S.; Emanuelli, T.; Izaguirry, A.; Weber Santos Cibin, F.; Rossini Augusti, P.; Casagrande Denardin, C. Goji Berry (Lycium Barbarum L.) Juice Reduces Lifespan and Premature Aging of Caenorhabditis Elegans: Is It Safe to Consume It? Food Research International 2021, 144, 110297. [Google Scholar] [CrossRef]
- WHO, W.H.O. Ageing.
- Trendelenburg, A.U.; Scheuren, A.C.; Potter, P.; Müller, R.; Bellantuono, I. Geroprotectors: A Role in the Treatment of Frailty. Mech Ageing Dev 2019, 180, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.E.; Lee, S.-J. v. Recent Progresses on Anti-Aging Compounds and Their Targets in Caenorhabditis Elegans. Transl Med Aging 2019, 3, 121–124. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant. Songklanakarin Journal of Science and Technology (SJST) 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic Biol Med 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Li, H.; Roxo, M.; Cheng, X.; Zhang, S.; Cheng, H.; Wink, M. Pro-Oxidant and Lifespan Extension Effects of Caffeine and Related Methylxanthines in Caenorhabditis Elegans. Food Chem X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, X.; Zhang, J.; Li, M.; Qi, Y.; Zhou, L. Calycosin Promotes Lifespan in Caenorhabditis Elegans through Insulin Signaling Pathway via Daf-16, Age-1 and Daf-2. J Biosci Bioeng 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Liao, V.H.-C.; Yu, C.-W.; Chu, Y.-J.; Li, W.-H.; Hsieh, Y.-C.; Wang, T.-T. Curcumin-Mediated Lifespan Extension in Caenorhabditis Elegans. Mech Ageing Dev 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Huang, C.-W.; Liao, W.-R.; How, C.M.; Yen, P.-L.; Wei, C.-C. Chronic Exposure of Zearalenone Inhibits Antioxidant Defense and Results in Aging-Related Defects Associated with DAF-16/FOXO in Caenorhabditis Elegans. Environmental Pollution 2021, 285, 117233. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Anthocyanins: Structural Characteristics That Result in Unique Metabolic Patterns and Biological Activities. Free Radic Res 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Yue, Y.; Li, S.; Shen, P.; Park, Y. Caenorhabditis Elegans as a Model for Obesity Research. Curr Res Food Sci 2021, 4, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, P.; Wang, P.; Zheng, S.; Qu, Z.; Liu, N. The Review of Anti-Aging Mechanism of Polyphenols on Caenorhabditis Elegans. Front Bioeng Biotechnol 2021, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, G.A.; Ashrafi, K. Investigating Connections between Metabolism, Longevity, and Behavior in Caenorhabditis Elegans. Trends in Endocrinology and Metabolism 2016, 27, 586–596. [Google Scholar] [CrossRef]
- Lee, S.A.; Lim, W.H.; Van Le, V.; Ko, S.R.; Kim, B.; Oh, H.M.; Ahn, C.Y. Lifespan Extension and Anti-Oxidant Effects of Carotenoid Pigments in Caenorhabditis Elegans. Bioresour Technol Rep 2022, 17. [Google Scholar] [CrossRef]
- Huang, C.; Xiong, C.; Kornfeld, K. Measurements of Age-Related Changes of Physiological Processes That Predict Lifespan of Caenorhabditis Elegans. Proc Natl Acad Sci U S A 2004, 101, 8084–8089. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Zhang, G.; Chen, X.; Wang, X. Itaconate Prolongs the Healthy Lifespan by Activating UPRmt in Caenorhabditis Elegans. Eur J Pharmacol 2022, 923, 174951. [Google Scholar] [CrossRef]
- Chow, D.K.; Glenn, C.F.; Johnston, J.L.; Goldberg, I.G.; Wolkow, C.A. Sarcopenia in the Caenorhabditis Elegans Pharynx Correlates with Muscle Contraction Rate over Lifespan. Exp Gerontol 2006, 41, 252–260. [Google Scholar] [CrossRef]
- Evason, K.; Collins, J.J.; Huang, C.; Hughes, S.; Kornfeld, K. Valproic Acid Extends Caenorhabditis Elegans Lifespan. Aging Cell 2008, 7, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Lakowski, B.; Hekimi, S. The Genetics of Caloric Restriction in Caenorhabditis Elegans. Proc Natl Acad Sci U S A 1998, 95, 13091–13096. [Google Scholar] [CrossRef] [PubMed]
- Liao, V.H.C.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-Mediated Lifespan Extension in Caenorhabditis Elegans. Mech Ageing Dev 2011, 132, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.R.; Hansen, M. Lessons from C. Elegans: Signaling Pathways for Longevity. Trends in Endocrinology & Metabolism 2012, 23, 637–644. [Google Scholar] [CrossRef]
- Vellai, T.; Takacs-Vellai, K.; Zhang, Y.; Kovacs, A.L.; Orosz, L.; Müller, F. Influence of TOR Kinase on Lifespan in C. Elegans. Nature 2003, 426, 620–620. [Google Scholar] [CrossRef]
- Cao, L.; Lee, S.G.; Park, S.-H.; Kim, H.-R. Sargahydroquinoic Acid (SHQA) Suppresses Cellular Senescence through Akt/MTOR Signaling Pathway. Exp Gerontol 2021, 151, 111406. [Google Scholar] [CrossRef]
- Wilkinson, D.S.; Taylor, R.C.; Dillin, A. Analysis of Aging in Caenorhabditis Elegans. In; 2012; pp. 353–381.
- Li, X.; Wang, X.; Wang, K.; Yang, X.; Liu, X.; Chen, J.; Li, J.; Wang, J.; Guo, Q.; Wang, H. Black Rice Anthocyanin Extract Enhances the Antioxidant Capacity in PC12 Cells and Improves the Lifespan by Activating IIS Pathway in Caenorhabditis Elegans. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2023, 265, 109533. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, Y.; Wang, C.; Zhou, W.; Shu, Y.; Zhang, K.; Zeng, X.; Guo, R. Lonicera Japonica Polysaccharides Improve Longevity and Fitness of Caenorhabditis Elegans by Activating DAF-16. Int J Biol Macromol 2023, 229, 81–91. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, B.; Li, Z.; Li, C.; Li, J. Zhuyeqing Liquor Promotes Longevity through Enhancing Stress Resistance via Regulation of SKN-1 and HSF-1 Transcription Factors in Caenorhabditis Elegans. Exp Gerontol 2023, 174, 112131. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, L.; Zhou, L. Oleanolic Acid Activates Daf-16 to Increase Lifespan in Caenorhabditis Elegans. Biochem Biophys Res Commun 2015, 468, 843–849. [Google Scholar] [CrossRef]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry Extract Ameliorates Oxidative Stress in Caenorhabditis Elegans via the SKN-1/Nrf2 Pathway. J Funct Foods 2020, 70, 103977. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Shan, S.; Wu, A.; Zhao, C.; Ye, X.; Zheng, X.; Zhu, R. Cyanidin-3-O-Glucoside Promotes Stress Tolerance and Lifespan Extension of Caenorhabditis Elegans Exposed to Polystyrene via DAF-16 Pathway. Mech Ageing Dev 2022, 207, 111723. [Google Scholar] [CrossRef] [PubMed]
- van de Klashorst, D.; van den Elzen, A.; Weeteling, J.; Roberts, M.; Desai, T.; Bottoms, L.; Hughes, S. Montmorency Tart Cherry (Prunus Cerasus L.) Acts as a Calorie Restriction Mimetic That Increases Intestinal Fat and Lifespan in Caenorhabditis Elegans. J Funct Foods 2020, 68, 103890. [Google Scholar] [CrossRef]
- Wang, S.; Xue, J.; Zhang, S.; Zheng, S.; Xue, Y.; Xu, D.; Zhang, X. Composition of Peony Petal Fatty Acids and Flavonoids and Their Effect on Caenorhabditis Elegans Lifespan. Plant Physiology and Biochemistry 2020, 155, 1–12. [Google Scholar] [CrossRef]
- Tambara, A.L.; de Los Santos Moraes, L.; Dal Forno, A.H.; Boldori, J.R.; Gonçalves Soares, A.T.; de Freitas Rodrigues, C.; Mariutti, L.R.B.; Mercadante, A.Z.; de Ávila, D.S.; Denardin, C.C. Purple Pitanga Fruit (Eugenia Uniflora L.) Protects against Oxidative Stress and Increase the Lifespan in Caenorhabditis Elegans via the DAF-16/FOXO Pathway. Food and Chemical Toxicology 2018, 120, 639–650. [Google Scholar] [CrossRef]
- Peixoto, H.; Roxo, M.; Krstin, S.; Röhrig, T.; Richling, E.; Wink, M. An Anthocyanin-Rich Extract of Acai (Euterpe Precatoria Mart.) Increases Stress Resistance and Retards Aging-Related Markers in Caenorhabditis Elegans. J Agric Food Chem 2016, 64, 1283–1290. [Google Scholar] [CrossRef]
- Sun, X.; Seeberger, J.; Alberico, T.; Wang, C.; Wheeler, C.T.; Schauss, A.G.; Zou, S. Açai Palm Fruit (Euterpe Oleracea Mart.) Pulp Improves Survival of Flies on a High Fat Diet. Exp Gerontol 2010, 45, 243–251. [Google Scholar] [CrossRef]
- Kampkötter, A.; Timpel, C.; Zurawski, R.F.; Ruhl, S.; Chovolou, Y.; Proksch, P.; Wätjen, W. Increase of Stress Resistance and Lifespan of Caenorhabditis Elegans by Quercetin. Comp Biochem Physiol B Biochem Mol Biol 2008, 149, 314–323. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Gómez-Orte, E.; Cabello, J.; Santos-Buelga, C. Deglycosylation Is a Key Step in Biotransformation and Lifespan Effects of Quercetin-3-O-Glucoside in Caenorhabditis Elegans. Pharmacol Res 2013, 76, 41–48. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Thirumurugan, K. Longevity Promoting Efficacies of Different Plant Extracts in Lower Model Organisms. Mech Ageing Dev 2018, 171, 47–57. [Google Scholar] [CrossRef]
- Martorell, P.; Forment, J.V.; de Llanos, R.; Montón, F.; Llopis, S.; González, N.; Genovés, S.; Cienfuegos, E.; Monzó, H.; Ramón, D. Use of Saccharomyces Cerevisiae and Caenorhabditis Elegans as Model Organisms To Study the Effect of Cocoa Polyphenols in the Resistance to Oxidative Stress. J Agric Food Chem 2011, 59, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- van de Klashorst, D.; van den Elzen, A.; Weeteling, J.; Roberts, M.; Desai, T.; Bottoms, L.; Hughes, S. Montmorency Tart Cherry (Prunus Cerasus L.) Acts as a Calorie Restriction Mimetic That Increases Intestinal Fat and Lifespan in Caenorhabditis Elegans. J Funct Foods 2020, 68, 103890. [Google Scholar] [CrossRef]
- Lashmanova, E.; Proshkina, E.; Zhikrivetskaya, S.; Shevchenko, O.; Marusich, E.; Leonov, S.; Melerzanov, A.; Zhavoronkov, A.; Moskalev, A. Fucoxanthin Increases Lifespan of Drosophila Melanogaster and Caenorhabditis Elegans. Pharmacol Res 2015, 100, 228–241. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Carotenoids from Mamey (Pouteria Sapota) and Carrot (Daucus Carota) Increase the Oxidative Stress Resistance of Caenorhabditis Elegans. Biochem Biophys Rep 2021, 26, 100989. [Google Scholar] [CrossRef] [PubMed]
- Aan, G.; Zainudin, M.; Karim, N.; Ngah, W. Effect of the Tocotrienol-Rich Fraction on the Lifespan and Oxidative Biomarkers in Caenorhabditis Elegans under Oxidative Stress. Clinics 2013, 68, 599–604. [Google Scholar] [CrossRef]
- Gómez-Linton, D.R.; Alavez, S.; Navarro-Ocaña, A.; Román-Guerrero, A.; Pinzón-López, L.; Pérez-Flores, L.J. Achiote (Bixa Orellana) Lipophilic Extract, Bixin, and δ-Tocotrienol Effects on Lifespan and Stress Resistance in Caenorhabditis Elegans. Planta Med 2021, 87, 368–374. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




