1. Introduction
Local vibration (LV) is a powerful tool for stimulating numerous receptors (e.g., cutaneous receptors, Golgi tendon organ, muscle spindles) by passively stretching the entire vibrated musculotendinous system [
1,
2,
3,
4]. When applied at high frequency (range between 80 and 100 Hz) and low amplitude (<0.5 mm) to a tendon or relaxed muscle, it mainly stimulates primary afferences (Ia) [
2,
5,
6]. The latter projected onto motor neurons via a monosynaptic reflex circuit, as well as a more complex poly-synaptic circuit, which can result in a tonic vibratory reflex contraction (TVR) [
7,
8,
9].
Prolonged exposure (20 – 30 minutes) to LV at rest to the plantar flexor [
10,
11,
12] or knee extensor muscles [
13,
14,
15,
16,
17,
18] in most cases leads to a loss of strength (for a review, refer [
24]) even though several studies have failed to demonstrate this effect [
20,
21,
22,
23]. The primary cause of this fatigue could be an intramuscular (peripheral) alteration beyond the neuromuscular junction [
24,
25]. However, this is still debatable as in many cases, markers of peripheral fatigue (e.g. the maximal M-wave response, the mechanical twitch obtained by peripheral electrical stimulation or the characteristics of this twitch) were not altered by the prolonged LV protocols [
10,
11,
20,
21,
23]. The second cause could be central (neural) alteration upstream of the neuromuscular junction. The magnitude of the exercise induced central fatigue is commonly evaluated by the level of voluntary activation through the use of interpolation twitch technique (ITT), electromyographic activity during maximal contraction, and early (less than the first 100ms from the onset of muscle contraction) rate of force development evaluations [
26,
27,
28]. The ITT technique with peripheral nerve stimulation [
10,
21] or corticospinal stimulation [
14,
23] was used in only 4 studies, and the results were never influenced by the applied LV protocol, regardless of strength loss. It seems that ITT, either with peripheral nerve stimulation [
10,
21] or corticospinal stimulation, is not affected after the application of different LV protocols, even if they lead to significant reduction of maximum strength . However, a decrease in electromyographic activity [
10,
11,
12,
15,
16,
17] or a reduction in the rate of torque development (RTD) [
15,
16,
29] were observed as soon as a loss of strength was observed.
Concomitant with the observed reduction of maximum strength after prolonger LV applications, an inactivation of α-motor units from the Ia afferents nerves is also observed, as it assessed through the H-reflex evaluation[
11,
20,
30]. This reduction in the H-reflex has long been attributed to presynaptic causes and has been suspected to be involved in the observed reduction of muscle strength [
3,
31,
32,
33]. However, several studies have recently highlighted a decrease in post-synaptic excitability (i.e., motor neuron excitability) following an acute LV protocol. This is reflected in a reduction of the thoracic motor evoked potential amplitude (TMEP) [
14,
29,
34], a motor unit type-dependent change in persistent inward current (i.e., increase for lower-threshold, decrease for higher-threshold motor units) [
35] and a reductions in motor unit discharge frequency [
13]. Note that a decrease in post LV motor neuron excitability appears to be accompanied by increased cortical excitability, estimated by the ratio of corticospinal track excitability (measured by transcutaneous magnetic stimulation) to motor neuron excitability [
14,
29]. Nevertheless, this increase in cortical excitability does not appear to be sufficient to counteract the negative effects of LV on maximal force production. As a result, the origin of this loss of strength would come from a potential increase in motor neuron recruitment thresholds and a decrease in firing rates, particularly of fast motor units, resulting in a decrease in the activation of the y-loop.
The majority of the results presented above were obtained for prolonged LV protocols (>20 minutes). However, according to our knowledge, there is a lack of data regarding the possible effect of short-termed LV protocols on maximal force production capacity. After a thrity second LV protocol, a reduction of the H reflex was observed in the soleus muscle [
30]. Furthermore, only after an application of an LV protocol lasted for over six minutes the excitability of flexor carpi radialis motor neurons (assessed with cervicomedullary motor evoked potential) was reduced [
36]. However, it is still unknow if these observations will be also true after short-termed LV protocol application in other major large locomotive muscles, like quadriceps. Thus, the aim of this study was to identify the shortest duration that would induce a reduction in the knee extensor muscles’ force and to confirm the central origin of such losses. We hypothesized that a minimum duration of 6 minutes would be necessary to induce a significant reduction of force.
2. Materials and Methods
2.1. Experimental Design
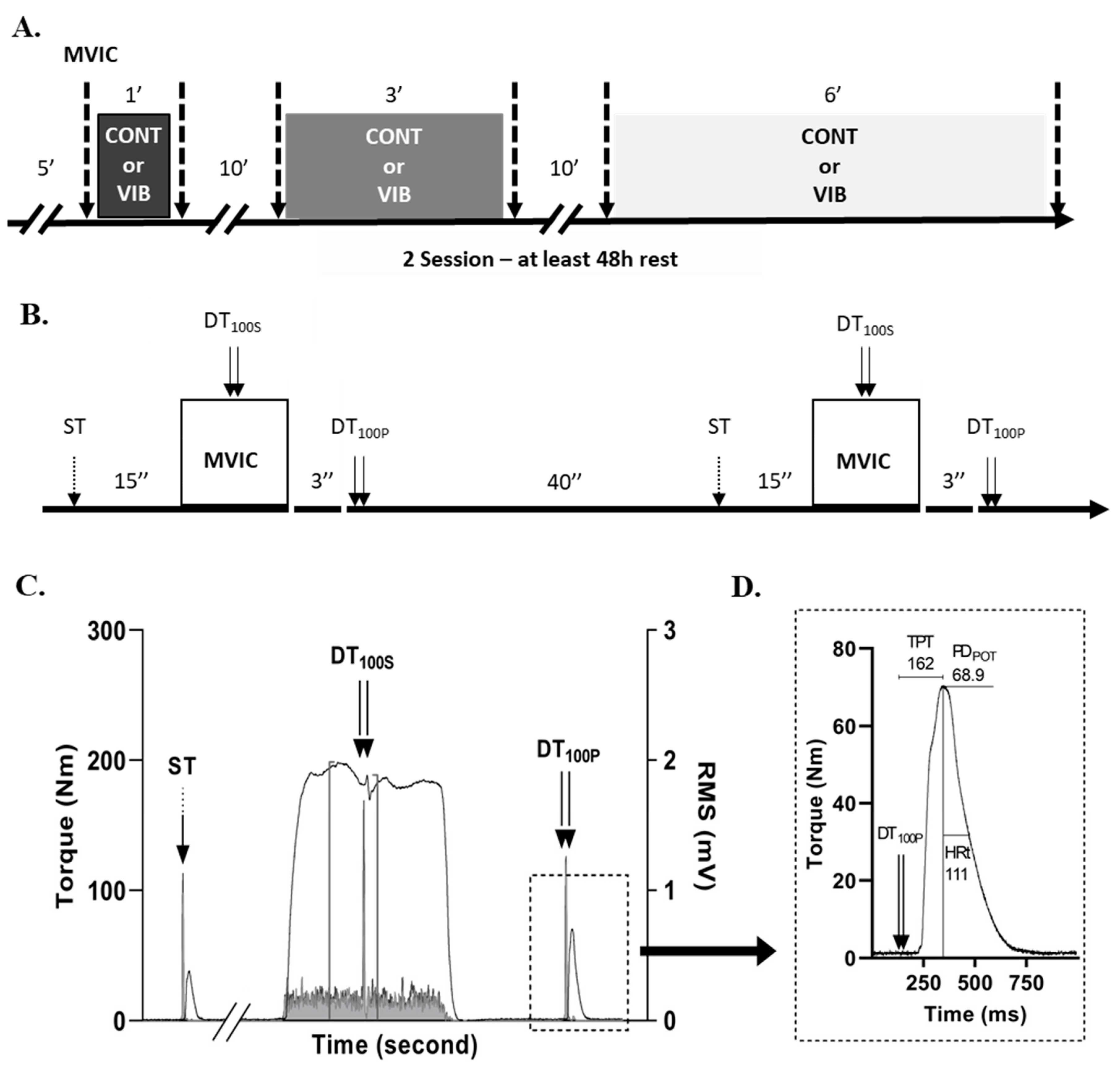
All participants performed two experimental sessions with at least 48 hours rest interval between them. Each experimental session started with a standardized warm-up. Then, participants performed maximal voluntary isometric contractions (MVIC) to measure quadriceps maximal strength before (PRE) and after (POST) 1, 3 and 6 minutes of local vibration (LV) or rest (CONT). The two conditions (i.e., LV or CONT) were performed in a random counterbalanced order between the two experiment sessions (
Figure 1A). To ensure restoration of the neuromuscular system in the event of experimental condition effects and to avoid the production of MVIC-related fatigue [
13,
37,
38], duration 1 and 3 minutes as well as duration 3 and 6 minutes were separated by 10 minutes of rest (
Figure 1A). LV was delivered on the quadricipital tendon at 100 Hz and 0.5mm displacement (Vibramoov, TechnoConcept). The electromyographic activity of the vastus lateralis (VL) and the rectus femoris (RF) were measured during the three durations in both CONT and LV condition. Voluntary activation level (VAL) was assessed with the interpolation twitch technique. In order to assess peripheral fatigue, the M wave associated with electrical single twitch (ST) stimulation and the parameters of the potentiated twitch post MVIC were analyzed.
2.2. Participants
Fourteen healthy participants were included in the experiment (age: 23 ± 1.3 years old, body weight: 73.5 ± 11.6 kg, height: 178.2 ± 9,5 cm). All participants were familiarized with knee isometric extension and free from neurological disease and musculoskeletal injury. The research was in accordance with the Declaration of Helsinki and approved by the local university ethics committee (CERUBFC-2021-11-23-041). Participants were informed of the research and signed a written consent before entering the research procedure.
2.3. Maximum Isometric Torque and Rate of Torque Development
Participants were placed on an isokinetic dynamometer (Biodex System 4, Biodex Medical Systems inc., Shirley, USA). The right leg was strapped to the rotation axis, and the position was noted down for the second session, as references: Knee angle was set at 70° (0°= full knee extension), hip flexion 110°. Movement of the upper body was limited with the help of a strap across the trunk and the waist.
MVIC was evaluated through 2 maximum efforts before and after each trial/block, with 1 min rest between them. Each contraction last for 4s with a superimposed electrical doublet (100 Hz) and a potentiated doublet about 3 seconds after the contraction [
39]. Moreover, a single electrical stimulation was delivered 5 seconds before each contraction to allow normalization of EMG signals a posteriori (
Figure 1B). Strong verbal encouragements were delivered throughout the effort. Furthermore, MVIC was assessed at least 30 seconds after the end of the experimental condition. Thus, last electrical stimulation was timed 2’30’’ after the end of the condition. Maximum torque was determined as the highest peak on the curve. The onset of contraction was determined as the time when the participant was reaching 2% of maximum torque, and the rate of torque development (RTD) was the level of torque (T
50) developed 50 ms after the onset of contraction. We haven't reported the RTD from 0 to 50ms because the value is numerically equivalent to the torque value at a specific time from 0 [
28]. All the data were recorded in the same file as the EMG data, through an analog-to-digital conversion system (MP150; Biopac Systems Inc., Goleta, CA, USA) at a sampling frequency of 2000 Hz, with a 30 Hz low-pass filter. In each time point, the T
50 was the mean performance of the two trials [
28]. In contrast, the other parameters described below were measured only on the trial that produced the maximum torque and were further used for statistical analysis.
2.4. Electrical Nerve Stimulation and M-Wave Amplitude:
M-waves were investigated for the VL and RF muscles. Electrical nerve stimulation was assessed on the femoral nerve, in the femoral triangle, with a stylus as anode and a 5x10cm anode patch. Electrical stimulation (Pulses duration: 1ms – Maximal output: 400V) is delivered by a constant current stimulator (stimulator (DS7A, Digitimer, Hertfordshire, UK). Firstly, the optimal position was found when, at a given intensity, the M-waves were the greatest. Then, the increment to establish the maximal M-waves stimulation intensity was assessed by increasing 10mA at a frequency of 0.2 Hz[
40,
41]. Afterward, 125% of the latter intensity was used as supramaximal stimulation during the session and was used to deliver: single twitch (ST), superimposed electrical doublet (DT
100S), and potentiated electrical doublet at 100 Hz (DT
100P). The peak-to-peak (PP) amplitudes (M wave) of both muscles were measured during ST application before the MVIC for each trial/block.
2.5. Electromyographic Activity
EMG activity of the right leg VL and RF was measured during MVIC with Ag/AgCl electrodes (diameter: 7mm, interelectrode distance: 20mm). Reference electrodes were set on the right patella. Electrodes were positioned on shaved and cleaned skin, and the exact location was noted for the second session. At rest, the root-mean-square (RMS) signal averaged over a 5-second window was monitored to be less than 3.5µV for each muscle.
EMG activity signal was measured during each contraction and experimental condition (i.e., 1, 3 and 6-minutes of LV or CONT) with a 2000 Hz sampling frequency using an analog-to-digital conversion system (MP150; Biopac Systems Inc., Goleta, CA, USA). The signal was amplified (gain = 500) and filtered (10-400 Hz). A bandpass filter (90-110 Hz) was applied to the raw EMG activity recorded during the LV or CONT condition. Data were stored and analyzed with a commercially available software (Acqknowledge MP150). From the raw EMG data, RMS was calculated to quantify the activity of the quadriceps using the following formula:
Computing of RMS during MVIC was realized on 25ms window (n = 50 points) with an overlap of 49 points. The same procedure was used to compute the RMS signal during the experimental conditions (i.e., LV or CONT) for each muscle with a time window of 60 seconds (n = 120.000 points) and no overlap. RMSMVIC refers to the average RMS signal recorded from 100ms to 0ms before peak force (window shifted if too close to DT100S) and RMSCOND refers to the average RMS signal recorded over 1, 3 or 6 minutes. Thus, the RMS were then normalized based on the peak-to-peak values from the M wave evaluation (RMSMVIC.M-1 and RMSCOND.M-1 ratios) measured before the MVIC for both muscles.
2.6. Interpolated Twitch Technic
The interpolated twitch technic (ITT) was used to calculate the voluntary activation level (VAL) during the MVIC. For ITT, doublet stimulation at 100 Hz was superimposed during the MVIC (DT
100S), 2s after the beginning of the onset of muscle contraction, and a second doublet potentiated at 100 Hz (DT
100P) was delivered at rest, approximatively 3 seconds after MVIC ends (see below for a method of electrical nerve stimulation,
Figure 1B). The superimposed peak doublet (PD
SUP) and the potentiated peak doublet (PD
POT) corresponded to the maximum torque signal recorded from stimulation to 250ms after stimulation. As DT
100S could not be superimposed every time at the maximal torque moment (T
MAX), Strojnik and Komi (1998) correction was used by incorporating the torque produced at the moment of stimulation (T
STIM) into the calculation method [
42]:
2.7. Potentiated Doublet Stimulation
In our experiment, peripheral fatigue markers were measured with DT
100P of 10ms width. Peak torque (PD
POT), time to peak torque (TPT) and half-relaxation time (HRt) were calculated from that electrical stimulation. PD
POT represents the peak torque recorded from the stimulation to 250ms after stimulation. TPT represents the time between the potentiated electrical doublet (DT
100P) delivery and reaching peak force. At last, HRt represents the time between the peak force of the twitch and a force level equal to half that peak (
Figure 1C and 1D). Sarcolemmal excitability was also estimated with peak-to-peak (PP) amplitude measurement of the single twitch delivered before the MVIC [
43].
2.8. Statistical Analysis
Raw data. The normal distribution of data was tested using the Shapiro-Wilk test on raw or log-transformed data. The transformation concerns raw data for the variables RMSCOND.M-1 for RF and VL muscle HRt and RMSMVIC.M-1 for RF and VL muscle. Repeated measures ANOVAs with three within-subject factors [Condition (CONT, LV) x Duration (1, 3, 6) x Time (PRE, POST)] on raw data for all variables were performed (i.e., MVIC, VAL, RMSMVIC.M-1 for RF and VL muscle, PDPOT, HRt, TPT, T50, PP RF and PP VL). Also, repeated measures ANOVAs with two within-subject factors [Condition (CONT, LV) x Duration (1, 3, 6)] were conducted on the RMSCOND.M-1 of the RF and VL muscle. For each ANOVA, the hypothesis of sphericity was verified using Mauchly's test, and the Greenhouse-Gasser correction was applied if the hypothesis was violated. Each significant main effect or interaction in repeated measures ANOVAs were followed by a post-hoc analysis using the HSD Tukey’s test.
Relative data. POST values were also expressed as a percentage of PRE to normalize the amplitude of changes between participants. The following formula was used for each duration (i.e., 1, 3, or 6 minutes):
The normality of these data was also checked using the Shapiro-wilk test. The variables T50, VAL, and RMSMVIC.M-1 of each muscle were transformed. Repeated measures ANOVAs with two within-subject factors [Condition (CONT, LV) x Duration (1, 3, 6)] were conducted on these variables. The treatment of the sphericity assumption and the post-hoc analysis for significant main effect or interaction were the same as for the raw data analysis. Despite the transformation, the MVIC, PDPOT, HRt, and TPT did not follow normal distribution, and Friedman ANOVAs were performed with one factor [Time (POST 1CONT, POST 3CONT, POST 6CONT, POST 1LV, POST 3LV, POST 6LV)]. Lastly, the grouped change observed in each condition irrespective of duration (i.e., POSTLV vs. POSTCONT) was computed. Normality was checked, and the two conditions were compared using Student's t tests for paired samples on log transform data (MVIC, VAL, RMSMVIC.M-1 for RF and VL muscle, T50, PP VL) or using Wilcoxon tests (PDPOT, TPT, HRt, PP RF). The amount of change was compared with the initial pre-treatment state using a one-sample student test or a one-sample Wilcoxon signed-rank test, depending on respect or non-respect of normality.
Statistica software (Statsoft, version 12, Tulsa, OK, United States) was used to perform statistical analyses. Significance was set at
p <.05. Partial Eta squared (ηp²) was used as a measure of effect size (ES) for the factors in each ANOVA. Small, medium, or large effects were considered for ηp² ≥.01, ≥.06, and ≥.14 respectively. G*Power (version 3.1.9.2, Universität Düsseldorf, Germany) was used to compute the Cohen’s
d ES for all significant pairwise comparison.
d ≥ .20, ≥ .50, and ≥ .80 represented small, medium, and large effect, respectively. The ES of the Friedman ANOVAs corresponded to the Kendall’s W coefficients and the ES for Wilcoxon matched pairs tests were computed as the Z-score divided by the square root of the pair number. Kendall's W coefficient and Z score interpretation was shared. Small, moderate, and large ES corresponded to values ≥.10, ≥.30, and ≥.50 respectively [
44].
3. Results
3.1. MVIC and Central Fatigue Markers
Raw data for MVIC, VAL, RMS
MVIC.M
-1 of both muscles, and T
50 are presented in
Table 1. Concerning the MVIC data, there was a significant
Condition ×
Time interaction (
F (1, 13) = 4.922,
p = .045, ηp² = .275). The MVIC level in PRE was higher in the CONT than in the LV condition (218.52 ± 78.63 N.m vs. 212.08 ± 76.03 N.m,
p = .032, ES = .419). However, no
Condition ×
Duration interaction (
p = .192), nor
Condition ×
Duration ×
Time interaction (
p = .379) were found. There was no significant interaction for the VAL (
p > .113), the RMS
MVIC.M
-1 of the RF muscle (
p > .611), the RMS
MVIC.M
-1 of the VL muscle (
p > .533), and the T
50 (
p > .147).
The same variables expressed as a change (cf. equation above) are presented in
Table 2. The
Time effect measured using Friedman ANOVA was not significant for the MVIC (
p = .113). Repeated measures ANOVA showed no significant effect of
Condition or
Condition ×
Duration interaction for VAL (
p = .125 and
p = .824), RMS
MVIC.M
-1 of the RF (
p = .845 and
p = .611), RMS
MVIC.M
-1 of the VL (
p = .533 and
p = .695) or T
50 (
p = .702 and
p = .673). Concerning theses central fatigue markers, no pairwise comparisons of grouped data were significant (
p > .108). Only for VAL in the CONT condition (-2.16 ± 2.59%) was significantly lower than the initial level (
p = .006, ES = -.878). The other markers did not differ from the initial level (
p >.087).
3.2. Peripheral Fatigue Markers
Raw data for PD
POT, TPT, HRt, and PP of each muscle are presented in
Table 1. There was no significant interaction for the PD
POT (
p > .147), PP of the RF (
p > .299), or PP of the VL muscle (
p > .399). Concerning the TPT data, there was a significant
Condition ×
Duration interaction (
F (1.418, 18.444) = 6.088,
p = .015, ηp² = .319). The TPT for the 6-minute duration was overall higher than the TPT for the 3-minute duration in the LV condition (138.96 ± 14.90 ms vs. 149.27 ± 20.81 ms,
p = .042, ES = .721). Other interactions were not significant (
p > .256). Concerning the HRt data, there was a significant
Condition ×
Duration interaction (
F (2. 26) = 4.322,
p = .024, ηp² = .250). However, post-hoc analysis showed no significant differences between the pairs. Other interactions were not significant (
p > .224).
The same variables expressed as a percentage change (cf. equation above) are presented in
Table 2. No
Time effect (Friedman ANOVAs) was observed for PD
POT (
p = .813). TPT (
p = .616), HRt (
p = .332), RF muscle PP (
p = .348) or that of the VL (
p = .316). Concerning theses peripheral fatigue markers, no significant pairwise comparisons were observed (
p > .300). No marker showed a significant change from the initial level (
p >.074).
3.3. Electromygraphic Activity during LV
Concerning both muscles, there was no effect of Condition (p > .102) or Duration (p > .142) nor Condition × Duration interaction (p > .336) for the RMSCOND.M-1.
Table 3.
Electromyographic activity (root mean square) of the rectus femoris (RMSCOND.M-1 RF) and vastus lateralis (RMSCOND.M-1 VL) muscle expressed as a percentage of the maximal M-wave recorded during the MVIC prior to recording for the 14 subjects. Data are reported for each duration (i.e., 1, 3, or 6 minutes) for the control (CONT) and local vibration (LV) conditions.
Table 3.
Electromyographic activity (root mean square) of the rectus femoris (RMSCOND.M-1 RF) and vastus lateralis (RMSCOND.M-1 VL) muscle expressed as a percentage of the maximal M-wave recorded during the MVIC prior to recording for the 14 subjects. Data are reported for each duration (i.e., 1, 3, or 6 minutes) for the control (CONT) and local vibration (LV) conditions.
| |
1 min |
3 min |
6 min |
| |
CONT |
LV |
CONT |
LV |
CONT |
LV |
| |
|
|
|
|
|
|
| RMSCOND.M-1 RF (%) |
.023 ± .013 |
.028 ± .019 |
.024 ± .02 |
.029 ± .024 |
.042 ± .042 |
.036 ± .037 |
| RMSCOND.M-1 VL (%) |
.056 ± .037 |
.051 ± .025 |
.056 ± .031 |
.061 ± .058 |
.084 ± 0.107 |
.084 ± .081 |
4. Discussion
The aim of the present study was to identify the shortest duration that would induce a reduction in the knee extensor muscles’ force and to confirm the central origin of such losses. The main finding of the present study was that the application of LV, lasted either 1 or 3 or 6 minutes do not affect negatively quadricep muscles’ force production, neither induce any central and/or peripheral fatigue.
Until now, the majority of the studies reporting significant reduction of maximum strength (from 4.3 [
15] to 17.4% [
17] for knee extension and from 5 [
10] to 19% [
12] for plantar flexion), have used long termed protocols, lasting between 20 and 30 minutes [
13,
14,
15,
16,
17,
18]. Considering that in the above studies as well as in the present study, the frequencies and the amplitudes of the LV protocols were similar (frequency: 70-120, amplitude: 0.2 - 2mm) [
10,
11,
12,
14,
15,
18,
29], it could be concluded that the observation of no significant force reduction, and the absence of central and peripherical fatique, as they evaluated in the present study, should be the outcome of the very short LV duration used in this research. We found no changes in markers of peripheral fatigue independent of experimental conditions (i.e., LV or CONT), as demonstrated in previous reaeach that have observed a loss of strength for longer LV duration.
Afferent feedback to the motoneuron pool is necessary for the recruitment of fast motor units during maximal contraction [
31,
45]. Impaired sensory feedback could easily leads to a stronger reduction in activation of muscle characterized by increased proportion of type II muscle fibers, compared to those with higher proportion of type I muscle fibers [
11,
12,
15]. This reduction in muscle EMG activity accompanied with a significant reduction of muscle force production [
20,
21,
22,
23]. More surprisingly, the level of voluntary activation remained unchanged when investigated despite the presence of loss of strength and reduced EMG activity [
10,
46]. For instance, Souron's study used transcranial magnetic stimulation to assess the level of voluntary activation. This method has several limitations, such as (i) using an estimation of the amplitude of potentiated stimulation to calculate the VAL, (ii) lower reproducibility, and (iii) a lower target specificity and stimulation intensity [
27]. These biases, inherent in the method used, may have led to the absence of any significant interaction between the two conditions (CONT vs. LV) in their work, despite the loss of force. Although not significant in the Herda
et al. study, a reduction in VAL was obtained in 10 of the 15 participants [
10]. Conversely, the reduction in RTD is regularly observed in association with the loss of strength [
15,
16,
29]. This result confirms the central origin of the loss of strength after LV and the difficulty in recruiting the fast fibres in producing an explosive contraction [
28]. In our case, the preservation of EMG activity levels, voluntary activation level, and RTD is consistent with the lack of force loss observed in our study.
The effect of LV on central nervous system excitability is increasingly well established [
34,
47]. A reduction in motor neuron excitability assessed at rest or during weak contractions by means of thoracic or cervicomedullary stimulation corroborates the loss of force for durations of 30 minutes [
29,
46]. Moreover, this reduction in motoneuronal excitability appears to be partly responsible for the reduction in spinal loop excitability assessed by the H reflex [
34,
36]. The maintenance of maximal force production and the level of voluntary activation in our study might imply that motoneuron excitability, and consequently the spinal loop, remains intact. In addition, only 30 seconds of LV applied on the soleus muscle tendon is sufficient to induce a reduction in the H-reflex. However, no loss of strength was found despite reduced H-reflex assessed in the study by Fry and Folland on the vastus medialis muscle after 30 minutes of LV [
20]. The same result was also observed for muscles involved in plantar flexion [
22]. These results weaken the hypothesis of a direct relationship between vibration-induced altered spinal loop excitability and the ability to produce maximum force. It should also be noted that an increase in cortical excitability has already been observed after the application of 30 minutes of LV to the quadriceps muscle (estimated by the MEP/Thoracix MEP ratio) [
29,
46], as well as an increase in corticospinal excitability (estimated by the MEP) during the application of LV to the same muscle [
48]. Consequently, the variables identified in our study do not allow us to determine whether the absence of force loss for the three durations tested results from the absence of changes in spinal excitability or from compensatory mechanisms at the level of the corticospinal pathway. In addition, other mechanisms modulating corticospinal excitability may be involved during contraction (e.g. neuromudulation, recurrent inhibition, or gamma loop contraction) and are not assessed by techniques performed at rest or during weak contraction.
It should be noted that our protocol did not induce TVR despite optimal application to the tendon. The latter, originating from mono and polysynaptic circuits. has already been documented for the quadriceps muscle [
9,
49]. The presence of this reflex contraction has only been reported in a single study measuring the effect of LV on maximum force production [
16]. The other studies did not mention and/or control for the potential presence of TVR. Thus, the absence of force loss in several studies, as well as the present one, could also be facilitated by the lack of this reflex contraction.
In conclusion, this study shows LV does not harm maximal force production in knee extensor muscles when the protocol is applied for 6 minutes or less. No changes appear to take place either above or below the neuromuscular junction. Even if changes in central nervous system excitability have already been reported for such short durations of LV applied to smaller muscle groups (e.g., Flexor Carpi Radialis or plantar flexor muscles), this seems insufficient for the knee extensor muscles. The evolution of motor neuronal excitability and that of the spinal loop for short periods of LV should be investigated for such muscle groups. A better understanding of the mechanisms and optimization of the vibration parameters used to induce neuromuscular fatigue would lead to improved application of LV in rehabilitation.
Author Contributions
Conceptualization. N.A. and C.P.; methodology. N.A., T.T., A.A., S.M., N.B., and C.P.; software. N.A. and S.Z.; validation. N.A., A.A., S.M., N.B. and C.P.; formal analysis. N.A. and S.Z.; investigation. S.Z., T.T. and N.A.; resources. S.Z., T.T., and N.A.; data curation. N.A.; writing—original draft preparation. N.A., T.T., and S.Z.; writing—review and editing. N.A., A.A., S.M., N.B. and C.P.; visualization. N.A., A.A., S.M., S.Z. and C.P.; supervision. C.P.; project administration. C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee of the local university (CERUBFC-2021-11-23-041).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the subjects for the time and energy they invested in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ribot-Ciscar, E.; Rossi-Durand, C.; Roll, J.P. Muscle Spindle Activity Following Muscle Tendon Vibration in Man. Neurosci. Lett. 1998, 258, 147–150. [CrossRef]
- Roll, J.P.; Vedel, J.P. Kinaesthetic Role of Muscle Afferents in Man, Studied by Tendon Vibration and Microneurography. Exp. Brain Res. 1982, 47, 177–190. [CrossRef]
- Hayward, L.F.; Nielsen, R.P.; Heckman, C.J.; Hutton, R.S. Tendon Vibration-Induced Inhibition of Human and Cat Triceps Surae Group I Reflexes: Evidence of Selective Ib Afferent Fiber Activation. Exp. Neurol. 1986, 94, 333–347. [CrossRef]
- Burke, D.; Hagbarth, K.-E.; Lofstedt, L.; Wallin, B.G. The Responses of Human Muscle Spindle Endings. J. Physiol. 1976, 261, 695–711.
- Roll, J.P.; Vedel, J.P.; Ribot, E. Alteration of Proprioceptive Messages Induced by Tendon Vibration in Man: A Microneurographic Study. Exp. Brain Res. 1989, 76, 213–222. [CrossRef]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L.; Wallin, B.G. The Responses of Human Muscle Spindle Endings to Vibration during Isometric Contraction. J. Physiol. 1976, 261, 695–711. [CrossRef]
- Gillies, J.D.; Burke, D.J.; Lance, J.W. Supraspinal Control of Tonic Vibration Reflex. J. Neurophysiol. 1971, 34, 302–309. [CrossRef]
- Desmedt, J.E.; Godaux, E. Mechanism of the Vibration Paradox: Excitatory and Inhibitory Effects of Tendon Vibration on Single Soleus Muscle Motor Units in Man. J. Physiol. 1978, 285, 197–207. [CrossRef]
- De Gail, P.; Lance, J.W.; Neilson, P.D. Differential Effects on Tonic and Phasic Reflex Mechanisms Produced by Vibration of Muscles in Man. J. Neurol. Neurosurg. Psychiatry 1966, 29, 1–11. [CrossRef]
- Herda, T.J.; Ryan, E.D.; Smith, A.E.; Walter, A.A.; Bemben, M.G.; Stout, J.R.; Cramer, J.T. Acute Effects of Passive Stretching vs Vibration on the Neuromuscular Function of the Plantar Flexors. Scand. J. Med. Sci. Sport. 2009, 19, 703–713. [CrossRef]
- Ushiyama, J.; Masani, K.; Kouzaki, M.; Kanehisa, H.; Fukunaga, T. Difference in Aftereffects Following Prolonged Achilles Tendon Vibration on Muscle Activity during Maximal Voluntary Contraction among Plantar Flexor Synergists. J. Appl. Physiol. 2005, 98, 1427–1433. [CrossRef]
- Yoshitake, Y.; Shinohara, M.; Kouzaki, M.; Fukunaga, T. Fluctuations in Plantar Flexion Force Are Reduced after Prolonged Tendon Vibration. J. Appl. Physiol. 2004, 97, 2090–2097. [CrossRef]
- Barrera-Curiel, A.; Colquhoun, R.J.; Hernandez-Sarabia, J.A.; DeFreitas, J.M. The Effects of Vibration-Induced Altered Stretch Reflex Sensitivity on Maximal Motor Unit Firing Properties. J. Neurophysiol. 2019, 121, 2215–2221. [CrossRef]
- Souron, R.; Besson, T.; McNeil, C.J.; Lapole, T.; Millet, G.Y. An Acute Exposure to Muscle Vibration Decreases Knee Extensors Force Production and Modulates Associated Central Nervous System Excitability. Front. Hum. Neurosci. 2017, 11. [CrossRef]
- Jackson, S.W.; Turner, D.L. Prolonged Muscle Vibration Reduces Maximal Voluntary Knee Extension Performance in Both the Ipsilateral and the Contralateral Limb in Man. Eur. J. Appl. Physiol. 2003, 88, 380–386. [CrossRef]
- Kouzaki, M.; Shinohara, M.; Fukunaga, T. Decrease in Maximal Voluntary Contraction by Tonic Vibration Applied to a Single Synergist Muscle in Humans. J. Appl. Physiol. 2000, 89, 1420–1424. [CrossRef]
- Konishi, Y.; Kubo, J.; Fukudome, A. Effects of Prolonged Tendon Vibration Stimulation on Eccentric and Concentric Maximal Torque and EMGs of the Knee Extensors. J. Sport. Sci. Med. 2009, 8, 548–552.
- Saito, A.; Ando, R.; Akima, H. Effects of Prolonged Patellar Tendon Vibration on Force Steadiness in Quadriceps Femoris during Force-Matching Task. Exp. Brain Res. 2016, 234, 209–217. [CrossRef]
- Souron, R.; Besson, T.; Millet, G.Y.; Lapole, T. Acute and Chronic Neuromuscular Adaptations to Local Vibration Training. Eur. J. Appl. Physiol. 2017, 117, 1939–1964. [CrossRef]
- Fry, A.; Folland, J.P. Prolonged Infrapatellar Tendon Vibration Does Not Influence Quadriceps Maximal or Explosive Isometric Force Production in Man. Eur. J. Appl. Physiol. 2014, 114, 1757–1766. [CrossRef]
- Cattagni, T.; Billet, C.; Cornu, C.; Jubeau, M. No Alteration of the Neuromuscular Performance of Plantar-Flexor Muscles After Achilles Tendon Vibration. J. Sport Rehabil. 2016, 26, 1–3. [CrossRef]
- Ekblom, M.M.N.; Thorstensson, A. Effects of Prolonged Vibration on H-Reflexes, Muscle Activation, and Dynamic Strength. Med. Sci. Sport. Exerc. 2011, 43, 1933–1939. [CrossRef]
- Farabet, A.; Souron, R.; Millet, G.Y.; Lapole, T. Changes in Tibialis Anterior Corticospinal Properties after Acute Prolonged Muscle Vibration. Eur. J. Appl. Physiol. 2016, 116, 1197–1205. [CrossRef]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [CrossRef]
- Place, N. Quantification of Central Fatigue: A Central Debate. Eur. J. Appl. Physiol. 2021, 121, 2375–2376. [CrossRef]
- Place, N.; Millet, G.Y. Quantification of Neuromuscular Fatigue: What Do We Do Wrong and Why? Sport. Med. 2020, 50, 439–447. [CrossRef]
- Dotan, R.; Woods, S.; Contessa, P. On the Reliability and Validity of Central Fatigue Determination. Eur. J. Appl. Physiol. 2021, 121, 2393–2411. [CrossRef]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of Force Development: Physiological and Methodological Considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [CrossRef]
- Kennouche, D.; Varesco, G.; Espeit, L.; Féasson, L.; Souron, R.; Rozand, V.; Millet, G.Y.; Lapole, T. Acute Effects of Quadriceps Muscle versus Tendon Prolonged Local Vibration on Force Production Capacities and Central Nervous System Excitability. Eur. J. Appl. Physiol. 2022, 122, 2451–2461. [CrossRef]
- Abbruzzese, M.; Minatel, C.; Reni, L.; Favale, E. Postvibration Depression of the H-Reflex as a Result of a Dual Mechanism: An Experimental Study in Humans. J. Clin. Neurophysiol. 2001, 18, 460–470. [CrossRef]
- Bongiovanni, L.G.; Hagbarth, K.E.; Stjernberg, L. Prolonged Muscle Vibration Reducing Motor Output in Maximal Voluntary Contractions in Man. J. Physiol. 1990, 423, 15–26. [CrossRef]
- Hultborn, H.; Meunier, S.; Morin, C.; Pierrot-Deseilligny, E. Assessing Changes in Presynaptic Inhibition of I a Fibres: A Study in Man and the Cat. J. Physiol. 1987, 389, 729–756. [CrossRef]
- Curtis, D.R.; Eccles, J.C. Synaptic Action during and after Repetitive Stimulation. J. Physiol. 1960, 150, 374–398. [CrossRef]
- Souron, R.; Baudry, S.; Millet, G.Y.; Lapole, T. Vibration-Induced Depression in Spinal Loop Excitability Revisited. J. Physiol. 2019, 597, 5179–5193. [CrossRef]
- Lapole, T.; Mesquita, R.N.O.; Baudry, S.; Souron, R.; Brownstein, C.G.; Rozand, V. Can Local Vibration Alter the Contribution of Persistent Inward Currents to Human Motoneuron Firing? J. Physiol. 2023, 601, 1467–1482. [CrossRef]
- Nito, M.; Yoshimoto, T.; Hashizume, W.; Shindo, M.; Naito, A. Vibration Decreases the Responsiveness of Ia Afferents and Spinal Motoneurons in Humans. J. Neurophysiol. 2021, 126, 1137–1147. [CrossRef]
- Ribot-Ciscar, E.; Vedel, J.P.; Roll, J.P. Vibration Sensitivity of Slowly and Rapidly Adapting Cutaneous Mechanoreceptors in the Human Foot and Leg. Neurosci. Lett. 1989, 104, 130–135. [CrossRef]
- Rozand, V.; Pageaux, B.; Marcora, S.M.; Papaxanthis, C.; Lepers, R. Does Mental Exertion Alter Maximal Muscle Activation? Front. Hum. Neurosci. 2014, 8, 1–10. [CrossRef]
- Garnier, Y.M.; Lepers, R.; Stapley, P.J.; Papaxanthis, C.; Paizis, C. Changes in Cortico-Spinal Excitability Following Uphill versus Downhill Treadmill Exercise. Behav. Brain Res. 2017, 317, 242–250. [CrossRef]
- Garnier, Y.M.; Paizis, C.; Lepers, R. Corticospinal Changes Induced by Fatiguing Eccentric versus Concentric Exercise. Eur. J. Sport Sci. 2019, 19, 166–176. [CrossRef]
- Babault, N.; Pousson, M.; Michaut, A.; Van Hoecke, J. Effect of Quadriceps Femoris Muscle Length on Neural Activation during Isometric and Concentric Contractions. J. Appl. Physiol. 2003, 94, 983–990. [CrossRef]
- Strojnik, V.; Komi, P. V. Neuromuscular Fatigue after Maximal Stretch-Shortening Cycle Exercise. J. Appl. Physiol. 1998, 84, 344–350. [CrossRef]
- Garnier, Y.M.; Lepers, R.; Dubau, Q.; Pageaux, B.; Paizis, C. Neuromuscular and Perceptual Responses to Moderate-Intensity Incline, Level and Decline Treadmill Exercise. Eur. J. Appl. Physiol. 2018, 118, 2039–2053. [CrossRef]
- Portney, L.G. Foundations of Clinical Research: Applications to Evidence-Based Practice.; Portney, L.G., Ed.; Fourth edi.; F. A. Davis Company, Philadelphia., 2020; ISBN 9780803661134.
- Bongiovanni, L.G.; Hagbarth, K.E. Tonic Vibration Reflexes Elicited during Fatigue from Maximal Voluntary Contractions in Man. J. Physiol. 1990, 423, 1–14. [CrossRef]
- Souron, R.; Besson, T.; McNeil, C.J.; Lapole, T.; Millet, G.Y. An Acute Exposure to Muscle Vibration Decreases Knee Extensors Force Production and Modulates Associated Central Nervous System Excitability. Front. Hum. Neurosci. 2017, 11, 8–10. [CrossRef]
- Pfenninger, C.; Grosboillot, N.; Digonet, G.; Lapole, T. Effects of Prolonged Local Vibration Superimposed to Muscle Contraction on Motoneuronal and Cortical Excitability. Front. Physiol. 2023, 14, 1–9. [CrossRef]
- Souron, R.; Oriol, M.; Millet, G.Y.; Lapole, T. Intermediate Muscle Length and Tendon Vibration Optimize Corticospinal Excitability During Knee Extensors Local Vibration. Front. Physiol. 2018, 9, 1–9. [CrossRef]
- Eklund, G.; Hagbarth, K.E. Normal Variability of Tonic Vibration Reflexes in Man. Exp. Neurol. 1966, 16, 80–92. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).