Submitted:
21 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Patients and Major Adverse Cardiovascular Events (MACE)
3.2. Prognostic Value of Galectin-3 Plasma Concentration Measured at Four Different Sites in AMI Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules. 2020, 10, 389. [CrossRef]
- Zaborska, B.; Sikora-Frąc, M.; Smarż, K.; Pilichowska-Paszkiet, E.; Budaj, A.; Sitkiewicz, D.; Sygitowicz, G. The Role of Galectin-3 in Heart Failure—The Diagnostic, Prognostic and Therapeutic Potential—Where Do We Stand? Int. J. Mol. Sci. 2023, 24, 13111.
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H.; Hiraide, T.; Fukuda, K.; Sano, M. IL (Interleukin)-10-STAT3-Galectin-3 axis is essential for osteopontin - producing reparative macrophage polarization after myocardial infarction. Circulation. 2018, 138, 2021-2035. [CrossRef]
- Li, M.; Yuan, Y.; Guo, K.; Lao, Y.; Huang, X.; Feng, L. Value of Galectin-3 in acute myocardial infarction. Am J Cardiovasc Drugs. 2020, 20, 333-342. [CrossRef]
- Di Tano, G.; Caretta, G.; De Maria, R.; Parolini, M.; Bassi, L.; Testa, S.; Pirelli, S. Galectin-3 predicts left ventricular remodelling after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. Heart. 2017, 103, 71-77. [CrossRef]
- Tian, L.; Chen, K.; Han, Z. Correlation between Galectin-3 and adverse outcomes in myocardial infarction patients: A Meta-Analysis. Cardiol Res Pract. 2020, 7, 7614327. [CrossRef]
- Agnello, L.; Bivona, G.; Lo Sasso, B.; Scazzone, C.; Bazan, V.; Bellia, C.; Ciaccio, M. Galectin-3 in acute coronary syndrome. Clin Biochem. 2017, 50, 797-803. [CrossRef]
- Yu, L.; Ruifrok, W.P.; Meissner. M.; Bos, E.M.; van Goor. H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail. 2013, 6, 107-117. [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int J Mol Sci. 2020, 21, 9232. [CrossRef]
- Wojciechowska, C.; Romuk, E.; Nowalany-Kozielska, E.; Jacheć. W. Serum Galectin-3 and ST2 as predictors of unfavorable outcome in stable dilated cardiomyopathy patients. Hellenic Journal of Cardiology. 2017, 58, 350-359. [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [CrossRef]
- Cheng, Z.; Cai, K.; Xu, C.; Zhan, Q.; Xu, X.; Xu, D.; Zeng, Q. Prognostic Value of Serum Galectin-3 in Chronic Heart Failure: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 783707. [CrossRef]
- Andrejic, O.M.; Vucic, R.M.; Pavlovic, M.; McClements, L.; Stokanovic, D.; Jevtovic-Stoimenov, T.; Nikolic, V. Association between Galectin-3 levels within central and peripheral venous blood, and adverse left ventricular remodeling after first acute myocardial infarction. Sci Rep, 2019. [CrossRef]
- Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; Jüni, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014, 35, 2541-619.
- Gagno, G.; Padoan, L.; Stenner, E.; Beleù, A.; Ziberna, F.; Hiche, C.; Paldino, A.; Barbati, G.; Biolo, G.; Fiotti, N.; et al. Galectin 3 and Galectin 3 binding protein improve the risk stratification after myocardial infarction. J Clin Med. 2019, 8, 570. [CrossRef]
- Asleh, R.; Enriquez-Sarano, M.; Jaffe, A.S.; Manemann, S.M.; Weston, S.A.; Jiang, R.; Roger, V.L. Galectin-3 levels and outcomes after myocardial infarction: a population - based study. J. Am. Coll. Cardiol. 2019, 73, 2286–2295.
- Li, M.; Guo, K.; Huang, X.; Feng, L.; Yuan, Y.; Li, J.; Lao, j.; Guo, Z. Association between serum Galectin-3 levels and coronary stenosis severity in patients with coronary artery disease. Front Cardiovasc Med. 2022, 9, 818162. [CrossRef]
- Tsai, T.H.; Sung, P.H.; Chang, L.T.; Sun, C.K.; Yeh, K.H.; Chung, S.Y.; Chua, S.; Chen, Y.L.; Wu, C.J.; Chang, H.W.; et al. Value and level of galectin-3 in acute myocardial infarction patients undergoing primary percutaneous coronary intervention. J Atheroscler Thromb. 2012; 19, 1073-82. [CrossRef]
- Lisowska, A.; Knapp, A.; Tycinska, A.; Motybel, E.; Kaminski, K., Swięcki, P.; Musial, W.J.; Dymicka-Piekarska, V. Predictive value of Galectin-3 for the occurrence of coronary artery disease and prognosis after myocardial infarction and its association with carotid IMT values in these patients: A mid-term prospective cohort study. Atherosclerosis. 2016, 246, 309-17. [CrossRef]
- Di Tano, G.; Caretta, G.; De Maria, R:; Bettari, L:; Parolini, M.; Testa. S.; Pirelli, S. Galectin-3 and outcomes after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. Biomarkers in Medicine. 2018, 12, 21–26. [CrossRef]
- Idzikowska, K.; Kacprzak, M.; Zielinska, M. The Prognostic Value of Cardiac Biomarkers in Patients with Acute Myocardial Infarction during and after Hospitalization. Rev. Cardiovasc. Med. 2022, 23, 320. [CrossRef]
- Tymińska, A.; Kapłon-Cieślicka, A.; Ozierański, K.; Budnik, M.; Wancerz, A:; Sypień, P.; Peller, M.; Maksym, J.; Balsam, P.; Opolski, G.; Filipiak, K.J. Association of galectin-3 and soluble ST2 with In-hospital and 1-year outcomes In patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Polish Archives of Internal Medicine. 2019; 129: 770–780. [CrossRef]
- Kang, S.H.; Moon, J.Y.; Kim, S.H.; Sung, J.H.; Kim, I.J.; Lim, S.W.; Cha, D.H.; Kim, W.J. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes in Koreans. Medicine (Baltimore). 2022, 101, e32579. [CrossRef]
- Cakar, M.A.; Gunduz, H.; Vatan, M.B.; Kocayigit, I.; Akdemir, R. The Effect of admission creatinine levels on one-year mortality in acute myocardial infarction. Sci. World J. 2011;(2012),. [CrossRef]
- Doost Hosseiny, A.; Moloi, S.; Chandrasekhar, J.; Farshid, A. Mortality pattern and cause of death in a long-term follow-up of patients with STEMI treated with primary PCI. Open Heart 2016, 3, e000405. [CrossRef]
- Ezubogly, M.; Akdeniz, B. Left ventricular ejection fraction in the prognosis of acute coronary syndromes. Int. J. Cardiol. 2017, 234, 137.
- Møller, J.E.; Hillis, G.S.; Oh, J.K.; Reeder, G.S.; Gersh, B.J.; Pellikka, P. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am. Heart J. 2006, 151, 419-425. [CrossRef]
- Perelshtein Brezinov, O.; Klempfner, R.; Zekry, S.B.; Goldenberg, I.; Kuperstein, R. Prognostic value of ejection fraction in patients admitted with acute coronary syndrome: A real world study. Medicine (Baltimore), 2017, 96, e6226.
- Margolis, G.; Khoury, S.; Ben-Shoshan, J.; Letourneau-Shesaf, S.; Flint, N.; Keren, G.; Shacham Y. Prognostic Implications of Mid-Range Left Ventricular Ejection Fraction on Patients Presenting With ST-Segment Elevation Myocardial Infarction. Am J Cardiol. 2017, 120, 186-190. [CrossRef]
- Chew, D.; Heikki, H.; Schmidt, G.; Kavanagh, K.M.; Dommasch, M.; Bloch Thomsen, P.E.; Sinnecker, D.; Raatikainen, P.; Exner, D.V. Change in Left Ventricular Ejection Fraction Following First Myocardial Infarction and Outcome. J Am Coll Cardiol EP. 2018, 4, 672–682. [CrossRef]
- Grandin EW, Jarolim P, Murphy SA, Ritterova, L.; Cannon, C.P.; Braunwald, E.; Morrow, D.A. Galectin-3 and the development of heart failure after acute coronary syndrome: pilot experience from PROVE IT-TIMI 22. Clin Chem. 2012, 58, 267–73. [CrossRef]
- Perea, R.J.; Morales-Ruiz, M.; Ortiz-Perez, J.T.; Bosch, X.; Andreu, D.; Borras, R.; Acosta, J.; Penela, D.; Prat-González, S., de Caralt, T.M.; et al. Utility of galectin-3 in predicting post-infarct remodeling after acute myocardial infarction based on extracellular volume fraction mapping. Int J Cardiol. 2016, 223, 458–464. [CrossRef]
- Wang, Q.; Huai, W.; Ye, X.; Pan, Y.; Yang, X.; Chen, M.; Ma, Q-B.; Gao, Y.; Zhang, Y. Circulating plasma galectin-3 predicts new-onset atrial fibrillation in patients after acute myocardial infarction during hospitalization. BMC Cardiovasc Disord. 2022, 22, 392. [CrossRef]
- Erdogan, O.; Karaayvaz, E.; Erdogan, T.; Panc, C.; Sarıkaya, R.; Oncul, A.; Bilge, A.K. A new biomarker that predicts ventricular arrhythmia in patients with ischemic dilated cardiomyopathy: Galectin-3. Revista Portuguesa de Cardiologia. 2021, 40, 829-83. [CrossRef]

| All patientsN = 59 | No MACE or deathN = 39 | MACE or deathN = 20 | p-value | |
|---|---|---|---|---|
| Galectin-3 at site: | ||||
| Aortic root (Q1, Q3), ng/ml | 9.2 (6.8-12.1) | 8.2 (5.9-9.9) | 13.9 (10.8-16.2) | <0.001 |
| Femoral/radial artery + SD, ng/ml | 9.72 ± 3.62 | 8.53 ± 3.46 | 11.55 ± 3.15 | 0.005 |
| Coronary sinus + SD, ng/ml | 9.74 ± 3.76 | 8.43 ± 2.57 | 12.56 ± 4.40 | 0.001 |
| Cubital vein + SD, ng/ml | 9.31 ± 3.35 | 8.05 ± 2.21 | 12.06 ± 3.80 | <0.001 |
| Age + SD, years | 65 ± 9 | 63 ± 8 | 69 ± 10 | 0.013 |
| Gender (male), n (%) | 42 (71) | 32 (78.0%) | 9 (50.0%) | 0.063 |
| BMI + SD, kg/m2 | 27.8 + 3.52 | 27.8 ± 3.4 | 27.8 ± 3.9 | 0.960 |
| Smoking, n (%) | 18 (31) | 14 (34) | 4 (22) | 0.540 |
| Diabetes mellitus, n (%) | 20 (34) | 14 (70) | 6 (30) | 0.999 |
| CVI, n (%) | 3 (5) | 2 (5) | 1 (6) | 0.999 |
| Hypertension, n (%) | 38 (64) | 24 (58) | 14 (78) | 0.238 |
| Hyperlipoproteinemia, n (%) | 17 (29) | 11 (27) | 6 (33) | 0.756 |
| STEMI, n (%) | 38 (64) | 23 (56) | 15 (83) | 0.044 |
| Anterior MI, n (%) | 26 (44) | 16 (41) | 10 (50) | 0.239 |
| Inferior MI, n (%) | 33 (56) | 23 (59) | 10 (50) | 0.969 |
| NSTEMI, n (%) | 21 (36) | 16 (41) | 5 (25) | 0.044 |
| Time from pain onset (Q1, Q3), hours | 10.0 (4.0-18.0) | 9.5 (4.0-20.0) | 8.5 (3.5-15.5) | 0.882 |
| AV block, n (%) | 3 (5) | 0 (0) | 3 (17) | 0.025 |
| VT/VF, n (%) | 7 (12) | 5 (12) | 2 (11) | 0.999 |
| AF, n (%) | 5 (8) | 2 (5) | 3 (17) | 0.160 |
| Systolic BP + SD, mmHg | 130 ± 28 | 137 ± 27 | 115 ± 26 | 0.005 |
| Diastolic BP + SD, mmHg | 75 ± 17 | 79 ± 17 | 68 ± 15 | 0.023 |
| HR + SD, bpm | 74 ± 14 | 76 ± 11 | 70 ± 21 | 0.272 |
| Urea (Q1, Q3), mmol/l | 6.3 (4.9-8.5) | 5.8 (4.6-6.9) | 9.6 (7.4-13.3) | 0.003 |
| Creatinine (Q1, Q3), μmol/l | 87.0 (78.0-100.0) | 86.0 (77.0-93.8) | 115.5 (79.8-167.8) | 0.044 |
| Creatinine clearance + SD, ml/min | 83.41 ± 27.17 | 91.79 ± 21.29 | 64.32 ± 29.95 | <0.001 |
| Cholesterol + SD, mmol/l | 5.68 ± 1.32 | 5.88 ± 1.19 | 5.22 ± 1.51 | 0.081 |
| HDL + SD, mmol/l | 1.13 ± 0.26 | 1.12 ± 0.21 | 1.15 ± 0.35 | 0.742 |
| LDL + SD, mmol/l | 3.66 ± 1.15 | 3.86 ± 1.02 | 3.19 ± 1.33 | 0.042 |
| Triglycerides (Q1, Q3), mmol/l | 1.7 (1.1-2.3)) | 1.7 (1.2-2.5) | 1.3 (1.0-2.0) | 0.303 |
| CK-MB (Q1, Q3), U/l | 24.0 (15.0-52.0) | 24.0 (16.2-54.0) | 25.0 (12.2-35.8) | 0.987 |
| CRP (Q1, Q3), mg/l | 5.0 (1.5-10.6) | 4.2 (1.2-10.1) | 5.2 (2.7-33.0) | 0.278 |
| Troponin T (Q1, Q3), ng/ml | 1.2 (0.2-7.3) | 1.1 (0.3-5.8) | 2.6 (0.1-5.2) | 0.593 |
| Pro-BNP (Q1, Q3), pg/ml | 459.5 (239.7-2182.7) | 372.5 (180.2-1979.5) | 2250.0 (275.2-6492.8) | 0.004 |
| Glycaemia (Q1, Q3), mmol/l | 6.2 (5.3-8.4) | 6.0 (5.3-7.0) | 8.2 (5.0-12.6) | 0.040 |
| Potassium (Q1, Q3), mmol/l | 4.3 (4.0-4.6) | 4.2 (3.9-4.7) | 4.5 (4.1-4.7) | 0.281 |
| Sodium (Q1, Q3), mmol/l | 139.0 (137.0-141.0) | 139.0 (137.2-141.0) | 139.5 (136.0-141.2) | 0.680 |
| RBC + SD, x1012/l | 4.61 ± 0.56 | 4.73 ± 0.51 | 4.30 ± 0.58 | 0.007 |
| Haemoglobin + SD, g/l | 137.43 ± 18.99 | 144.44 ± 14.62 | 120.53 ± 17.91 | <0.001 |
| Leukocyte count (Q1, Q3), x109/l | 9.6 (8.6-12.0) | 9.4 (8.4-11.6) | 10.1 (7.4-12.9) | 0.657 |
| Platelet count + SD, x109/l | 238.03 ± 65.60 | 232.73 ± 55.37 | 250.822 ± 80.62 | 0.406 |
| LVEF + SD, % | 52 ± 5 | 53 ± 5 | 50 ± 6 | 0.157 |
| EDV + SD, mm | 77.62 ± 22.65 | 76.82 ± 21.73 | 79.53 ± 25.33 | 0.683 |
| ESV + SD, mm | 38.25 ± 13.38 | 38.8 (10.2-45.8) | 38.0 (31.5-45.8) | 0.392 |
| E/A ratio (Q1, Q3) | 0.7 (0.6-0.8) | 0.7 (0.6-0.8) | 0.7 (0.6-1.0) | 0.925 |
| E/E` ratio (Q1, Q3) | 7.9 (6.6-10.0) | 7.8 (6.5-9.7) | 8.0 (6.6-12.2) | 0.263 |
| LA + SD, mm | 37.72 ± 4.93 | 38.20 ± 4.95 | 36.59 ± 4.84 | 0.262 |
| Number of coronary lesions + SD | 1.67 + 0.87 | 1.68 ± 0.82 | 1.65 ± 1.00 | 0.887 |
| 1-vessel CAD, n (%) | 11 (19) | 8 (21) | 3 (15) | 0.869 |
| 2-vessel CAD, n (%) | 20 (34) | 15 (38) | 5 (25) | 0.601 |
| 3-vessel CAD, n (%) | 28 (47) | 16 (41) | 12 (60) | 0.029 |
| Furosemide, n (%) | 18 (31) | 9 (22) | 9 (53) | 0.030 |
| Spironolactone, n (%) | 10 (17) | 5 (12) | 5 (29) | 0.139 |
| ACE inhibitors, n (%) | 42 (71) | 32 (78) | 10 (59) | 0.197 |
| Beta-blockers, n (%) | 43 (73) | 33 (80) | 10 (59) | 0.107 |
| Calcium channel antagonists, n (%) | 7 (12) | 3 (7) | 4 (23) | 0.178 |
| Amiodarone, n (%) | 13 (22) | 8 (19) | 5 (29) | 0.494 |
| DAPT, n (%) | 53 (90) | 39 (95) | 14 (82) | 0.144 |
| Ticagrelor, n (%) | 33 (56) | 26 (63) | 7 (41) | 0.151 |
| Trimetazidine, n (%) | 25 (42) | 18 (44) | 7 (41) | 0.999 |
| Statins, n (%) | 54 (92) | 39 (95) | 15 (88) | 0.573 |
| UFH/LMWH, n (%) | 55 (93) | 40 (98) | 15 (94) | 0.999 |
| Event | n (%) |
|---|---|
| MACE, n (%) | 20 (100) |
| Death, n (%) | 5 (25) |
| Re-AMI, n (%) | 1 (5) |
| Cerebrovascular insult, n (%) | 4 (20) |
| Re-hospitalization due to heart failure, n (%) | 5 (25) |
| Re-hospitalization due to malignant arrhythmias, n (%) | 5 (25) |
| Univariate analysis | OR (95%CI) | p-value | R2 | HL test p-value |
|---|---|---|---|---|
| Galectin-3 at site: | ||||
| Aortic root | 1.277 (1.076-1.517) | 0.005 | 0.255 | 0.581 |
| Femoral/radial artery | 1.309 (1.068-1.604) | 0.009 | 0.224 | 0.556 |
| Coronary sinus | 1.422 (1.155-1.750) | 0.001 | 0.342 | 0.48695 |
| Cubital vein | 1.566 (1.225-2.000) | <0.001 | 0.405 | 0.454 |
| Age (years) | 1.089 (1.014-1.169) | 0.018 | 0.145 | 0.289 |
| Gender (male) | 0.281 (0.086-0.918) | 0.036 | 0.103 | 0.301 |
| STEMI | 3.913 (0.980-15.625) | 0.053 | 0.101 | 0.300 |
| NSTEMI | 0.256 (0.064-1.020) | 0.053 | 0.101 | 0.300 |
| 3-vessel CAD, n (%) | 3.375 (1.110-12.669) | 0.033 | 0.115 | 0.256 |
| Systolic BP (mmHg) | 0.968 (0.945-0.992) | 0.009 | 0.186 | 0.081 |
| Diastolic BP (mmHg) | 0.958 (0.922-0.996) | 0.030 | 0.126 | 0.553 |
| Urea (mmol/l) | 1.546 (1.167-2.048) | 0.002 | 0.296 | 0.616 |
| Creatinine (μmol/l) | 1.034 (1.008-1.061) | 0.010 | 0.264 | 0.004 |
| Creatinine clearance (ml/min) | 0.954 (0.927-0.982) | 0.001 | 0.305 | 0.220 |
| Cholesterol (mmol/l) | 0.677 (0.432-1.062) | 0.090 | 0.074 | 0.464 |
| LDL (mmol/l) | 0.591 (0.349-1.000) | 0.050 | 0.100 | 0.672 |
| Pro-BNP (pg/ml) | 1.000 (1.000-1.001) | 0.022 | 0.176 | 0.549 |
| Glycaemia (mmol/l) | 1.270 (1.043-1.547) | 0.017 | 0.172 | 0.109 |
| RBC count (x1012/l) | 0.227 (0.070-0.739) | 0.014 | 0.167 | 0.913 |
| Haemoglobin (g/l) | 0.914 (0.869-0.961) | <0.001 | 0.439 | 0.382 |
| Furosemide | 4.000 (1.198-13.357) | 0.024 | 0.122 | 0.516 |
| Multivariateanalysis | OR (95%CI) | p-value | R2 | HL test p-value |
| Gal-3 level at aortic roota | 1.228 (1.011-1.491) | 0.038 | 0.621 | 0.440 |
| Haemoglobin (g/l) | 0.821 (0.699-0.965) | 0.017 | 0.621 | 0.440 |
| Gal-3 level Femoral/radial arterya | 3.438 (1.275-9.265) | 0.015 | 0.846 | 0.943 |
| Haemoglobin (g/l) | 0.860 (0.765-0.966) | 0.011 | 0.846 | 0.943 |
| Creatinine clearance (ml/min) | 0.908 (0.829-0.994) | 0.036 | 0.846 | 0.943 |
| Gal-3 level coronary sinusa | 1.044 (0.663-1.644) | 0.851 | 0.519 | 0.858 |
| Haemoglobin (g/l) | 0.927 (0.874-0.984) | 0.012 | 0.519 | 0.858 |
| Gal-3 level cubital veina | 1.163 (0.694-1.948) | 0.566 | 0.519 | 0.860 |
| Haemoglobin (g/l) | 0.927 (0.874-0.984) | 0.012 | 0.519 | 0.860 |
| Urea (mmol/l) | 1.521 (1.039-2.226) | 0.031 | 0.519 | 0.860 |
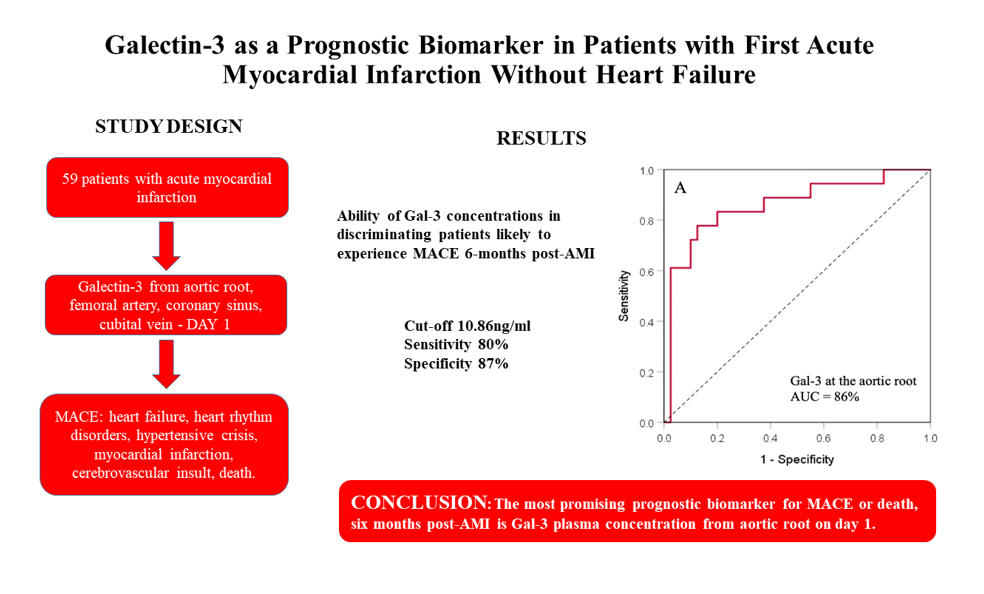
| Gal-3 at site | AUC (95% CI) | SE | p-value | Cut-off (ng/ml) | Sn (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| Aortic root | 0.858 (0.744-0.973) | 0.058 | <0.001 | 10.86 | 80% | 87% | 76% | 89% |
| Femoral artery | 0.742 (0.596-0.888) | 0.074 | 0.006 | 10.18 | 70% | 77% | 61% | 83% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





