Submitted:
20 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Recombinant Antigens
Adjuvant mixtures
Antigenic preparations
Animals, immunization and collections
ELISA
Avidity index (AI)
Neutralization Index
Immunoblotting
Enzyme-linked ImmunoSpot (ELISpot)
Statistical analysis
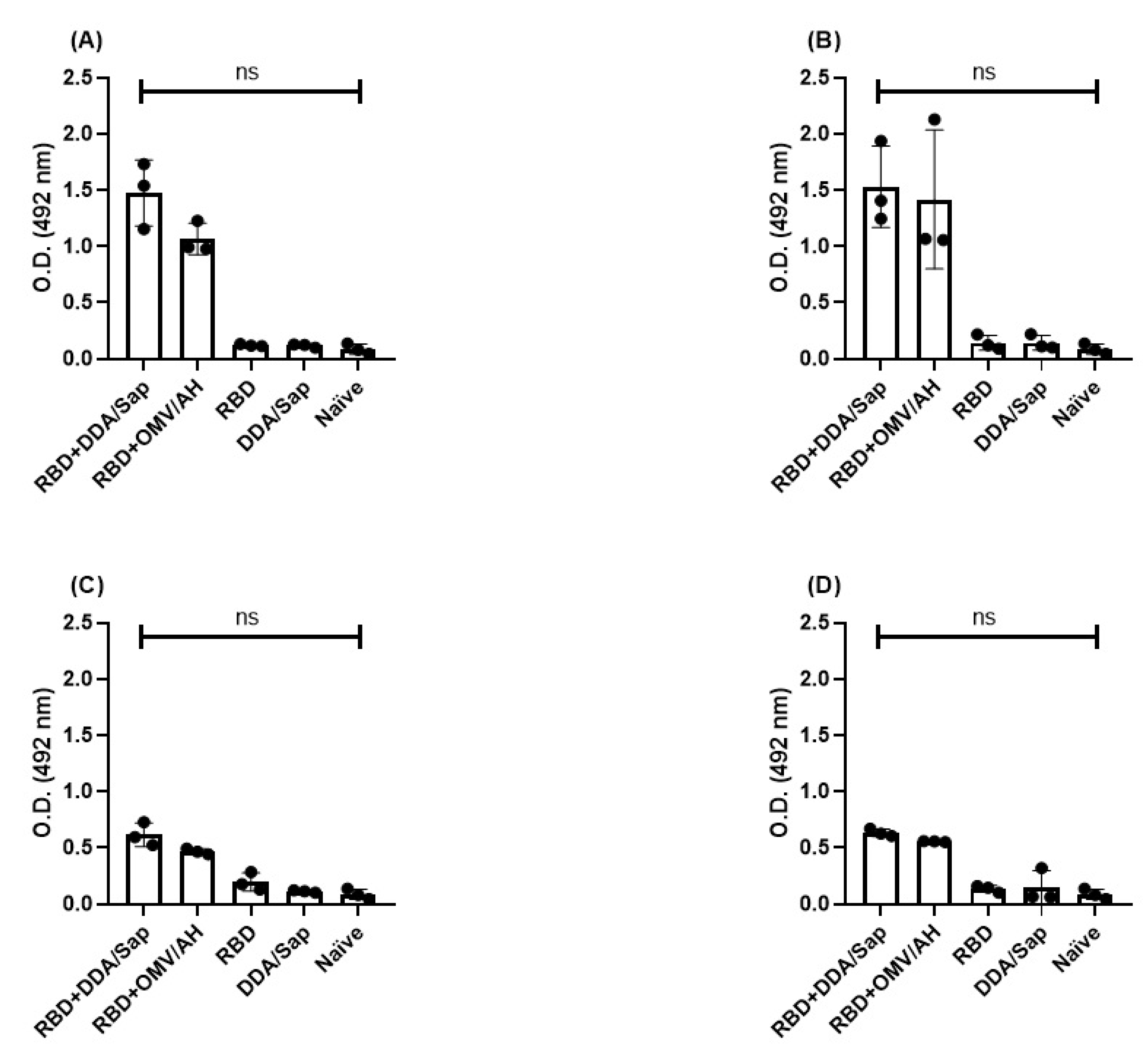
3. Results
IgM antibodies and avidity
| 21 days | 47 days | 176 days | 368 days | |
|---|---|---|---|---|
| RBD+DDA/Sap | 28.77 | 60.86 | 71.70 | 43.72 |
| RBD+OMV/AH | 20.81 | 47.05 | 77.10 | 64.72 |
IgG persistence and avidity
| O.D (450nm) | IgG—Day 368 | ||||
| RBD+DDA/Sap | RBD+OMV/AH | RBD | DDA/Sap | Naive | |
| 1.792 | 1.161 | 0.106 | 0.054 | 0.0795 | |
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | |
|---|---|---|---|---|---|
| RBD+DDA/Sap | 83.2 | 63.9 | 56.7 | 48.2 | 10.24 |
| RBD+OMV/AH | 43.72 | 62.67 | 37.5 | 44.49 | 12.12 |
Neutralization against Omicron variant
| Pre-immune | 21 days | 47 days | 176 days | |
|---|---|---|---|---|
| RBD+DDA/Sap | 14.78 | 18.62 | 21.48 | 20.37 |
| RBD+OMV/AH | 12.26 | 21.95 | 27.60 | 26.90 |
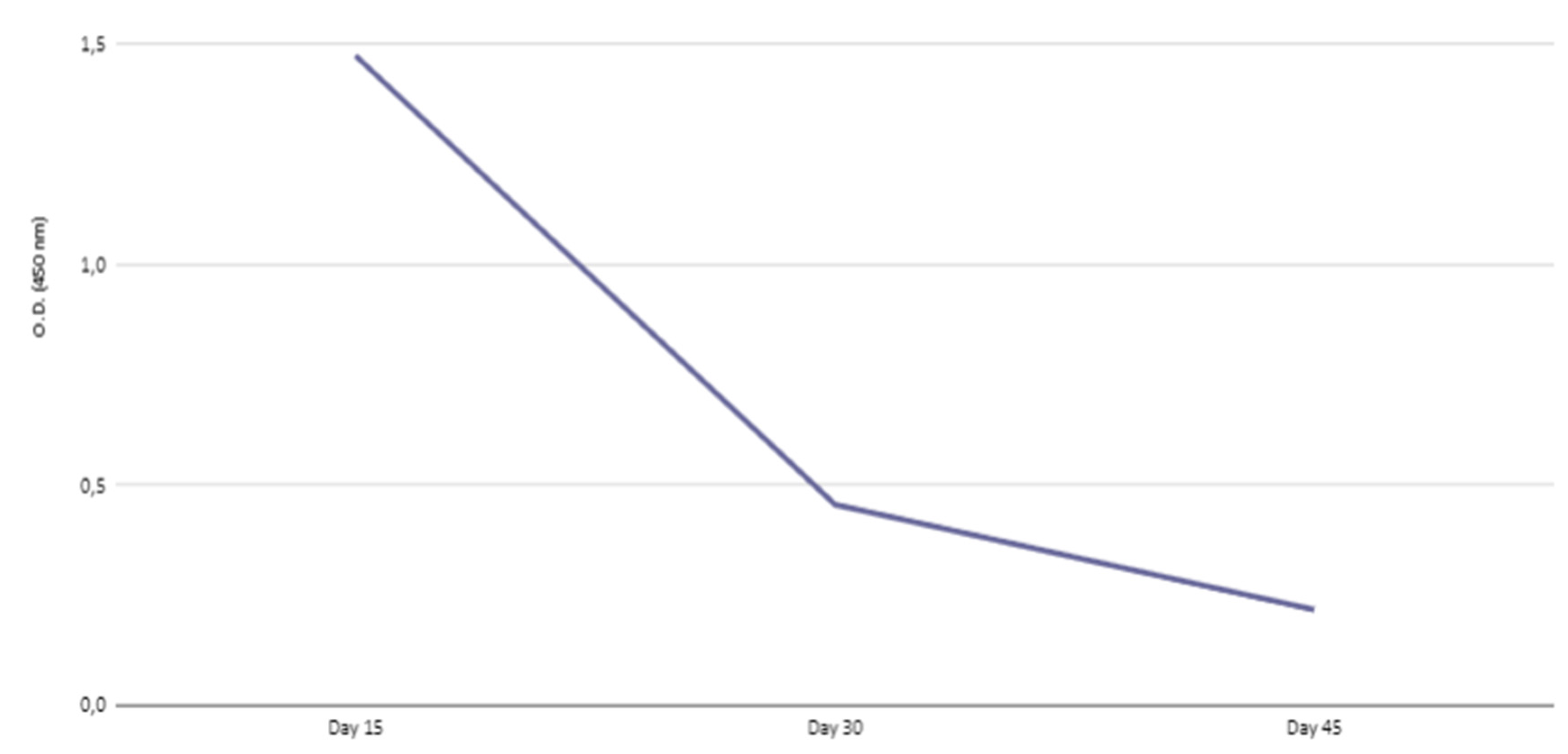
Kinetics of IgG transferred to offspring
| Days after birth | |||
|---|---|---|---|
| 15 | 30 | 45 | |
| IgG | 56.06 | 39.48 | 31.57 |
| IgG1 | 66.34 | 26.54 | 26.77 |
| IgG2a | 62.90 | NR | NR |
| IgG2b | 62.69 | NR | NR |
| IgG3 | 68.69 | 41,75 | NR |
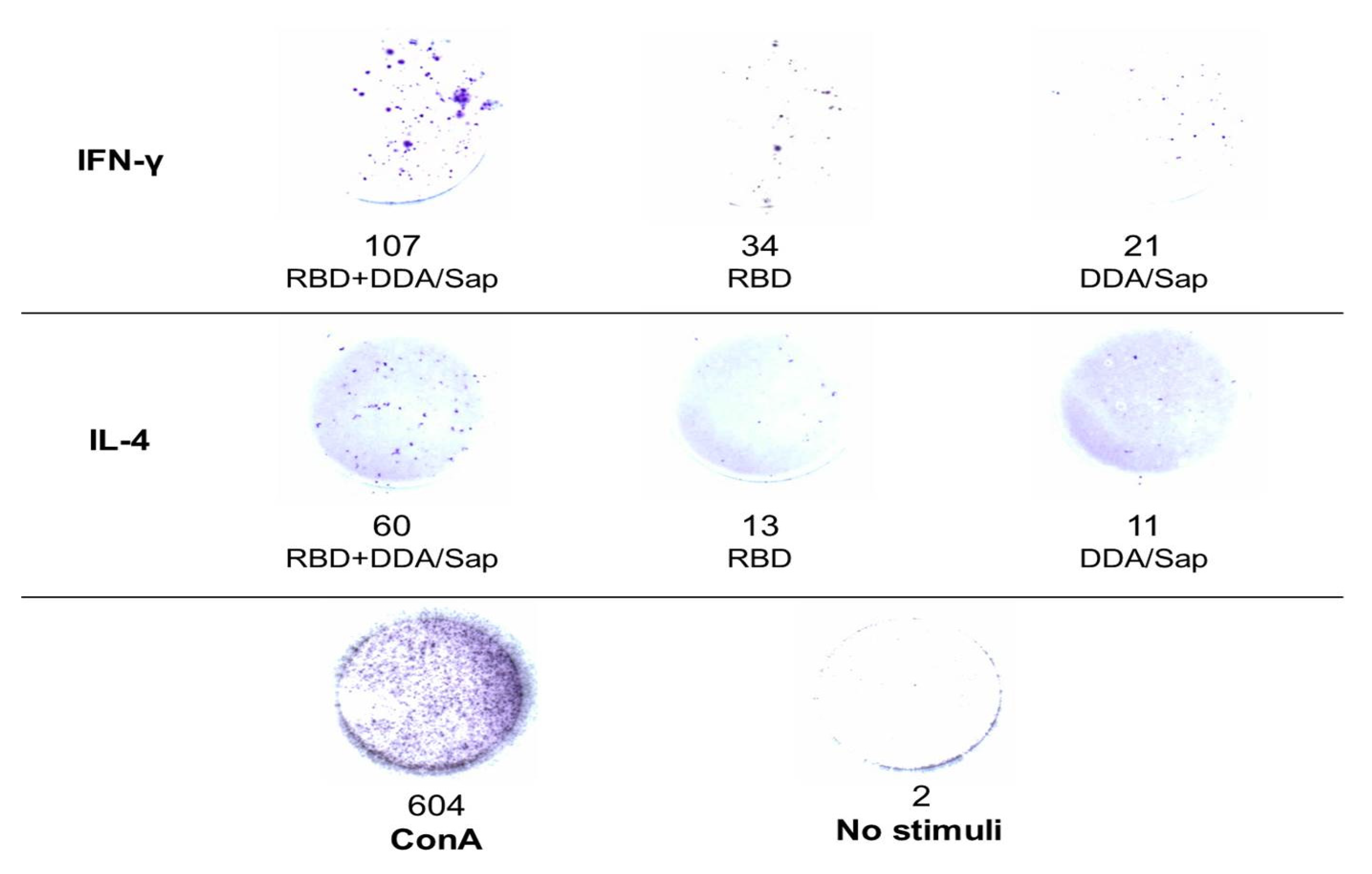
IL-4 and IFN-Υ secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koutsakos, M.; Ellebedy, A.H. Immunological imprinting: Understanding COVID-19. Immunity 2023, 56, 909–913. [Google Scholar] [CrossRef]
- Konyak, B.M.; Sharma, M.; Kharia, S.; Pandey, R.P.; Chang, C.-M. A Systematic Review on the Emergence of Omicron Variant and Recent Advancement in Therapies. Vaccines 2022, 10, 1468. [Google Scholar] [CrossRef]
- Chong, W.C.; Chellappan, D.K.; Shukla, S.D.; Peterson, G.M.; Patel, R.P.; Jha, N.K.; Eri, R.D.; Dua, K.; Tambuwala, M.M.; Shastri, M.D. An Appraisal of the Current Scenario in Vaccine Research for COVID-19. Viruses 2021, 13, 1397. [Google Scholar] [CrossRef]
- Krammer, F. The role of vaccines in the COVID-19 pandemic: what have we learned? Semin. Immunopathol. 2023, 1–18. [Google Scholar] [CrossRef]
- Marshall, C.L.B.; Kaplowitz, E.; Ibroci, E.; Chung, K.; Gigase, F.A.J.M.; Lieber, M.L.; Graziani, M.; Ohrn, S.B.; Lynch, J.; Castro, J.B.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Titer Levels in Pregnant Individuals After Infection, Vaccination, or Both. Obstetrics & Gynecology 2023, 141, 1199–1202. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.D.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; Mancini, A.; et al. COVID-19 Infection in Children and Infants: Current Status on Therapies and Vaccines. Children 2022, 9, 249. [Google Scholar] [CrossRef]
- Agolli, A.; Agolli, O.; Velazco, D.F.S.; Ahammed, R.; Patel, M.; Cardona-Guzman, J.; Garimella, R.; Rummaneethorn, N.; Bista, S.; Abreu, R.; et al. Fetal Complications in COVID-19 Infected Pregnant Woman: A Systematic Review and Meta-Analysis. Avicenna J. Med. 2021, 11, 200–209. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Burry, L.; Tabbara, N. Role of maternal COVID-19 vaccination in providing immunological protection to the newborn. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 42, 58–70. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Tan, K.; Li, R.; Huang, X.; Liu, Q. Outer Membrane Vesicles: Current Status and Future Direction of These Novel Vaccine Adjuvants. Front. Microbiol. 2018, 9, 783. [Google Scholar] [CrossRef]
- Portilho, A.I.; Correa, V.A.; Cirqueira, C.d.S.; De Gaspari, E. Intranasal and Intramuscular Immunization with Outer Membrane Vesicles from Serogroup C Meningococci Induced Functional Antibodies and Immunologic Memory. Immunol. Investig. 2022, 51, 2066–2085. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.B.; Prudencio, C.R.; De Gaspari, E. Experimental studies using OMV in a new platform of SARS-CoV-2 vaccines. Hum. Vaccines Immunother. 2021, 17, 2965–2968. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.B.; De Gaspari, E. Avidity assay to test functionality of anti-SARS-Cov-2 antibodies. Vaccine 2021, 39, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Lincopan, N.; Espíndola, N.M.; Vaz, A.J.; da Costa, M.H.B.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine 2009, 27, 5760–71. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-X.; Xie, Y.; Ye, Y.-P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef]
- Correa, V.A.; Portilho, A.I.; De Gaspari, E. Immunological Effects of Dimethyldioctadecylammonium Bromide and Saponin as Adjuvants for Outer Membrane Vesicles from Neisseria meningitidis. Diseases 2022, 10, 46. [Google Scholar] [CrossRef]
- De Almeida, A.F.; De Gaspari, E. Dioctadecyldimethylammonium bromide (DODAB-BF) as a new adjuvant for maternal-fetal immunization in mice against Neisseria meningitidis: evaluation of humoral response. Pathog. Dis. 2017, 76. [Google Scholar] [CrossRef]
- Santos, F.A.d.O.; Lincopan, N.; De Gaspari, E. Evaluation of intranasal and subcutaneous route of immunization in neonatal mice using DODAB-BF as adjuvant with outer membrane vesicles of Neisseria meningitis B. Immunobiology 2018, 223, 750–760. [Google Scholar] [CrossRef]
- Lima, G.G.; Portilho, A.I.; De Gaspari, E. Adjuvants to increase immunogenicity of SARS-CoV-2 RBD and support maternal–fetal transference of antibodies in mice. Pathog. Dis. 2022, 80. [Google Scholar] [CrossRef]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Tan, J.; Bhavsar, D.; Capuano, C.; Kirkpatrick, E.; et al. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef]
- da Costa, H.H.M.; Orts, D.J.B.; Moura, A.D.; Duarte-Neto, A.N.; Cirqueira, C.S.; Réssio, R.A.; Kanamura, C.T.; Miguita, K.; Ferreira, J.E.; Santos, R.T.M.; et al. RBD and Spike DNA-Based Immunization in Rabbits Elicited IgG Avidity Maturation and High Neutralizing Antibody Responses against SARS-CoV-2. Viruses 2023, 15, 555. [Google Scholar] [CrossRef] [PubMed]
- De Gaspari, E.N.; Zollinger, W.D. Expression of class 5 antigens by meningococcal strains obtained from patients in Brazil and evaluation of two new monoclonal antibodies. Braz. J. Infect. Dis. 2001, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Granoff, D.M.; Maslanka, S.E.; Carlone, G.M.; Plikaytis, B.D.; Santos, G.F.; Mokatrin, A.; Raff, H.V. A Modified Enzyme-Linked Immunosorbent Assay for Measurement of Antibody Responses to Meningococcal C Polysaccharide That Correlate with Bactericidal Responses. Clin. Diagn. Lab. Immunol. 1998, 5, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Chackerian, B.; Lowy, D.R.; Schiller, J.T. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J. Clin. Investig. 2001, 108, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Júnior, D.M.; Araújo, J.A.P.; Catelan, T.T.T.; de Souza, A.W.S.; Cruvinel, W.d.M.; Andrade, L.E.C.; da Silva, N.P. Sistema imunitário - parte II: fundamentos da resposta imunológica mediada por linfócitos T e B. Rev. Bras. de Reum. 2010, 50, 552–580. [Google Scholar] [CrossRef]
- Kober, C.; Manni, S.; Wolff, S.; Barnes, T.; Mukherjee, S.; Vogel, T.; Hoenig, L.; Vogel, P.; Hahn, A.; Gerlach, M.; et al. IgG3 and IgM Identified as Key to SARS-CoV-2 Neutralization in Convalescent Plasma Pools. PLOS ONE 2022, 17, e0262162. [Google Scholar] [CrossRef]
- Gasser, R.; Cloutier, M.; Prévost, J.; Fink, C.; Ducas. ; Ding, S.; Dussault, N.; Landry, P.; Tremblay, T.; Laforce-Lavoie, A.; et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep. 2021, 34, 108790. [Google Scholar] [CrossRef]
- Knies, A.; Ladage, D.; Braun, R.J.; Kimpel, J.; Schneider, M. Persistence of humoral response upon SARS-CoV-2 infection. Rev. Med Virol. 2021, 32, e2272. [Google Scholar] [CrossRef]
- Alharbi, N.K.; A Al-Tawfiq, J.; Alwehaibe, A.; Alenazi, M.W.; Almasoud, A.; Algaisi, A.; A Alhumaydhi, F.; Hashem, A.M.; Bosaeed, M.; A Alsagaby, S. Persistence of Anti-SARS-CoV-2 Spike IgG Antibodies Following COVID-19 Vaccines. Infect. Drug Resist. 2022, ume 15, 4127–4136. [Google Scholar] [CrossRef]
- Costa, C.; Migliore, E.; Galassi, C.; Scozzari, G.; Ciccone, G.; Coggiola, M.; Pira, E.; Scarmozzino, A.; La Valle, G.; Cassoni, P.; et al. Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers. Vaccines 2022, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Pirahmadi, S.; Zakeri, S.; Mehrizi, A.A.; Djadid, N.D.; Raz, A.-A.; Sani, J.J. Combining Monophosphoryl Lipid A (MPL), CpG Oligodeoxynucleotide (ODN), and QS-21 Adjuvants Induces Strong and Persistent Functional Antibodies and T Cell Responses against Cell-Traversal Protein for Ookinetes and Sporozoites (CelTOS) of Plasmodium falciparum in BALB/c Mice. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Tross, D.; Klinman, D.M. Effect of CpG Oligonucleotides on Vaccine-Induced B Cell Memory. J. Immunol. 2008, 181, 5785–5790. [Google Scholar] [CrossRef]
- Bauer, G. High avidity of vaccine-induced immunoglobulin G against SARS-CoV-2: potential relevance for protective humoral immunity. Explor. Immunol. 2022, 2, 133–156. [Google Scholar] [CrossRef]
- Webster, R.G. The immune response to influenza virus. 3. Changes in the avidity and specificity of early IgM and IgG antibodies.. 1968, 14, 39–52. [Google Scholar]
- Khatri, I.; Staal, F.J.T.; van Dongen, J.J.M. Blocking of the High-Affinity Interaction-Synapse Between SARS-CoV-2 Spike and Human ACE2 Proteins Likely Requires Multiple High-Affinity Antibodies: An Immune Perspective. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Kudriavtsev, A.V.; Vakhrusheva, A.V.; Novoseletsky, V.N.; Bozdaganyan, M.E.; Shaitan, K.V.; Kirpichnikov, M.P.; Sokolova, O.S. Immune Escape Associated with RBD Omicron Mutations and SARS-CoV-2 Evolution Dynamics. Viruses 2022, 14, 1603. [Google Scholar] [CrossRef]
- da Costa, C.H.S.; de Freitas, C.A.B.; Alves, C.N.; Lameira, J. Assessment of mutations on RBD in the Spike protein of SARS-CoV-2 Alpha, Delta and Omicron variants. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Hale, M.; Netland, J.; Chen, Y.; Thouvenel, C.D.; Smith, K.N.; Rich, L.M.; Vanderwall, E.R.; Miranda, M.C.; Eggenberger, J.; Hao, L.; et al. IgM antibodies derived from memory B cells are potent cross-variant neutralizers of SARS-CoV-2. J. Exp. Med. 2022, 219. [Google Scholar] [CrossRef]
- Wu, J.Y.; Gardner, B.H.; I Murphy, C.; Seals, J.R.; Kensil, C.R.; Recchia, J.; A Beltz, G.; Newman, G.W.; Newman, M.J. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J. Immunol. 1992, 148, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Maeda, D.L.N.F.; Batista, M.T.; Pereira, L.R.; Cintra, M.d.J.; Amorim, J.H.; Mathias-Santos, C.; Pereira, S.A.; Boscardin, S.B.; Silva, S.d.R.; Faquim-Mauro, E.L.; et al. Adjuvant-Mediated Epitope Specificity and Enhanced Neutralizing Activity of Antibodies Targeting Dengue Virus Envelope Protein. Front. Immunol. 2017, 8, 1175. [Google Scholar] [CrossRef]
- Khurana, S.; Chearwae, W.; Castellino, F.; Manischewitz, J.; King, L.R.; Honorkiewicz, A.; Rock, M.T.; Edwards, K.M.; Del Giudice, G.; Rappuoli, R.; et al. Vaccines with MF59 Adjuvant Expand the Antibody Repertoire to Target Protective Sites of Pandemic Avian H5N1 Influenza Virus. Sci. Transl. Med. 2010, 2, 15ra5–15ra5. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, E.; Ianni, E.; Frigimelica, E.; Petracca, R.; Galli, G.; Scorza, F.B.; Norais, N.; Laera, D.; Giusti, F.; Pierleoni, A.; et al. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xiao, Y.; Zhu, M.; Chen, Y.-H. HIV epitope-peptides in aluminum adjuvant induced high levels of epitope-specific antibodies. Int. Immunopharmacol. 2001, 1, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrands, I.; Agger, E.M.; Olsen, A.W.; Korsholm, K.S.; Andersen, C.S.; Jensen, K.T.; Andersen, P. Cationic Liposomes Containing Mycobacterial Lipids: a New Powerful Th1 Adjuvant System. Infect. Immun. 2005, 73, 5817–5826. [Google Scholar] [CrossRef]
- Lindblad, E.B.; Elhay, M.J.; Silva, R.; Appelberg, R.; Andersen, P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 1997, 65, 623–629. [Google Scholar] [CrossRef]
- Mayorga, O.; Muñoz, J.E.; Lincopan, N.; Teixeira, A.F.; Ferreira, L.C.S.; Travassos, L.R.; Taborda, C.P. The role of adjuvants in therapeutic protection against paracoccidioidomycosis after immunization with the P10 peptide. Front. Microbiol. 2012, 3, 154. [Google Scholar] [CrossRef]
- Klinguer-Hamour, C.; Libon, C.; Plotnicky-Gilquin, H.; Bussat, M.-C.; Revy, L.; Nguyen, T.; Bonnefoy, J.-Y.; Corvaı̈a, N.; Beck, A. DDA adjuvant induces a mixed Th1/Th2 immune response when associated with BBG2Na, a respiratory syncytial virus potential vaccine. Vaccine 2002, 20, 2743–2751. [Google Scholar] [CrossRef]
- Hjertner, B.; Bengtsson, T.; Morein, B.; Paulie, S.; Fossum, C. A novel adjuvant G3 induces both Th1 and Th2 related immune responses in mice after immunization with a trivalent inactivated split-virion influenza vaccine. Vaccine 2018, 36, 3340–3344. [Google Scholar] [CrossRef]
- Yu, H.; Worrall, L.J.; Berger, T.; Petric, M.; Lin, B.H.; Vuckovic, M.; Robb, C.S.; Le, Q.; Kenward, C.; Dai, C.; et al. Identification of an Optimized Receptor-Binding Domain Subunit Vaccine against SARS-CoV-2. J. Immunol. 2023, 211, 981–993. [Google Scholar] [CrossRef]
- Mekonnen, D.; Mengist, H.M.; Jin, T. SARS-CoV-2 subunit vaccine adjuvants and their signaling pathways. Expert Rev. Vaccines 2021, 21, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Algaissi, A.; Lai, C.-C.; Chang, C.-K.; Lin, J.-S.; Wang, Y.-S.; Chang, B.-H.; Chang, Y.-C.; Chen, W.-T.; Fan, Y.-Q.; et al. Subunit vaccines with a saponin-based adjuvant boost humoral and cellular immunity to MERS coronavirus. Vaccine 2023, 41, 3337–3346. [Google Scholar] [CrossRef]
- Morein, B.; Abusugra, I.; Blomqvist, G. Immunity in neonates. Veter- Immunol. Immunopathol. 2002, 87, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Baez, I.M.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age. New Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef]
- Dattner, I.; Goldberg, Y.; Katriel, G.; Yaari, R.; Gal, N.; Miron, Y.; Ziv, A.; Sheffer, R.; Hamo, Y.; Huppert, A. The role of children in the spread of COVID-19: Using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLOS Comput. Biol. 2021, 17, e1008559. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine 2003, 21, 3382–3388. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E.; et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19–Associated Hospitalization in Infants Aged <6 Months — 17 States, July 2021–January 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 264–270. [Google Scholar] [CrossRef]
- Food D, Administration, Vaccines, Committee RBPA. FDA briefing document: EUA amendment request for Pfizer-BioNTech COVID-19 vaccine for use in children 6 months through 4 years of age. 2022. Available from: https://www.fda.gov/media/159195/download. 1591.
- Wen, J.; Du, X.; Li, A.; Zhang, S.; Shen, S.; Zhang, Z.; Yang, L.; Sun, C.; Li, J.; Zhu, S. Dilemmas and options for COVID-19 vaccination in children. Ital. J. Pediatr. 2023, 49, 1–10. [Google Scholar] [CrossRef]
- DUNCAN JR, et al. Maternal antibody transfer to neonatal mice: enhancement by glucocorticoid administratio. Clin Diagn Lab Immunol 2006, 13, 970–973.
- Simister, N.E.; Story, C.M. Human placental Fc receptors and the transmission of antibodies from mother to fetus. J. Reprod. Immunol. 1997, 37, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, J.; Fontannaz, P.; de Tejada, B.M.; Othenin-Girard, V.; Chilin, A.; Lemaître, B.; Blanchard-Rohner, G.; Siegrist, C.-A.; Eberhardt, C.S. Influence of Timing of Maternal Pertussis Immunization on the Avidity of Transferred Antibodies in Term and Preterm Neonates. Clin. Infect. Dis. 2023, 77, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Cacciottolo, M.; Li, Y.; Nice, J.B.; LeClaire, M.J.; Twaddle, R.; Mora, C.L.; Adachi, S.Y.; Young, M.; Angeles, J.; Elliott, K.; et al. Nanograms of SARS-CoV-2 spike protein delivered by exosomes induce potent neutralization of both delta and omicron variants. PLOS ONE 2023, 18, e0290046. [Google Scholar] [CrossRef]
- Boelig, R.C.; Chaudhury, S.; Gromowski, G.D.; Mayer, S.; King, J.; Aghai, Z.H.; Bergmann-Leitner, E. Reduced maternal immunity and vertical transfer of immunity against SARS-CoV-2 variants of concern with COVID-19 exposure or initial vaccination in pregnancy. Front. Immunol. 2023, 14, 1216410. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





