Submitted:
21 September 2023
Posted:
25 September 2023
You are already at the latest version
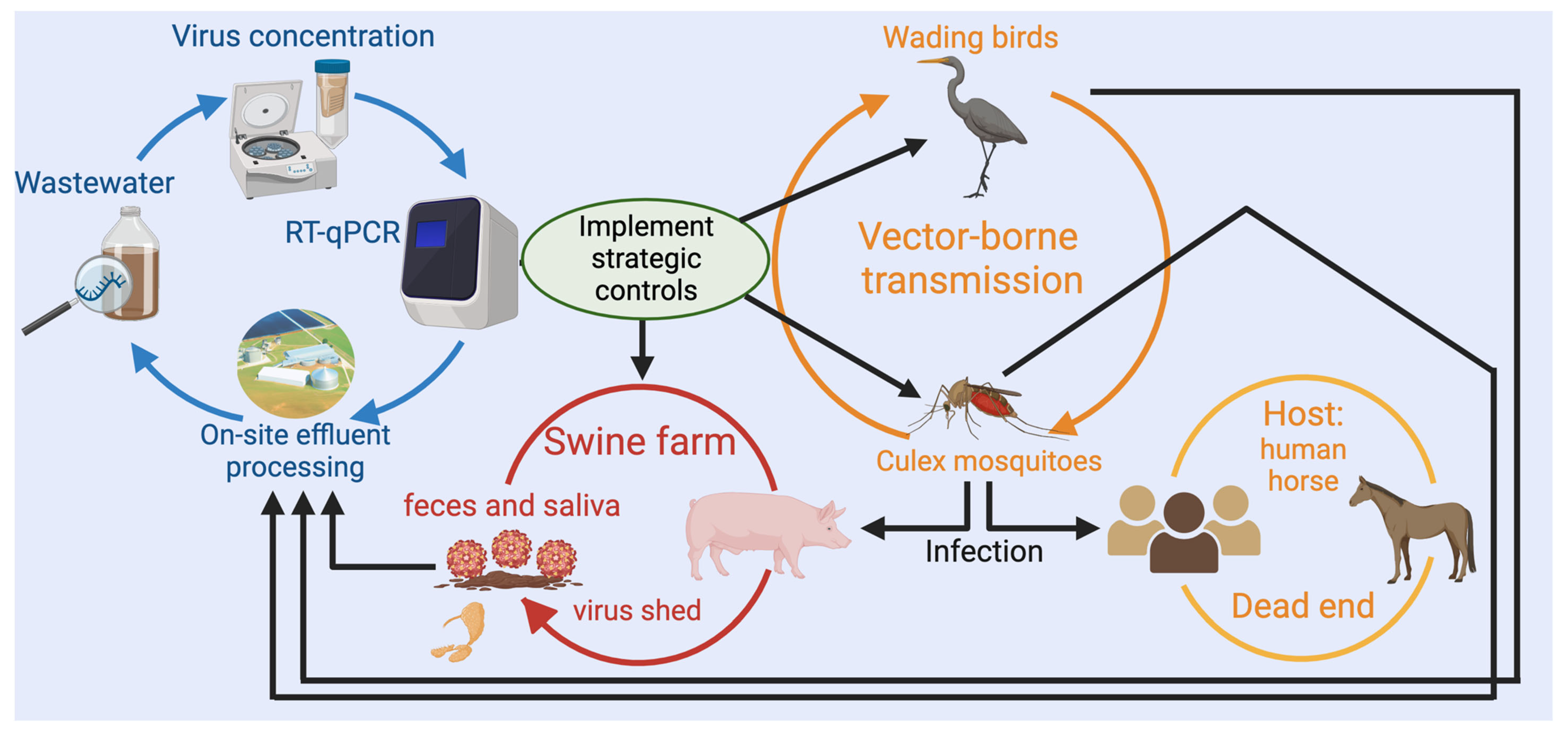
Abstract
Keywords:
References
- Ahmed, W.; Bertsch, P.M.; Bivins, A.; Bibby, K.; Farkas, K.; Gathercole, A.; Haramoto, E.; Gyawali, P.; Korajkic, A.; McMinn, B.R.; et al. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020, 739, 139960. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Tscharke, B.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Choi, P.; Clarke, L.; Dwyer, J.; Edson, J.; Nguyen, T.M.H.; et al. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: A temporal case study. Sci. Total Environ. 2021, 761, 144216. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; Food and Agriculture Organization of the United Nations: Rome, 2012. [Google Scholar]
- Allen, T.; Murray, K.A.; Zambrana-Torrelio, C.; Morse, S.S.; Rondinini, C.; Di Marco, M.; Breit, N.; Olival, K.J.; Daszak, P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Auerswald, H.; Ruget, A.-S.; Ladreyt, H.; In, S.; Mao, S.; Sorn, S.; Tum, S.; Duong, V.; Dussart, P.; Cappelle, J.; et al. Serological Evidence for Japanese Encephalitis and West Nile Virus Infections in Domestic Birds in Cambodia. Front. Veter- Sci. 2020, 7, 15. [Google Scholar] [CrossRef]
- Bernstein, A.S.; Ando, A.W.; Loch-Temzelides, T.; Vale, M.M.; Li, B.V.; Li, H.; Busch, J.; Chapman, C.A.; Kinnaird, M.; Nowak, K.; et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci. Adv. 2022, 8, eabl4183. [Google Scholar] [CrossRef]
- Bivins, A.; North, D.; Ahmad, A.; Ahmed, W.; Alm, E.; Been, F.; Bhattacharya, P.; Bijlsma, L.; Boehm, A.B.; Brown, J.; et al. Wastewater-Based Epidemiology: Global Collaborative to Maximize Contributions in the Fight Against COVID-19. Environ. Sci. Technol. 2020, 54, 7754–7757. [Google Scholar] [CrossRef]
- Carlson, C.J.; Albery, G.F.; Merow, C.; Trisos, C.H.; Zipfel, C.M.; Eskew, E.A.; Olival, K.J.; Ross, N.; Bansal, S. Climate change increases cross-species viral transmission risk. Nature 2022, 607, 555–562. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Fogel, R.; Limson, J. Overview of Diagnostic Methods, Disease Prevalence and Transmission of Mpox (Formerly Monkeypox) in Humans and Animal Reservoirs. Microorganisms 2023, 11, 1186. [Google Scholar] [CrossRef]
- Diamond, M.B.; Keshaviah, A.; Bento, A.I.; Conroy-Ben, O.; Driver, E.M.; Ensor, K.B.; Halden, R.U.; Hopkins, L.P.; Kuhn, K.G.; Moe, C.L.; et al. Wastewater surveillance of pathogens can inform public health responses. Nat. Med. 2022, 28, 1992–1995. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic spillover: Understanding basic aspects for better prevention. Genet. Mol. Biol. 2021, 44, e20200355. [Google Scholar] [CrossRef]
- Fan, V.Y.; Jamison, D.T.; Summers, L.H. Pandemic risk: How large are the expected losses? Bull. World Health Organ. 2018, 96, 129–134. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. 2023. Animal production. Available online: https://www.fao.org/animal-production/en/.
- Gibbs, E.P.J. The evolution of One Health: a decade of progress and challenges for the future. Veter- Rec. 2014, 174, 85–91. [Google Scholar] [CrossRef]
- Gilbert, W.; Thomas, L.; Coyne, L.; Rushton, J. Review: Mitigating the risks posed by intensification in livestock production: the examples of antimicrobial resistance and zoonoses. Animal 2021, 15, 100123. [Google Scholar] [CrossRef]
- Gubernot, D.M.; Boyer, B.L.; Moses, M.S. Animals as Early Detectors of Bioevents: Veterinary Tools and a Framework for Animal-Human Integrated Zoonotic Disease Surveillance. Public Heal. Rep. 2008, 123, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Hall-Mendelin, S.; Jansen, C.C.; Cheah, W.Y.; Montgomery, B.L.; Hall, R.A.; Ritchie, S.A.; Hurk, A.F.V.D. Culex annulirostris (Diptera: Culicidae) host feeding patterns and Japanese encephalitis virus ecology in northern Australia. J. Med Èntomol. 2012, 49, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Wahaab, A.; Nawaz, M.; Khan, S.; Nazir, J.; Liu, K.; Wei, J.; Ma, Z. Potential Role of Birds in Japanese Encephalitis Virus Zoonotic Transmission and Genotype Shift. Viruses 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Hassard, F.; Smith, T.R.; Boehm, A.B.; Nolan, S.; O'Mara, O.; Di Cesare, M.; Graham, D. Wastewater surveillance for rapid identification of infectious diseases in prisons. Lancet Microbe 2022, 3, e556–e557. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P.K. Livestock and global change: Emerging issues for sustainable food systems. Proc. Natl. Acad. Sci. 2013, 110, 20878–20881. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Colling, A.; Gurung, R.B.; Allen, J. The potential of diagnostic point-of-care tests (POCTs) for infectious and zoonotic animal diseases in developing countries: Technical, regulatory and sociocultural considerations. Transbound. Emerg. Dis. 2021, 68, 1835–1849. [Google Scholar] [CrossRef]
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. 2013, 110, 8399–8404. [Google Scholar] [CrossRef]
- Jori, F.; Hernandez-Jover, M.; Magouras, I.; Dürr, S.; Brookes, V.J. Wildlife–livestock interactions in animal production systems: what are the biosecurity and health implications? Anim. Front. 2021, 11, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Karesh, W.B.; Dobson, A.; Lloyd-Smith, J.O.; Lubroth, J.; Dixon, M.A.; Bennett, M.; Aldrich, S.; Harrington, T.; Formenty, P.; Loh, E.H.; et al. Ecology of zoonoses: Natural and unnatural histories. Lancet 2012, 380, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Ronquillo, N.; Belda-Ferre, P.; Alvarado, D.; Javidi, T.; Longhurst, C.A.; Knight, R. High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSystems 2021, 6, e00045–21. [Google Scholar] [CrossRef] [PubMed]
- Kilaru, P.; Hill, D.; Anderson, K.; Collins, M.B.; Green, H.; Kmush, B.L.; A Larsen, D. Wastewater Surveillance for Infectious Disease: A Systematic Review. Am. J. Epidemiology 2022, 192, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Ahmed, W.; Bibby, K.; Carducci, A.; Gerba, C.P.; Hamilton, K.A.; Haramoto, E.; Rose, J.B. SARS-CoV-2 in wastewater: State of the knowledge and research needs. Sci. Total Environ. 2020, 739, 139076. [Google Scholar] [CrossRef]
- Lord, J.S.; Gurley, E.S.; Pulliam, J.R.C. Rethinking Japanese Encephalitis Virus Transmission: A Framework for Implicating Host and Vector Species. PLoS Negl. Trop. Dis. 2015, 9, e0004074. [Google Scholar] [CrossRef]
- Lebov, J.; Grieger, K.; Womack, D.; Zaccaro, D.; Whitehead, N.; Kowalcyk, B.; MacDonald, P. A framework for One Health research. One Heal. 2017, 3, 44–50. [Google Scholar] [CrossRef]
- Lee, W.L.; Gu, X.; Armas, F.; Leifels, M.; Wu, F.; Chandra, F.; Chua, F.J.D.; Syenina, A.; Chen, H.; Cheng, D.; et al. Monitoring human arboviral diseases through wastewater surveillance: Challenges, progress and future opportunities. Water Res. 2022, 223, 118904. [Google Scholar] [CrossRef]
- Lee, W.L.; Gu, X.; Armas, F.; Leifels, M.; Wu, F.; Chandra, F.; Chua, F.J.D.; Syenina, A.; Chen, H.; Cheng, D.; et al. Monitoring human arboviral diseases through wastewater surveillance: Challenges, progress and future opportunities. Water Res. 2022, 223, 118904. [Google Scholar] [CrossRef]
- McMahon, B.J.; Morand, S.; Gray, J.S. Ecosystem change and zoonoses in the Anthropocene. Zoonoses Public Heal. 2018, 65, 755–765. [Google Scholar] [CrossRef]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Gao, T.; Ellehoj, E.; Li, Q.; Qiu, Y.; Maal-Bared, R.; Sikora, C.; Tipples, G.; Diggle, M.; Hinshaw, D.; et al. Wastewater-Based Surveillance Is an Effective Tool for Trending COVID-19 Prevalence in Communities: A Study of 10 Major Communities for 17 Months in Alberta. ACS ES&T Water 2022, 2, 2243–2254. [Google Scholar] [CrossRef]
- Perry, B.D.; Grace, D.; Sones, K. Current drivers and future directions of global livestock disease dynamics. Proc. Natl. Acad. Sci. 2011, 110, 20871–20877. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; levy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Adhikari, S.; Zhang, S.; Solomon, T.B.; Lipponen, A.; Islam, M.A.; Thakali, O.; Sangkham, S.; Shaheen, M.N.F.; Jiang, G.; Haramoto, E.; Mazumder, P.; Malla, B.; Kumar, M.; Pitkanen, T.; Sherchan, S.P. . Tracing COVID-19 trails in wastewater: A systematic review of SARS-CoV-2 Surveillance with viral variants. Water 2023, 15, 1018. [Google Scholar] [CrossRef]
- Toribio-Avedillo, D.; Gómez-Gómez, C.; Sala-Comorera, L.; Rodríguez-Rubio, L.; Carcereny, A.; García-Pedemonte, D.; Pintó, R.M.; Guix, S.; Galofré, B.; Bosch, A.; et al. Monitoring influenza and respiratory syncytial virus in wastewater. Beyond COVID-19. Sci. Total. Environ. 2023, 892, 164495–164495. [Google Scholar] [CrossRef]
- Toribio-Avedillo, D.; Gómez-Gómez, C.; Sala-Comorera, L.; Rodríguez-Rubio, L.; Carcereny, A.; García-Pedemonte, D.; Pintó, R.M.; Guix, S.; Galofré, B.; Bosch, A.; et al. Monitoring influenza and respiratory syncytial virus in wastewater. Beyond COVID-19. Sci. Total. Environ. 2023, 892, 164495–164495. [Google Scholar] [CrossRef]
- WHO. Expanded programme on immunization global eradication of poliomyelitis by the year 2000. Wkly. Epidemiol. Rec. 1988, 63, 161–162. [Google Scholar]
- Williams, D.T.; MacKenzie, J.S.; Bingham, J. ; 2019. Flaviviruses. In Diseases of Swine (eds J.J.; Zimmerman, L.A.; Karriker, A.; Ramirez, K.J.; Schwartz, G.W.; Stevenson and J.; Zhang).
- Wilson, W.J. Isolation of enteric Bacilli from sewage and water and its bearing on epidemiology. BMJ 1933, 2, 560–562. [Google Scholar] [CrossRef]
- World Bank. (2010). People, Pathogens and Our Planet, Vol 1: Towards a One Health Approach for Controlling Zoonotic Diseases. Report 50833-GLB.
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Calamari, D.; Bagnati, R.; Schiarea, S.; Fanelli, R. Cocaine in surface waters: a new evidence-based tool to monitor community drug abuse. Environ. Health 2005, 4, 14. [Google Scholar] [CrossRef]
| Viruses | Family | Genus | Genome type | Epidemiology | Source | Transmission | Mortality and morbidity | Hosts |
|---|---|---|---|---|---|---|---|---|
| African swine fever virus (ASFV) Non-zoonotic |
Asfarviridae | Asfivirus. | Enveloped DNA | Endemic in Africa, Europe, Asia and the Carribean | Blood, tissues, secretions and excretions of sick and dead animals | Direct and indirect contact between infected and susceptible animals; indirect transmission via contaminated fomites including trucks, feed, people, equipment and possibly some flies; swill feeding; Vector-borne transmission: soft ticks of the genus Ornithodoros (Africa) | Mortality rates up to 100% in domestic pigs | Domestic and wild Sus scrofa; warthogs (Phacochoerus spp.), bush pigs (Potamochoerus spp.) and giant forest hogs (Hylochoerus meinertzhageni); Ticks of belonging to the Ornithodoros moubata complex |
| Bluetongue virus (BTV) Non-zoonotic |
Reoviridae | Orbivirus | Non-enveloped dsRNA | Occurs on all continents except Antarctica. Endemic in many parts of the world, including Europe, Asia, Australia and North America | Infected Culicoides spp, blood, semen, placenta; | Vector-borne transmission: midges of the genus Culicoides | Mortality rates of 30-70% in susceptible sheep, with morbidity rates close to 100%; mortality rates up to 90% in wild deer and antelopes; cattle have often higher infection rates than sheep | Domestic and wild ruminants including sheep, goats, cattle, buffaloes, deer, most species of African antelope and other Artiodactyla such as camels |
| Dengue virus (DENV) Zoonotic |
Flaviviridae | Flavivirus | Enveloped ssRNA | Endemic in many parts of the world, especially in tropical parts of America, Africa, South-East Asia. | Infected mosquito vectors; humans and primates | Mosquito-borne transmission: mosquito of the genus Aedes | Many DENV infections are asymptomatic or produce only mild illness. In severe cases, dengue can be fatal | Humans are the main host. Infection also occurred in pigs, marsupials, bats, birds, horses, bovid, rodent and dogs |
| Foot-and-mouth disease virus (FMDV) |
Picornaviridae | Aphthovirus | Non-enveloped ssRNA | Endemic in parts of Asia, Africa, the Middle East and South America (sporadic outbreaks in free areas) | All secretions and excretions from acutely infected animals including expired air, saliva, milk, urine, feces, semen, fluid from FMD-associated vesicles, and in amniotic fluid and aborted fetuses in sheep | Direct and indirect contact between infected and susceptible animals; indirect transmission via contaminated fomites; airborne spread | Mortality rates of 1-5% in adult animals, and more than 20% in young animals. Morbidity can reach 100% in susceptible populations | Cloven-footed livestock species such as cattle, sheep and pigs. |
| Hepatitis E virus (HEV) Zoonotic |
Hepeviridae | Orthohepevirus | Non-enveloped ssRNA | Worldwide but most common in east and south Asia | Feces | Fecal-oral route. | Case fatality rates around 1-3% in human, and 10-30% among pregnant women; mortality rates around 1% in chicken | Pigs, wild boars, cows, deer, rabbits, chicken, camels, contaminated shellfish and humans |
| Influenza type A viruses (e.g., H1N1, H5N1, H7N9, H3N2) Zoonotic |
Orthomyxoviridae | Alphainfluenzavirus | Enveloped ssRNA | Endemic in all parts of the world | Saliva, nasal secretions and faeces of infected animals | Direct and indirect contact between infected animals; indirect transmission via contaminated fomites including feed, equipment and other materials; Airborne; droplet | Case fatality rates around 56% in human; mortality up to 90-100% in chicken for highly pathogenic subtypes | Wild aquatic birds, mammals. |
| Japanese encephalitis virus (JEV) Zoonotic |
Flaviviridae | Flavivirus | Enveloped ssRNA | Endemic in eastern, south-eastern, and southern Asian countries, and western India, western Pacific region including the eastern Indonesian archipelago, Papua New Guinea and Northern Australia | Infected mosquito vectors, water birds including the family Ardeidae (herons and egrets), pigs are an amplifying host | Vector-borne transmission cycle among mosquitoes (Culex spp.), birds and swine. Humans, horses and other species considered dead end hosts | Case morbidity in horses 1-1.4% but fatality rates in these cases from 5-15% to 30-40% in severe epizootics; human case morbidity 1% but case fatality rates can be up to 30%; morbidity and mortality rate near 0% in adult swine but can reach 100% in non-immune piglets infected in utero | Wild water birds of the family Ardeidae, pigs, human and horse (dead-end host) |
| Lumpy skin disease virus (LSDV) Non-zoonotic |
Poxviridae | Capripoxvirus | Enveloped DNA | Endemic in most African partscountries, parts of the Middle East, south-east Europe, the Balkans, Caucasus, Russian Federation and large parts of Asia | Skin nodules, scabs, crusts (contain relatively high amount of LDSV); blood, saliva, ocular, nasal discharge and semen of infected animals | Arthropod vector-borne transmission (mechanical): mosquitoes (e.g. Culex mirificens and Aedes natrionus), biting flies (e.g. Stomoxys calcitrans and Biomyia fasciata) and male ticks (Riphicephalus appendiculatus and Amblyomma hebraeum) |
Mortality rates of 1-5% and morbidity rates of 10-20% in cattle | Cattle (Bos indicus and B. taurus), water buffalo (Bubalus bubalis) |
| Porcine epidemic diarrhea virus (PEDV) Non-zoonotic |
Coronaviridae | Alphacoronavirus | Enveloped ssRNA | Endemic in Europe, large parts of Asia and the Americas | Faeces of infected animals | Direct transmission via faecal-oral route; indirect transmission via contaminated fomites including trucks, feed, equipment and people | Mortality rates less than 5 % in adult and fattening pigs, less than 10 % in piglets older than 10 days, up to 100 % in sucking piglets, morbidity rates up to 100% | Pigs |
| Porcine reproductive and respiratory syndrome virus (PRRSV) Non-zoonotic |
Arteriviridae |
Arterivirus | Enveloped ssRNA | Endemic in pig-raising areas of Europe, North America, parts of Asia | Body fluids and secretions of infected animals | Direct and indirect contact between infected and susceptible animals; indirect transmission via contaminated fomites including equipment and insects; transplacental; potential wind-borne |
Mortality rates of 10% in pregnant sows, 20% in finishers, 70% in nursery pigs and 100% in suckling pigs | Domestic pigs |
| Rift Valley fever virus (RVFV) Zoonotic |
Phenuiviridae | Phlebovirus | Enveloped ssRNA | Endemic mainly in many sub-Saharan African countries, Egypt, the Arabian Peninsula (South-west) and some Indian Ocean Islands. Usually presents in epizootic form following heavy rains/flooding | Eggs of infected mosquitoes, blood, secretions and tissues of infected animals | Arthropod-borne transmission, direct contact with the blood, secretions and tissue of infected animals; Airborne | Mortality rates of 70-100 % in young lambs and goats; 20-70% in sheep and calves; less than 10% in adult goats, cattle, buffalo, inapparent in pigs, dogs, cats, Asian monkeys, and humans; influenza-like syndrome in humans | Domestic and wild animals including cattle, sheep, goats, monkeys, wild ruminants, antelope, domestic carnivores. Humans are highly susceptible but dead end hosts |
| West Nile virus (WNV) Zoonotic |
Flaviviridae | Flavivirus | Enveloped ssRNA | Endemic in many parts of the world, particularly in Africa, Middle East, West Asia, Australia, and parts of Europe | Infected mosquito vectors; bird reservoirs | Vector-borne transmission cycle between mosquitoes (genus Culex) and birds. Mammals including horses are dead-end hosts | Case fatality rates of 23-57% in equids, close to 100% in mammals other than horses; mortality rates around 20-60% in geese; typically mild symptoms in human and horse, but can cause neurological disease. | Birds, reptiles, amphibians, mammals and marsupials, mosquitoes, and ticks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





