1. Introduction
Type 2 diabetes and hypertension are acute health problems of today’s world. The percentage of people with type 2 diabetes is continuously increasing and worldwide there are at least 422 million people with diabetes and 1.28 billion adults with hypertension according to the WHO. Many scientists are working to find the primary causes which initiate the development of insulin resistance, a starting condition of type 2 diabetes. Using various inbred mouse strains, Nelson et al. determined the effects of diet on the development of insulin resistance primarily in muscle, liver and white adipose tissue [
1] and Mejhert and Ryden commented on this report [
2]. Petersen and Shulman published a comprehensive and detailed review on the “mechanism of insulin action and insulin resistance” and came to the conclusion that the “sheer complexity of biological systems means that any effort to understand insulin resistance with a unified, succinct and straightforward model may be a fool’s errand” [
3]. Galicia-Garcia et al. [
4] proposed that type 2 diabetes is caused by a combination of two primary factors, namely a defective insulin secretion of the pancreatic β-cells and the inability of insulin sensitive tissues to respond appropriately to insulin, and they concluded that there is still a long way to go to fully understand insulin resistance.
The mechanism of insulin action, which is a prerequisite to determine defects in the action of insulin, appears to be nearly completely solved: Insulin binds to its receptor, whose intrinsic tyrosine kinase is in this way activated; this kinase auto-phosphorylates itself and then phosphorylates primarily the insulin receptor substrates IRS1-4 and several Shc proteins [
5,
6]. The IRS proteins are suggested to regulate metabolism, whereas Shc proteins appear to be involved in the regulation of proliferation by insulin. The IRS proteins are considered to be docking proteins to which various enzymes bind via Src-homology-2 (SH-2) domains and send signals by phosphorylation cascades further into a cell. Additionally, the IRS proteins are inactivated by serine/threonine phosphorylation by several different protein ser/thr kinases [
5].
Earl Sutherland articulated many years ago that a decrease in the strength of the insulin signal will increase the strength of the counterregulatory signal leading to dominance of the regulations triggered by cyclic AMP [
7]. This view has not been adequately pursued. A characteristic of insulin resistance is that it progresses slowly and gradually for reasons not fully understood at the present time. Inhibition of single proteins/enzymes such as the IRS proteins cannot explain this slow progression, though they play a crucial role in the signal-transduction. Current thinking is that protein phosphorylation cascades are of primary importance [
5,
6], but fails to consider that insulin antagonizes glucagon action. Insulin rapidly reverses cyclic AMP-triggered protein ser/thr phosphorylation. The key player in this scenario is the natural cyclic AMP antagonist, prostaglandyl-inositol cyclic phosphate (cyclic PIP) [
8,
9]. The synthesis of cyclic PIP is stimulated by insulin and also noradrenaline. In type 2 diabetic and also hypertensive rodents a reduced cyclic PIP synthesis was found [
8]. The effect of decreasing cyclic PIP synthesis and the consequently increasing cyclic AMP synthesis on the development of insulin resistance is the topic of this report.
3. The Natural Cyclic AMP Antagonist Cyclic PIP
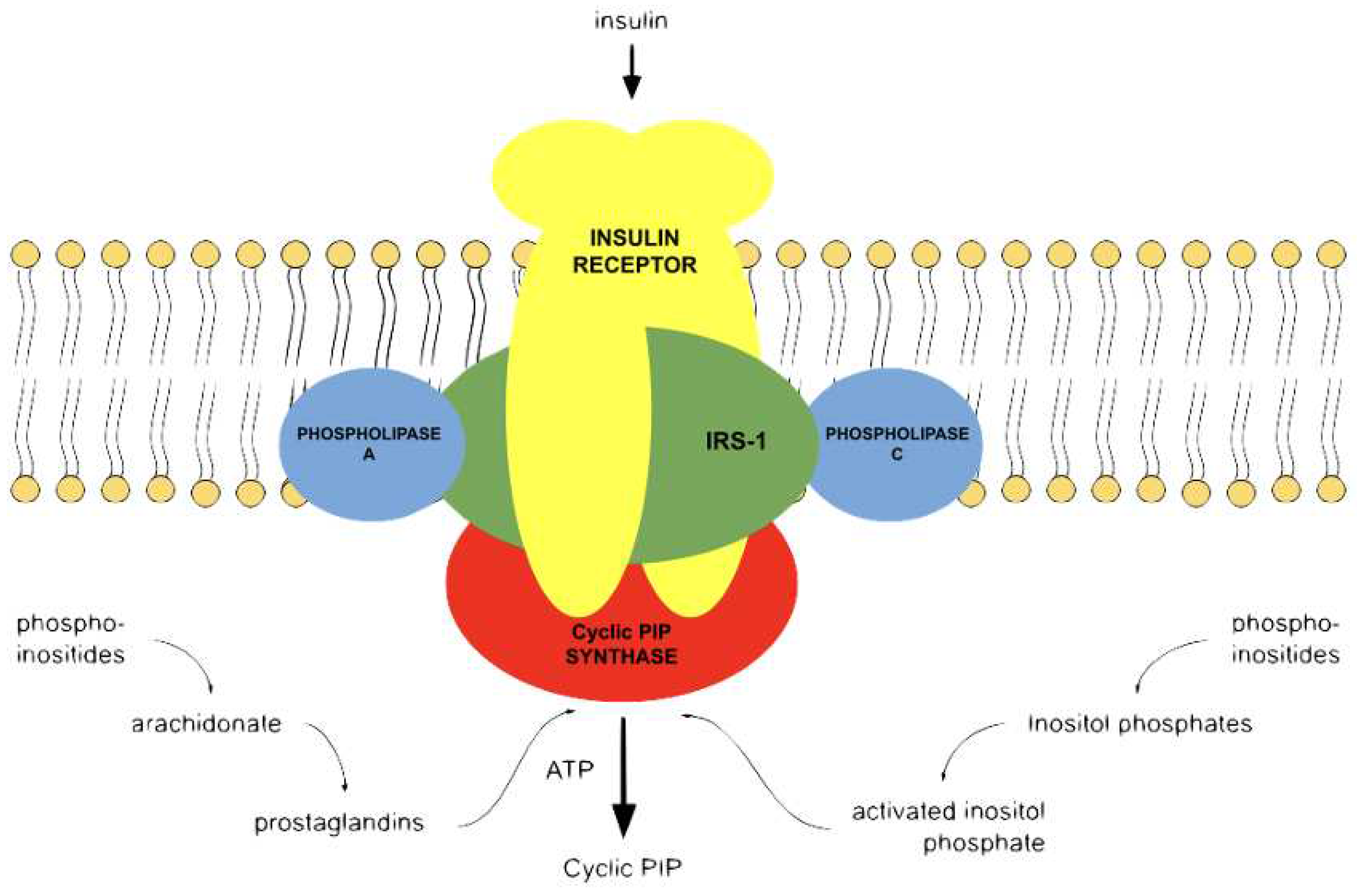
The search for an antagonist to cyclic AMP started in the laboratory of the late Earl Sutherland. Cyclic PIP was primarily isolated from rat livers, which were extra-corporally perfused with buffer and stimulated with noradrenaline or insulin, homogenized within 1 to 3 minutes then put under denaturing conditions to minimize the enzymatic degradation of cyclic PIP. From 2 kg of rat livers approximately 60 g of water-soluble, low molecular compounds were obtained, which also contained cyclic PIP. A rough calculation indicated that 500.000 to 1.000.000 molecules of this extract contain 1 molecule of cyclic PIP. But more challenging was the finding that cyclic PIP is one of the most labile molecules of this extract. It decays by 80% within 30 minutes held at an ionic strength comparable to a 0.5 molar sodium chloride solution, approximately a 3-fold concentrated physiological saline solution. The labilities of cyclic PIP are connected to the tension of the 5-ring phosphodiester, which is the most labile bond of cyclic PIP, as well as the allyl-ether bond combining two secondary alcohols and the beta-keto-hydroxy structure of the C5-ring of prostaglandin E (PGE). Chemically it is O-(prostaglandyl E)-(15-4′) (
myo-inositol 1′:2′-cyclic phosphate) [
8]. It is biosynthesized from PGE and ‘activated inositol phosphate’ by cyclic PIP synthase [
8], which is active in a tyrosine phosphorylated form [
13]. The synthesis of cyclic PIP is also stimulated by adrenergic α
1- and α
2-receptors [
14]. This result contradicts the present view on the action of adrenergic α
1- and α
2-receptor action, as discussed in [
14,
15]. The adrenergic alpha-receptors are transmembrane, G protein-coupled receptors, thus cyclic PIP synthase should be activated also by a G protein [
13]. It is not known whether there are two different variants of cyclic PIP synthase, but it is known that the biosynthesis of the substrates for cyclic PIP synthesis involves phospholipase A2 and phospholipase C, both of which are activated by both these modes of activation as summarized in [
15]. The synthesis of cyclic AMP from ATP is a one-step reaction and ATP is generally always present in a high enough amount in all cells to warrant maximal synthesis of cyclic AMP. In contrast, the biosynthesis of cyclic PIP needs at least five reaction steps (
Figure 1) and both substrates are obtained from membrane-bound lipids [
15].
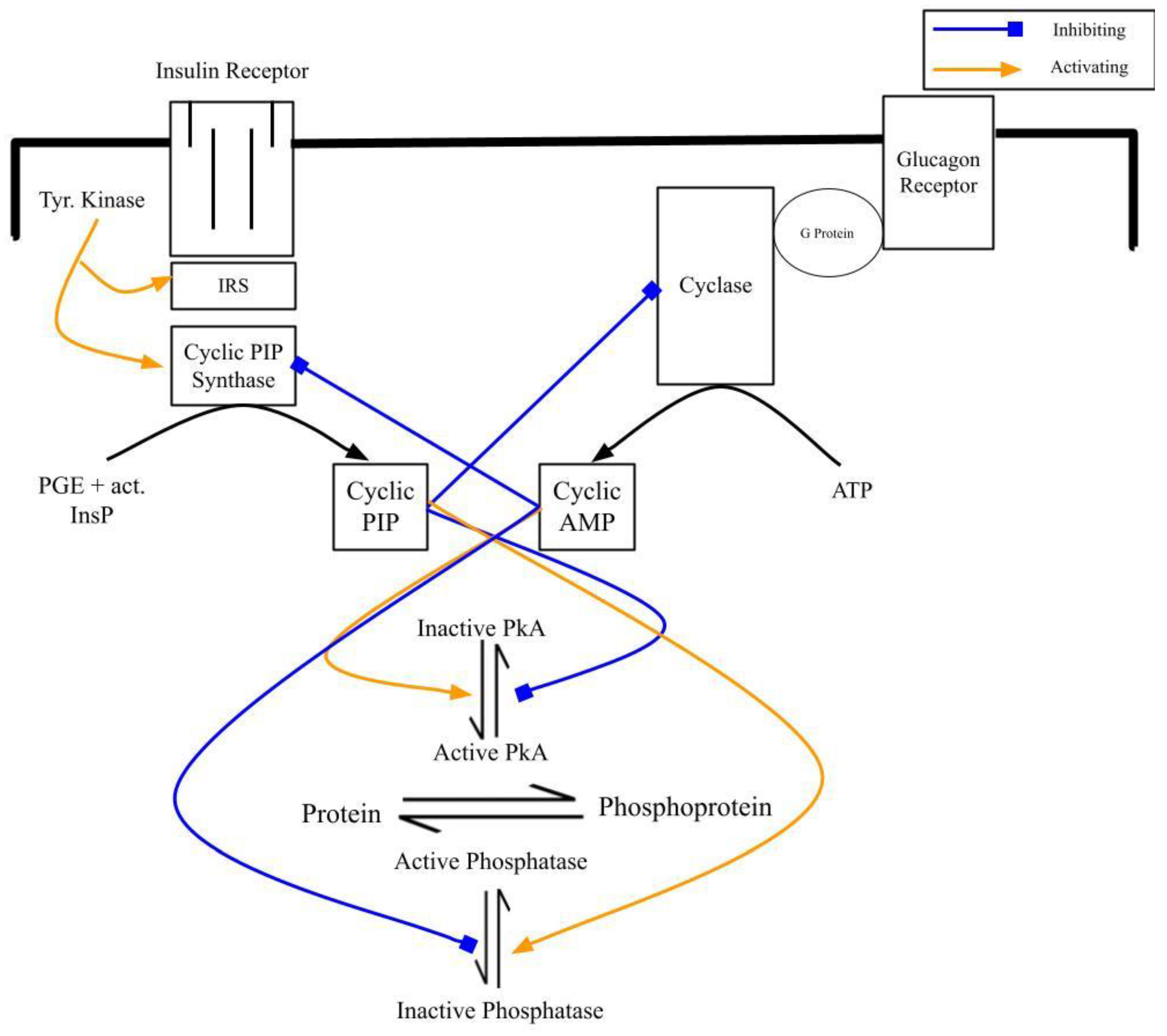
The primary action of cyclic AMP is to activate cyclic AMP-dependent protein kinase (PKA), whereas cyclic PIP inhibits PKA. The PKA is activated by increasing concentrations of cyclic AMP (10
-8 to 10
-6 molar) at least 10-fold. Addition of increasing concentrations of cyclic PIP leads to increasing inhibition of basal and cyclic AMP-activated PKA. For an equal percentage of inhibition of basal and cyclic AMP-activated PKA approximately 4-times more cyclic PIP is needed in case of the cyclic AMP-activated kinase [
14,
16]. The two regulators, cyclic AMP and cyclic PIP, perfectly regulate the activity of PKA. It was to assume that protein ser/thr phosphatase, the counter-regulatory enzyme to PKA, would also be regulated by these two compounds. The phosphatase is a rather labile enzyme. Therefore, scientists decided to isolate and characterize the catalytic subunits of this class of enzymes [
17]. For this reason, the holoenzyme of protein ser/thr phosphatase was partly purified. The obtained enzyme has a slightly higher molecular weight than PKA and it is 7-fold activated by cyclic PIP and completely inhibited by cyclic AMP in the physiological action range [
8]. As was the case with cyclic AMP [
10], further modes of action of cyclic PIP are likely to be found in the coming years as chemical synthesis of cyclic PIP allows additional studies.
5. Illnesses Connected with Decreased Synthesis of Cyclic PIP
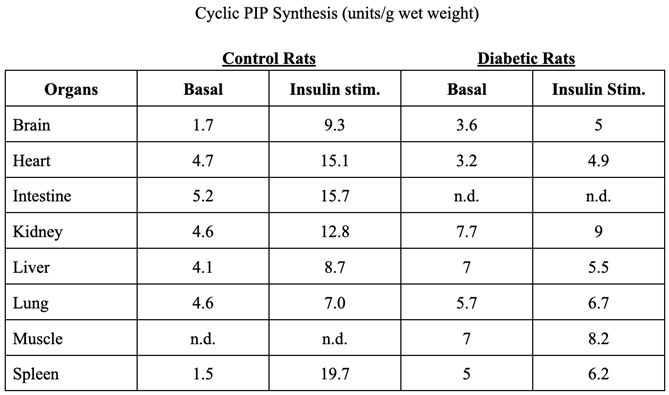
The stimulation of cyclic PIP synthesis decreases in low dose streptozotocin diabetic rats [
18,
23] and additionally, streptozotocin-induced type 2 diabetes in rats can be improved by feeding arachidonic acid [
24], the substrate for prostaglandin and cyclic PIP synthesis. Furthermore, inhibition of cyclic PIP synthesis by non-steroidal anti-inflammatory drugs (NSAIDs) leads to a metabolic state of the treated rats, which is comparable to type 2 diabetes at the onset of the illness [
21]. Glucose tolerance tests showed that in these treated rats the blood insulin levels increased at least threefold higher as compared to the untreated control rats. Cyclic PIP inhibits the release of insulin in accordance with insulin’s action. A reduced synthesis of cyclic PIP leads to a decreasing inhibition of insulin secretion from the pancreatic β-cells and thus to increased blood insulin levels [
21]. Additionally, these animals had 50% higher blood glucose levels, which declined slower in comparison with the values from untreated control rats. Accordingly, liver and muscle of these treated rats had highly reduced glycogen contents. These results correlate with the stimulation of glucose uptake by cyclic PIP in adipocytes [
21].
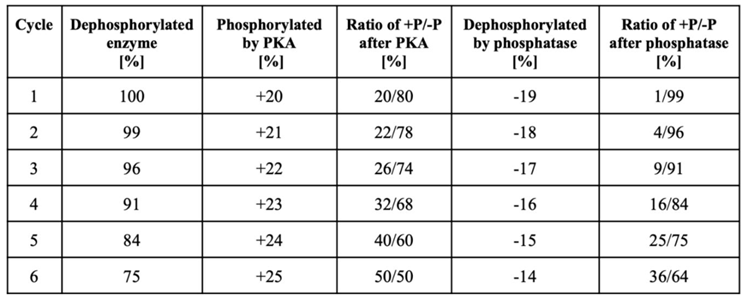
Why and how does decreased stimulation of cyclic PIP synthesis cause insulin resistance? In the introduction, some present views on insulin resistance were mentioned. These reports describe metabolic conditions, which show the extent of derailment of metabolism in the course of the development of type 2 diabetes. However, the events that initiated the derailment have not been identified and are mostly not discussed. In particular, the action of cyclic AMP and the existence and action of cyclic PIP are rarely considered. But it has been recognized that many different pathways are derailed in the case of the development of insulin resistance [
1,
3,
4,
25]. However, since insulin triggers a multitude of regulations in all the different organs of a body, one could realize that a single, essential derailment of insulin’s signal transduction, such as the gradual loss of stimulation of cyclic PIP synthase will result in a gradual decrease of all these many regulations stimulated by insulin. The simplified model-calculation of
Table 2 may visualize how the regulation of the metabolism can derail as result of a decreasing stimulation of cyclic PIP synthesis. Interconvertible enzymes are at the start 100% in the de-phospho-form and cyclic AMP-activated PKA converts 20% of these enzymes to the phospho-form. On a successive insulin stimulation, because of the lower activation of cyclic PIP synthase, the protein phosphatase is less active and only 19% of the phosphorylated enzymes are dephosphorylated, and 1% remain in the phospho-form. On a second ‘phosphorylation cycle’ the kinase is 1% more active and the protein phosphatase accordingly 1% less active because of the mutual inhibition of the two regulators and the increasing dominance of cyclic AMP (as discussed above). The result is that 4% of the enzymes remain after this second ‘phosphorylation cycle’ in the phospho-form and after the 6th ‘phosphorylation cycle’ 36% of the enzymes remain in the phospho-form. This calculation illustrates that the regulation of the metabolism will gradually and consistently get more and more out of balance and continuously increasing amounts of interconvertible enzymes will stay in the phosphorylated from. Thus, catabolic regulations will dominate more and more and anabolic regulations will decrease comparably. Certainly, in reality this will not be a straight forward, linear development, because parameters such as time and concentrations of key stimulators will vary. Finally, the magnitude of the activity-changes will be much smaller than in this brief model of
Table 2, where an arbitrary 1% change was applied, and the progress of the derailment will accordingly be slower. Regardless of these details, this development will progress comparably to a temperature curve, but slowly and continuously over prolonged times, until metabolism is increasingly stuck in the catabolic state.
As mentioned in the introduction, Galicia-Garcia et al. recently concluded that type 2 diabetes is caused by a defective insulin secretion and an inability of insulin responsive tissues to respond to insulin [
4]. It could have been recognized that these defects are related to the decreasing action of cyclic PIP. Firstly, cyclic PIP inhibits the release of insulin from pancreatic β-cells [
8] and in case of a vanishing stimulation of cyclic PIP synthesis the shut off of insulin’s secretion seizes and the resulting continuous excretion of insulin will gradually lead to an exhaustion of the pancreatic β-cells [
26]. Secondly, all organs/cells of a body are responsive to insulin and not just skeletal muscles, liver and white adipose cells, and, so far as cyclic PIP is the intracellular executor of many actions of insulin [
8], these functions will vanish in case of a decreasing cyclic PIP synthesis.
What are reasons which cause the decreasing stimulation of cyclic PIP synthesis? 1) One possibility is that needed substrates can be limiting. This problem should play a minor role in the synthesis of ‘activated inositol phosphate’, since generally there should be no deficit of
myo-inositol intake by food. However, an increasing number of reports show that polycystic ovarian syndrome, gestational diabetes mellitus and metabolic syndrome can be improved by increased intake of inositol [
27]. Since
myo-inositol is a structural component of cyclic PIP, one may ask if this could correlate with an improved synthesis of cyclic PIP, however, presently it is not known which defect in the path from myo-inositol via phosphatidylinositol to activated inositol phosphate can be improved by increased intake of
myo-inositol. 2) Substrate deficiency will more likely play a role in case of the synthesis of prostaglandins. The supply of lipids containing polyunsaturated fatty acids as omega-6 fatty acids can be limiting, especially since humans cannot synthesize these acids themselves and are dependent on their sources of food. Along these lines, pain relievers of the NSAID-family inhibit the biosynthesis of prostaglandins which impair the synthesis of cyclic PIP [
21], and more recently was reported that streptozotocin-induced type 2 diabetes can be improved by support with arachidonic acid [
24]. 3) Too many humans have high stress levels that result from problems of daily life such as too long exposures to too much noise and to challenges at work [
28], causing elevated cyclic AMP levels [
29]. 4) Another consideration is that regulatory mechanisms decline with aging. Though not finally proven, key hormones such as dehydroepiandrosterone, growth hormone, insulin-like growth factor-1 and testosterone appear to be involved in aging and in insulin action [
30]. Presently, the mechanisms of action of these hormones are still under investigation. Pataky, Young and Nair recommend in their review on aging that caution be taken in treatment with these hormones, since too many negative side effects of these hormonal replacements could outweigh the positive effects. They suggest that, presently, it is better to maximally apply physical exercise and calorie restrictions, since this way of lifestyle has positive effects on aging and on insulin sensitivity and has no negative side effects, though the mechanisms of action of lifestyle-changes are still largely unknown. These hormones start to decrease at around the age of 30 by around 1 to 2% annually [
30]. This could be a further factor affecting the slow development of type 2 diabetes over many years. Unfortunately, not enough is known about the biochemical mechanism of action of these hormones and how their decrease might lead to type 2 diabetes and hypertension and which one of these hormones has an effect on the stimulatory mechanism of cyclic PIP synthesis.
In summary, the development of an imbalance in the mutual inhibition of the synthesis of cyclic PIP and cyclic AMP, as outlined above (
Figure 2), will lead to a derailment in the regulation of metabolism. There appear to be compensatory mechanisms, which may slow down this derailment of the metabolism. Matching with cyclic PIP action, insulin inhibits skeletal muscle PKA as shown, for instance in healthy Rhesus monkeys [
31]. Diabetes, apart from the disrupted insulin signaling, moreover leads to a defective signaling of adrenergic β-receptors. In the focus stays the not adequately responsive PKA [
32,
33,
34]. The decreasing cyclic PIP synthesis leads initially to increasing cyclic AMP levels, whose actions appear to be subsequently opposed by an unresponsive PKA in a, presently, not finally discovered way. If such a down-regulation can improve diabetic conditions or if an unresponsive PKA makes the treatment of diabetes more complicated has to be found out.
There are reports that type-2 diabetes is curable through lifestyle changes in diet and exercise. Certainly, there are environmental conditions which can be altered to improve the metabolic situation. However, other factors such as the aging process need to be better understood. Of particular note here are the four hormones discussed to have effects on aging and insulin action. Such studies would lead to substantial progress in understanding insulin resistance. This report discusses only the effects of decreasing stimulation of cyclic PIP synthesis with respect to the regulation of the equilibrium between the de-phospho- and phospho-form of interconvertible enzymes. When in the coming years further regulatory properties of cyclic PIP are better characterized, then further aspects of the development of insulin resistance may be found.