Introduction
Peripheral artery disease (PAD) is a progressive disease caused mainly by atherosclerosis and characterized by stenosis and/or occlusion of the arteries, with the lower extremities more commonly affected than the upper extremities.1 End-stage PAD often presents with chronic limb-threatening ischemia (CLTI), defined by the presence of ischemic rest pain and severe tissue loss such as ulcers or gangrene, according to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II 2007).2 An all-cause mortality rate of 22% has been reported within 12 months of the diagnosis, related mostly to cardiovascular and peripheral complications (i.e., infections).3−5
First-line treatment of CLTI relies on lower limb revascularization, either through endovascular techniques or surgical bypass.6 According to two published meta-analyses, the rate of limb salvage was similar between the two aforementioned approaches.7,8 In patients with multiple comorbidities or a low chance of successful revascularization due to advanced disease in the tibial arteries, pedal arteries, or small arteries of the foot (otherwise known as “desert foot”), there are often no options available for successful revascularization, leading to primary amputation being considered the only solution. Patients with small artery disease (SAD), including those with diabetes, end-stage renal disease, and thromboangiitis obliterans, are estimated to account for around 20% of patients with PAD, and their prognosis, including 20% mortality at 6 months, is noted to be worse than those with less severe disease.2,9,10
Alternative treatment options for patients with no-option CLTI have been described, including spinal cord stimulation, lumbar sympathectomy, intermittent pneumatic compression, hyperbaric oxygen therapy, stem cell therapy, and prostanoid therapy, though the outcomes have been poor in trials of these approaches.11,12 For this group of patients, deep venous arterialization (DVA) has been reported as a promising alternative to amputation.
Interpreting DVA
The notion of DVA is that perfusion of the peripheral ischemic tissues and the plantar venous arch is achieved through arterialization of the disease-free venous bed by creating an arterial-deep venous shunt that enables wound healing.12 Femoral arteriovenous anastomosis was first described in dogs in 1881 and in humans in 1894 by Francois-Frank, with poor rates of wound healing and limb salvage and several complications.13,14 Afterwards, several studies were published investigating the technique, but after 1916, interest in the field diminished.15 Later in 1977, the modern version of the procedure was introduced by Sheil, who described the anastomosis of an arterialized great saphenous vein to the dorsal venous arch of the foot.16 Potential mechanisms of action for this procedure include reversed blood flow in the arterioles and capillaries permitting tissue nutrition and neovascularization stimulated by angiogenic factors leading to a remodeling of the vascular distribution system of the foot.17−19
There are various ways in which DVA can be performed, including surgical, endovascular, or hybrid approaches. Surgical DVA was pioneered by Herb Dardik. Proximal anastomosis over the great saphenous vein (GSV) and the most distal patent artery (popliteal artery or superficial femoral artery) is performed.20 Limb salvage for surgical DVA has been reported in the literature to be 70%.21 The evolution of endovascular technology has allowed for the development of percutaneous approaches, and interest in this approach has increased as this less invasive approach reduces the burden on the multiple comorbidities that are often present in patients with no-option CLTI.
The purpose of this review is to present the technique of DVA, focused on its percutaneous approaches, analyse how it is performed, and address all pertinent issues by exploring the most up-to-date available data.
Percutaneous DVA (pDVA)
Benefits of pDVA
The lack of options for end-stage CLTI patients highlights pDVA as a last option for limb salvage when amputation seems to be the only option remaining. The major advantage of this approach is that it offers a minimally invasive option for patients with adequate endoluminal access. This in turn leads to lower periprocedural risks and the absence of wound creation, which is additionally important in an already ischemic leg.22
Adverse effects and Disadvantages of pDVA
As with any percutaneous catheterization, possible adverse effects of pDVA include vascular complications at the access site (i.e., bleeding events, hematoma), arterial and venous thromboembolic events (myocardial infarction, stroke, pulmonary embolism, etc.), contrast-induced nephropathy, or renal failure.22 Apart from this, stent placement between the arterial and venous systems may lead to complications such as thrombosis and circuit stenosis. Also, if using prolonged balloon inflation to disrupt valves, valve effacement may not be as successful as in open approaches and those approaches that utilize a valvulotome.14
The advantages and adverse effects of pDVA are summarized in
Table 1.
pDVA Technique
Different methods are used to perform pDVA, but in each method, there are three important steps: firstly, formation of an arterio-venous (AV) fistula; secondly, disruption of venous valves so that flow reversal is possible; and thirdly, prevention of shunting of arterial blood through the AV fistula and other interconnecting veins. For the creation of an arterio-venous (AV) fistula, re-entry devices can be used as well as catheters, techniques involving snares (venous arterialization simplified technique; VAST), or double punctures (AV spear).23−25 However, up to 20% of these procedures can fail because of the inability to create the arteriovenous (AV) communication. For instance, when the vessel wall is heavily calcified, the wire tip or needle cannot penetrate when a penetration wire or reentry device is used.26,27 The LimFlow system (LimFlow SA, Paris, France) is currently the only registered device with which a percutaneous DVA procedure can be performed in its entirety, overcoming the challenge of AV fistula creation. However, it may increase the overall procedure cost, and regulatory approval is pending in several markets.28
Selection Criteria for pDVA Intervention
DVA is performed on Rutherford class 5 or 6 patients with absence of an evident distal target vessel for bypass or endovascular intervention and at least one patent tibial artery in the proximal segment.14 As reported by Kum et al., general factors, angiographic factors, and wound-related factors should be the three focus areas in order to select patients suitable to undergo pDVA.29 In particular, concerning general factors, patients with poor cardiac function, pre-existing coronary lesions, and high output fistulas for dialysis access should be excluded. The same is true for patients with deep venous thrombosis (DVT). With regards to angiographic factors, it is important to ensure the suitability of crossing vessels in order to optimize inflow. This means that several anatomical factors, such as the presence of possible angulation and fascia, should be considered and evaluated. With respect to wound factors, patients with acute limb ischemia, systemic sepsis, and wounds around the distal retrograde access site should be considered unsuitable.
DVA Techniques
Several described methodologies for deep vein arterialization have been described in the literature, though there has not been much standardization of procedural steps outside of the LimFlow trials. Techniques include a combination of open surgical, hybrid, and fully percutaneous approaches. Results across all reports have been promising, demonstrating the viability of the arterialization of deep veins for restoring blood flow to the lower limb and potentially saving it from major amputation. Open surgical DVA is achieved by pairing a surgical arteriovenous bypass with the physical effacement of the valves of the pedal loop vein. This technique has been shown to have comparable patency and limb salvage rates as surgical pedal bypass.30 Hybrid superficial venous arterialization, involves making an in-situ open surgical anastomosis from the Greater Saphenous Vein to the Popliteal Artery followed by valve lysis and distal endovascular focalization of flow.31,32 Additionally, a single-stage approach has been described which involves open bypass with a stent to the venous outflow followed by percutaneous valve lysis.33 Finally, the fully percutaneous approach utilizes tibial arteries as the donor vessel to create an arteriovenous crossing into the tibial vein, which is lined with a covered stent to focalize blood flow into the pedal veins, the valves of which are effaced percutaneously. pDVA can be performed using off-the-shelf devices or the proprietary LimFlow System, which is commercially available in Europe and the United States.
Benefits of LimFlow System Purpose-Built Devices
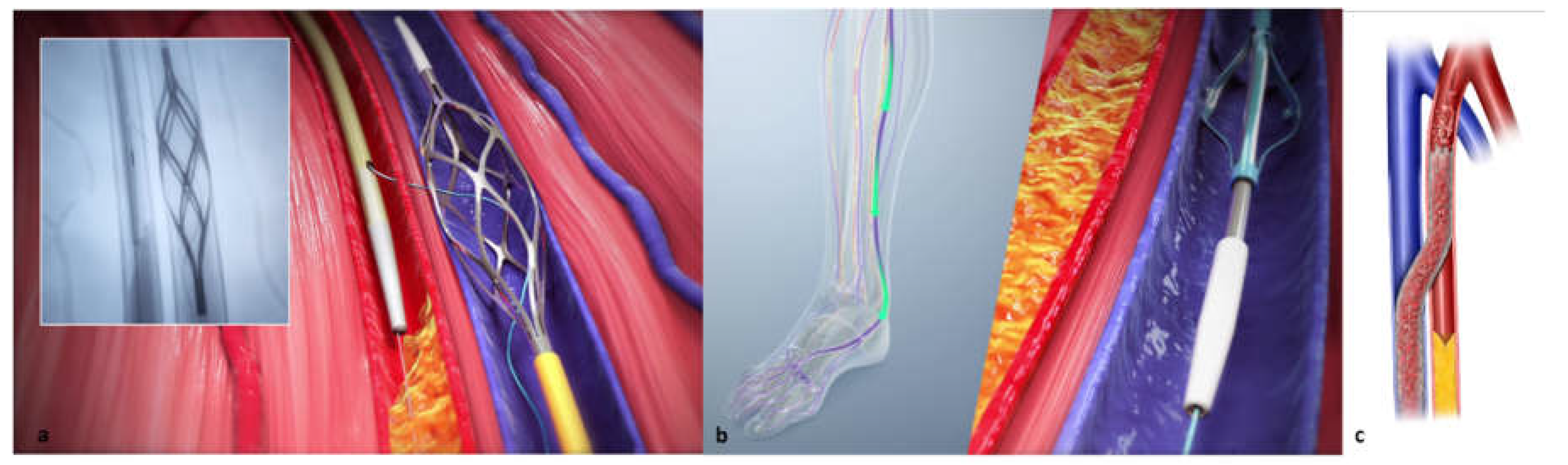
The LimFlow System consists of a series of purpose-built devices, including crossing catheters for the creation of an arteriovenous fistula, a retrograde valvulotome, and tapered and straight stent grafts. The arterial crossing catheter has a needle with a 10mm reach that is deployed through the arterial wall into a 6mm self-expanding nitinol basket deployed in the paired vein. This radiopaque target allows for easy AV alignment and wire capture for the arteriovenous crossing. The retrograde valvulotome lyses the valves in veins below the crossover point, down into the pedal venous loop, allowing forward blood flow. Utilizing the valvulotome reduces the trauma to the vessel wall, avoiding trauma-induced stenosis in the outflow veins that can occur if using a balloon to efface the valves.29 The tapered crossing stent allows for optimal sizing for both the artery and vein, and the straight extension stents are blocking smaller veins from stealing flow back up the leg, focalizing the blood flow into the foot.
pDVA Procedure Using the LimFlow System
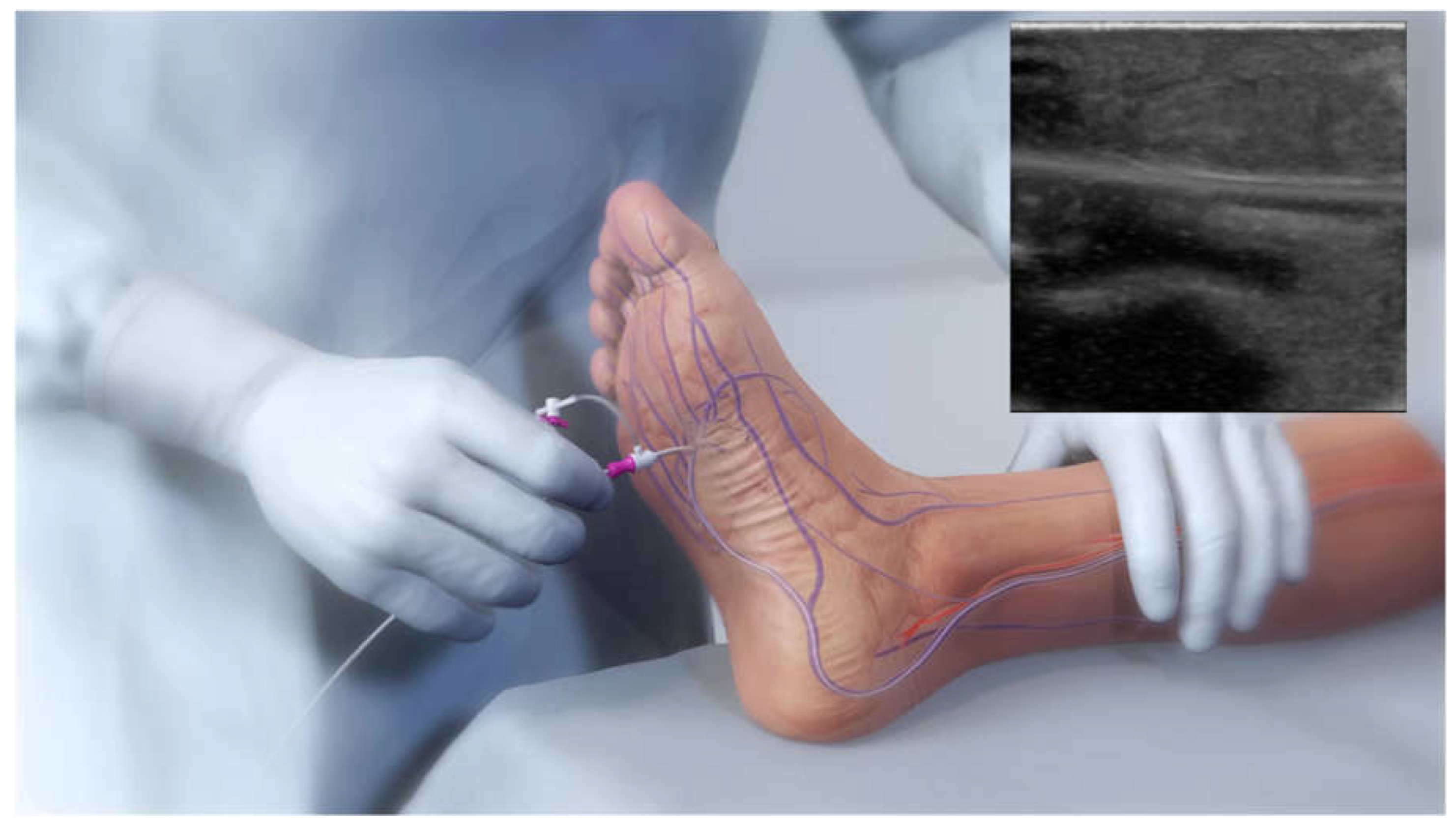
Venous access is achieved in the lateral plantar vein (
Figure 1), and the venous catheter is advanced up the vein to the intended crossing point in the tibials, which is selected after simultaneous digital subtraction angiography (DSA) and venography to identify the shortest distance between the vessels. After achieving antegrade femoral arterial approach, an arterial crossing catheter is inserted and advanced down the artery to the below-the-knee crossing point. When the two catheters are aligned, artery-to-vein crossing is achieved by advancing the needle from the arterial crossing catheter into the basket of the venous catheter. This is followed by advancing a guidewire through the venous access at the bottom of the foot. The connection is then dilated by a small angioplasty balloon, allowing the passage of other devices. Then the 4F over-the-wire push valvulotome serves to render the venous valves incompetent, which allows reversal flow in the vein, bringing oxygenated blood to the foot. Self-expanding stent-grafts are then placed from the level of the ankle to just below the crossing point, creating a conduit for continuous blood flow and covering venous collaterals. Finally, a tapered, self-expanding, covered stent graft is deployed to optimize the transition and flow from the artery into the vein, preventing cardiac overload as well as leakage at the crossover point.
Figure 2 demonstrates an illustration of the basic procedural steps. Procedural time ranges from 90 minutes to 4 hours. After the procedure, patients are kept on lifelong antiplatelet therapy (oral clopidogrel or aspirin) in combination with warfarin or direct oral anticoagulants for 3 months. A follow-up protocol for monitoring blood flow to the affected area is necessary using duplex ultrasound.
29 The system earned the CE mark in 2016 and FDA approval in 2023 and is available in Europe and the United States.
Notably, an important observation should be taken into consideration when using the LimFlow device: prior to the creation of the AV fistula, patients may necessitate arterial and/or venous interventions (i.e., arterioplasty, venoplasty).34
Studies with the LimFlow Device
PROMISE I
PROMISE I was a single-arm, multicenter pilot study and the first published report concerning the safety and efficacy of the LimFlow system in the United States. 32 “no option” CLTI patients were encompassed. Technical success was reported at 97%. Reinterventions were performed in 52% of patients, with most of them (88%) occurring within the first 3 months. The wound healing status of fully healed or healing was 67% and 75% at 6 and 12 months, respectively. The amputation-free survival rates (AFS) at 30-days, 6-months, and a year following the procedure were found to be 91%, 74%, and 70%, respectively.35
PROMISE II
PROMISE II was a multi-center, prospective, single-arm study for the assessment of the safety and efficacy of DVA using the LimFlow, encompassing 105 no-option CLTI patients treated in the USA. The primary endpoint of the trial was AFS at 6 months. Secondary outcomes include the healing of chronic wounds caused by ischemia. Published data from the trial report a technical success rate of 99%. At 6 months, 66.1% of the patients had AFS. Limb salvage (avoidance of above-ankle amputation) was attained in 67 patients (76.0% by Kaplan-Meier analysis). Complete wound healing was reported in 25% of patients, and 51% were in the process of healing.36
ALPS
The ALPS study was a multi-center study that aimed to evaluate retrospectively 32 no-option CLTI patients treated with the LimFlow procedure. The primary endpoint of the trial was AFS at 6 months, while secondary outcomes comprised wound healing, survival rate, and limb salvage at 6, 12, and 24 months. Achieved technical success was 96.9%. Recorded AFS at 6, 12, and 24 months was 83.9%, 71%, and 67.2%, respectively. The reported percentages for limb salvage at 6, 12, and 24 months were 86.8%, 79.8%, and 79.8%, respectively, while for complete wound healing, they were 36.6%, 68.2%, and 72.7%. 17 patients needed reintervention for occlusion.37
PROMISE International
This study is a single-arm, open-label, prospective study with a twelve-month follow-up period. It encompassed 50 no-option CLTI patients. The primary endpoint will be AFS, and secondary outcomes will include complete wound healing, primary and secondary patency, limb salvage, renal function, and technical and procedural success.22
PROMISE UK
PROMISE UK (NCT03807661) is an ongoing multicenter post-market national trial that has already enrolled 28 patients. Primary outcomes will be AFS throughout 1 year. Secondary outcomes comprise wound healing, primary and secondary patency, limb salvage, procedural and technical success, as well as quality of life.
PROMISE III
PROMISE III (NCT05313165) is a prospective, multicenter, single-arm study with an estimated enrollment of 100 patients. Primary outcomes will be AFS throughout 6 months. Secondary outcomes include various measures such as limb salvage, change in Rutherford classification, wound healing, freedom from contrast-induced nephropathy, as well as procedural and technical success.
The purpose and status of studies in the treatment of CLTI with the LimFlow device are summarized in
Table 2.
Discussion
Percutaneous DVA is considered the last-chance treatment for no-option patients with CLTI. In this way, palliative amputation, which is linked to a high risk of peri-procedural and 1-year mortality, is avoided. Due to the lack of a reproducible and standardized procedure, venous arterialization has been attempted in multiple settings using tools and techniques currently available (i.e., “off-label”). In studies where alternate techniques of venous arterialization have been used, technical and clinical success varied from 77% to 100% and from 29% to 75%, respectively.38 A multicenter retrospective study in Japan, the DEPARTURE JAPAN Study, reports the results of pDVA using the off-the-shelf technique. The VAST was used in 83.3% of the cases. The technical success rate of percutaneous deep venous arterialization was 88.9%, and the amputation-free survival rates at 6 and 12 months were 55.6% and 49.4%, respectively. Most of the studies focus on AFS because it provides both a measure of the safety (mortality) and effectiveness (limb salvage) of the procedure.35 Published studies demonstrate that pDVA using the LimFlow System can be performed with good technical success and high AFS rates at 6 and 12 months. More precisely, to date, according to the published data, technical success and AFS at 6 months are supposed to be over 96% and 66%, respectively. We should underline that, whichever method is used, the right equipment (sheaths, wires, catheters, balloons, and stents) is crucial. In this way, experts will be able to effectively manage possible complications and reduce the time of the procedure. A potential complication related to the pDVA procedure is bleeding at the vessel puncture when crossing is attempted with non-dedicated devices, which could lead to compartment syndrome. No complications were reported in the studies evaluating the LimFlow device.27 However, rates of secondary interventions to optimize flow and wound healing are relatively high when compared with conventional arterial revascularization. This could be explained by the advanced and diffuse atherosclerotic disease of the target population.35 Reinterventions can be endovascular or/and surgical and are reported for occlusion, asymptomatic stenosis, infection of the stent-graft, and bleeding from a superficial vein adjacent to the granulating wound. A variety of techniques and devices are used during reintervention, including thrombolysis, mechanical thrombectomy, DCB angioplasty, and stenting.38
Challenges during pDVA using the LimFlow System include navigation through a complex pedal venous system and management of venous spasm. Pre-procedure ultrasound or venography contributes to identifying the venous anatomy and optimizing case planning.35 Apart from that, close monitoring of patients after the LimFlow procedure and prompt adjustments to postprocedural care play an essential role in order to achieve a good and long-term result.
Finally, despite the upfront cost of the LimFlow System, the costs, prevalence, and mortality rate of CLTI qualify pDVA with the LimFlow System as a cost-effective and “high-value” treatment compared to the status quo treatment.39
Conclusion
The evolution of endovascular technology has allowed for the development of percutaneous deep vein arterialization (pDVA) approaches. The lack of options for CLI patients highlights pDVA as a last option for limb salvage when amputation seems to be the only appropriate option. The advantage of pDVA is its minimally invasive nature, which in turn permits the absence of wounds and lower rates of infections. The novel technique of the LimFlow device combines the advantages of surgical DVA with those of a minimally invasive procedure. Published data concerning the pDVA procedure with the LimFlow system demonstrate encouraging results concerning technical success, wound healing, and limb salvage. With the LimFlow device, vessel crossing, and valve disruption can be easily performed. Reinterventions are common due to the advanced and diffuse atherosclerotic disease of the target population. Long-term results can be achieved by optimizing the nutritive flow towards the ischemic tissue and by close postprocedural monitoring for any changes in the patient’s clinical condition (swelling, pain, infection).
References
- Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018;275:379-381. [CrossRef]
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45 Suppl S:S5-67. [CrossRef]
- Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg 2015;62(6):1642-51 e3. [CrossRef]
- Uccioli L, Meloni M, Izzo V, Giurato L, Merolla S, Gandini R. Critical limb ischemia: current challenges and future prospects. Vasc Health Risk Manag 2018;14:63-74. [CrossRef]
- Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14. [CrossRef]
- Bradbury AW, Moakes CA, Popplewell M, et al.; BASIL-2 Investigators. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. Lancet. 2023 ;401(10390):1798-1809. 27 May. [CrossRef] [PubMed]
- Albers M, Romiti M, Brochado-Neto FC, Pereira CA. Meta-analysis of alternate autologous vein bypass grafts to infrapopliteal arteries. J Vasc Surg 2005;42(3):449-55. [CrossRef]
- Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg 2008;47(5):975-981. [CrossRef]
- Faglia E, Clerici G, Clerissi J, et al. Long-term prognosis of diabetic patients with critical limb ischemia: a population-based cohort study. Diabetes Care 2009;32(5):822-7. [CrossRef]
- Ho VT, Gologorsky R, Kibrik P, et al. Open, percutaneous, and hybrid deep venous arterialization technique for no-option foot salvage. Journal of Vascular Surgery 2020;71(6):2152-2160. [CrossRef]
- Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019;69(6S):3S-125S e40. [CrossRef]
- Schreve MA, Vos CG, Vahl AC, et al. Venous Arterialisation for Salvage of Critically Ischaemic Limbs: A Systematic Review and Meta-Analysis. European Journal of Vascular and Endovascular Surgery 2017;53(3):387-402. [CrossRef]
- Francois-Franck, M. Note à propos de la communication de M Raimond Petit sur la susture artério-veneuse. Compt Rend Hebd Soc Biol 1896;48:150.
- Ho VT, Gologorsky R, Kibrik P, et al. Open, percutaneous, and hybrid deep venous arterialization technique for no-option foot salvage. J Vasc Surg 2020;71(6):2152-2160. [CrossRef]
- Szilagyi DE, Jay GD, 3rd, Munnell ER. Femoral arteriovenous anastomosis in the treatment of occlusive arterial disease. AMA Arch Surg 1951;63(4):435-51. (In eng). [CrossRef]
- Sheil, GR. Treatment of critical ischaemia of the lower limb by venous arterialization : an interim report. Br J Surg 1977;64(3):197-9. [CrossRef]
- Ozek C, Zhang F, Lineaweaver WC, et al. Arterialization of the venous system in a rat lower limb model. Br J Plast Surg 1997;50(6):402-7. [CrossRef]
- Graham AM, Baffour R, Burdon T, et al. A demonstration of vascular proliferation in response to arteriovenous reversal in the ischemic canine hind limb. J Surg Res 1989;47(4):341-7. [CrossRef]
- Baffour R DR, Burdon T, Sniderman A, Common A, Graham A et al. An angiographic study of ischaemia as a determinant of neovascularisation in arteriovenous reversal. Surg Gynaecol Obstet 1988;166:28-32.
- Chang HW, Hsu CH, Jhang YC, Wang CC, Chang CT, Chen HC. Percutaneous Deep Vein Arterialization for a Chronic Limb-Threatening Ischemia Patient in Taiwan. Acta Cardiol Sin 2021;37(4):434-437. [CrossRef]
- Saab FA, Mustapha JA, Ansari M, et al. Percutaneous Deep Venous Arterialization: Treatment of Patients with End-Stage Plantar Disease. Journal of the Society for Cardiovascular Angiography & Interventions 2022;1(6). [CrossRef]
- Schreve MA, Lichtenberg M, Ünlü Ç, et al. PROMISE international; a clinical post marketing trial investigating the percutaneous deep vein arterialization (LimFlow) in the treatment of no-option chronic limb ischemia patient. CVIR Endovascular 2019;2(1). [CrossRef]
- Ysa A, Lobato M, Mikelarena E, et al. Homemade Device to Facilitate Percutaneous Venous Arterialization in Patients With No-Option Critical Limb Ischemia. J Endovasc Ther 2019;26(2):213-218. [CrossRef]
- Ichihashi S, Shimohara Y, Bolstad F, Iwakoshi S, Kichikawa K. Simplified Endovascular Deep Venous Arterialization for Non-option CLI Patients by Percutaneous Direct Needle Puncture of Tibial Artery and Vein Under Ultrasound Guidance (AV Spear Technique). Cardiovasc Intervent Radiol 2020;43(2):339-343. [CrossRef]
- Migliara B, Cappellari TF. A Novel Technique to Create an Arteriovenous Fistula During Total Percutaneous Deep Foot Venous Arterialisation Using an IVUS Guided Catheter. Eur J Vasc Endovasc Surg 2018;55(5):735. [CrossRef]
- Gandini R, Merolla S, Scaggiante J, et al. Endovascular Distal Plantar Vein Arterialization in Dialysis Patients With No-Option Critical Limb Ischemia and Posterior Tibial Artery Occlusion: A Technique for Limb Salvage in a Challenging Patient Subset. J Endovasc Ther 2018;25(1):127-132. [CrossRef]
- Ichihashi S, Iwakoshi S, Nakai T, et al. Role of Percutaneous Deep Venous Arterialization for Patients with Chronic Limb-threatening Ischemia. Interv Radiol (Higashimatsuyama) 2023;8(2):97-104. [CrossRef]
- Kum S, Tan YK, Schreve MA, et al. Midterm Outcomes From a Pilot Study of Percutaneous Deep Vein Arterialization for the Treatment of No-Option Critical Limb Ischemia. J Endovasc Ther 2017;24(5):619-626. [CrossRef]
- Kum S, Huizing E, Schreve MA, et al. Percutaneous deep venous arterialization in patients with critical limb ischemia. J Cardiovasc Surg (Torino) 2018;59(5):665-669. [CrossRef]
- Schreve MA, Minnee RC, Bosma J, Leijdekkers VJ, Idu MM, Vahl AC. Comparative study of venous arterialization and pedal bypass in a patient cohort with critical limb ischemia. Ann Vasc Surg 2014;28(5):1123-7. [CrossRef]
- Montero-Baker M, Sommerset Rvt J, Miranda JA. How I Do It: Hybrid Superficial Venous Arterialization and Endovascular Deep Venous Arterialization. Journal of Vascular Surgery Cases, Innovations and Techniques 2023:101160. [CrossRef]
- Ferraresi R, Casini A, Losurdo F, et al. Hybrid Foot Vein Arterialization in No-Option Patients With Critical Limb Ischemia: A Preliminary Report. J Endovasc Ther 2019;26(1):7-17. [CrossRef]
- Alexandrescu V, Ngongang C, Vincent G, Ledent G, Hubermont G. Deep calf veins arterialization for inferior limb preservation in diabetic patients with extended ischaemic wounds, unfit for direct arterial reconstruction: preliminary results according to an angiosome model of perfusion. Cardiovasc Revasc Med 2011;12(1):10-9. [CrossRef]
- Mustapha JA, Saab FA, Clair D, Schneider P. Interim Results of the PROMISE I Trial to Investigate the LimFlow System of Percutaneous Deep Vein Arterialization for the Treatment of Critical Limb Ischemia. J Invasive Cardiol 2019;31(3):57-63.
- Clair DG, Mustapha JA, Shishehbor MH, et al. PROMISE I: Early feasibility study of the LimFlow System for percutaneous deep vein arterialization in no-option chronic limb-threatening ischemia: 12-month results. J Vasc Surg 2021;74(5):1626-1635. [CrossRef]
- Shishehbor MH, Powell RJ, Montero-Baker MF, et al. Transcatheter Arterialization of Deep Veins in Chronic Limb-Threatening Ischemia. N Engl J Med 2023;388(13):1171-1180. [CrossRef]
- Schmidt A, Schreve MA, Huizing E, et al. Midterm Outcomes of Percutaneous Deep Venous Arterialization With a Dedicated System for Patients With No-Option Chronic Limb-Threatening Ischemia: The ALPS Multicenter Study. J Endovasc Ther 2020;27(4):658-665. [CrossRef]
- Takagi T, Miyamoto A, Ohura N, Yamauchi Y. Percutaneous deep venous arterialization with balloon angioplasty salvaged a life-threatening critical limb in a hemodialysis patient after repeated pedal angioplasty failed: A case report. Clin Case Rep 2023;11(6):e7589. [CrossRef]
- Pietzsch J, M E, BP G, PA S. Cost-Effectiveness of Percutaneous Deep Vein Arterialization for Patients With No-Option Chronic Limb-Threatening Ischemia: An Exploratory Analysis Based on the PROMISE I Study. J CRIT LIMB ISCHEM 2021;1(4):E148-E157.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).